Abstract
We combined tissue culture and flow cytometry to assess the activities of various temperatures, chemicals, and disinfectants on the viability and infectivity of spores of Encephalitozoon intestinalis. Surfanios and benzalkonium chloride, disinfectants currently used in the hospital, were remarkably efficient in destroying spore viability and infectivity.
Microsporidia are emerging pathogens in immunocompromised patients (2, 3, 11). No treatment is yet available, and preventive measures should be implemented, including the use of physical or chemical methods to destroy or inactivate infective spores present in the environment (4, 9). Tissue culture methods have already been used to assess the infectivity of spores exposed to various temperatures or disinfectants (6, 8, 10, 12). This technique is not fully appropriate, however, for assessing spore viability. In this study, we combined tissue culture and a flow cytometry method to assess the viability and infectivity of spores of Encephalitozoon intestinalis that had been exposed to various temperatures and disinfectants.
The strain of E. intestinalis used in this study was obtained from a human immunodeficiency virus-infected patient. It was maintained in U-373 human glioblastoma cells (MG-ATCC-HTB 17) in 75-cm2 culture flasks. Every other day from day 10 postinfection, spores were harvested from the supernatant and stored at 4°C until use.
Effect of different temperatures.
One milliliter of a spore suspension adjusted to 107 spores/ml in phosphate-buffered saline (PBS) was subjected to various temperatures (37, 4, or −20°C) over 15 days. Spores resuspended in culture medium plus 10% dimethyl sulfoxide were frozen at −196 or −80°C for 15 days. After treatment, spores were washed three times with PBS, resuspended in 1 ml of RPMI, and then titrated in tissue culture.
Exposure to chemical and disinfectants.
One milliliter of a spore suspension adjusted to 107 spores/ml was centrifuged for 10 min at 3,500 × g. Pellets were resuspended in 250 μl of various chemicals or disinfectants (Table 1). After exposure, spores were washed three times with PBS; then the pellet was resuspended in 1 ml of RPMI. The spore suspension was adjusted to 2 × 106/ml prior to culture titration and flow cytometry.
TABLE 1.
Effect of a 5-min exposure to chemical agents and disinfectants on infectivity and viability of spores of E. intestinalis
| Treatment | % of spores recovered | Culture titration
|
Flow cytometry
|
|||||
|---|---|---|---|---|---|---|---|---|
| Mean titera:
|
Pc | % Reduction | % Viabilityb:
|
% Reduction | ||||
| Before treatment | After treatment | Before treatment | After treatment | |||||
| HCl, 0.1 N | 84 | 90.5 (64-128) | 22.6 (16-32) | 0.0001 | 75 | 79 | 50 | 37 |
| NaOH, 0.1 N | 86 | 84.4 (64-128) | 34.3 (32-64) | 0.002 | 59 | 79 | 54 | 32 |
| Surfanios, 0.2% | 9 | 97.0 (64-256) | 0 (0-0) | <10−4 | 100 | 80 | 23 | 79 |
| Benzalkonium chloride | Aggregated spores | 147.8 (128-256) | NDd | ND | ND | ND | ND | |
| Sodium hypochlorite (chlorine, 36 g/liter) | Spores destroyed | 256 (256-256) | ND | ND | ND | ND | ND | |
| Ethanol, 70% | 43 | 106.2 (64-256) | 0 (0-0) | <10−4 | 100 | 78 | 23 | 71 |
Geometric mean (range) of the titer from 10 to 15 titrations.
Percent nonfluorescent (viable) spores from a count of 10,000 spores.
Wilcoxon rank-sum test.
ND, not done.
Assessment of infectivity by tissue culture.
Ninety-six-well tissue culture plates were seeded with U-373-MG cells in RPMI medium. Serial twofold dilutions of treated and untreated (control) spores, ranging from 1 to 1.10−5, were prepared in RPMI. Each of five replicate culture wells was infected with 50 μl of each spore dilution; culture plates were then incubated at 37°C for 8 days without changing the medium. On day 8 postinfection, plates were examined under an inverted microscope (×250). The last dilution (titer) for which there was at least one parasitic focus of infection was recorded for each series of dilutions.
Assessment of viability by flow cytometry.
Ten microliters of a freshly prepared solution of propidium iodide (Sigma, Saint Quentin, France) at 10 mg/liter in PBS was added to 500 μl of each spore suspension (treated and controls) and then incubated at 37°C in the dark for 30 min. Analyses were performed with a Beckman Coulter Epics XL apparatus. Ten thousand cells were analyzed, and light scatter and fluorescence signals were collected, assuming that fluorescent spores were dead and nonfluorescent spores were viable (1).
Statistical analysis.
Titration data are presented as geometric mean values and ranges from at least two separate experiments (i.e., 10 to 15 replicate titrations) except for the temperature of −20°C (one experiment, three replicate cultures). Results obtained for each disinfectant and temperature were compared to controls by using the Wilcoxon rank-sum test.
The culture method used for titration of E. intestinalis spore suspensions was reproducible, as 65 separate titrations of untreated spore suspensions yielded a mean final titer of 6.9 log2 ± 0.7, i.e., a coefficient of variation of 10.6%. Assessment of viability by cytometry was also reproducible, since the percentage of viable spores, estimated from 10,000 counts of events in seven separate experiments, ranged from 71 to 81% (mean, 78.4% ± 3.4%).
Storage at low temperatures for 2 weeks resulted in a marked decrease of infectivity, even in the presence of dimethyl sulfoxide. Mean titers of spore suspensions exposed to −20, −80, and −196°C were reduced by 99.6, 99.3, and 99.3%, respectively (P < 0.001 for the three temperatures). These results are in agreement with those of Shadduck and Polley (8), obtained with Encephalitozoon cuniculi, and those of Koudela et al. (6), who found that freezing rendered E. intestinalis spores noninfective for SCID mice. For short-term storage of spores, we confirmed that 4°C was optimal (7), since no reduction (0%) of infectivity was observed compared to controls. In contrast, exposure at 37°C resulted in 100% loss of infectivity. A brief exposure of 5 min at 60 or 100°C resulted in 100% loss of infectivity. This confirms that boiling definitively inactivates the spores (5).
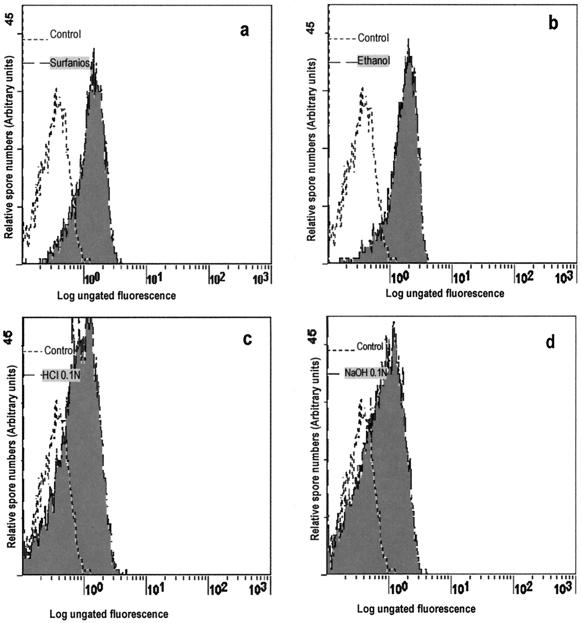
Exposure of spores for 5 min to HCl or NaOH had a significant effect on infectivity (75 and 59% reduction, respectively) and reduced viability by 37 and 32%, respectively (Table 1; Fig. 1). Surfanios and ethanol resulted in 100% reduction of infectivity compared to controls. Reducing the time of exposure to ethanol to 30 s was equally efficient, whereas exposure for 10 s resulted in 62% reduction of infectivity (data not shown). The examination of spore viability by flow cytometry confirmed the remarkable efficacy of ethanol and Surfanios (Fig. 1). For benzalkonium and for sodium hypochlorite, viability assessment was not feasible because spores were aggregated or destroyed during exposure.
FIG. 1.
Assessment of the viability of spores of E. intestinalis by flow cytometry (fluorescence histograms). Spores were exposed to Surfanios for 5 min (a), 70% ethanol for 5 min (b), 0.1 N HCl for 5 min (c), or 0.1 N NaOH for 5 min (d).
Thus, both methods demonstrated the activity of 70% ethanol, a result which is in agreement with those reported by Shadduck and Polley for E. cuniculi (8). Similarly, we confirmed the activity of sodium hypochlorite on spores of E. intestinalis (12).
In addition, we show that two disinfectants routinely used in the hospital are remarkably efficient. Benzalkonium chloride, a bactericidal and fungicidal quaternary amine, produced a rapid aggregation of spores. This effect is of interest in terms of clinical practice, as this disinfectant is routinely used for rapid hand disinfection. Surfanios is a combination of amino acid hydrochloride, anion chelators, and didecyldimethylammonium chloride which is effective against bacteria, viruses, and fungi. Here, we show that Surfanios combined direct destruction of spores and inhibition of their infectivity and viability. This finding is relevant in term of hospital hygiene, as Surfanios is used for cleaning and disinfection of surfaces in hospital wards.
REFERENCES
- 1.Borel, E., M. Mayençon, K. Kaiser, S. Picot, and F. Peyron. 1998. Fluorogenic detection of viable Toxoplasma gondii. Parasite 5:371-373. [DOI] [PubMed] [Google Scholar]
- 2.Bryan, R. T., and D. A. Schwartz. 1999. Epidemiology of microsporidiosis, p. 502-516. In M. Wittner and L. M. Weiss (ed.), The microsporidia and microsporidiosis. ASM Press, Washington, D.C.
- 3.Didier, E. S., K. F Snowden, and J. A. Shadduck. 1998. Biology of microsporidia species infecting mammals. Adv. Parasitol. 40:283-320. [DOI] [PubMed] [Google Scholar]
- 4.Dowd, S. E., C. P. Gerba, and I. L. Pepper. 1998. Confirmation of the human pathogenic microsporidia Enterocytozoon bieneusi, Encephalitozoon intestinalis, and Vittaforma corneae in water. Appl. Environ. Microbiol. 64:3332-3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Green, L. C., P. J. LeBlanc, and E. S. Didier. 2000. Discrimination between viable and dead Encephalitozoon cuniculi (microsporidian) spores by dual staining with Sytox Green and Calcofluor White M2R. J. Clin. Microbiol. 38:3811-3814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koudela, B., S. Kucerova, and T. Hudcovic. 1999. Effect of low and high temperatures on infectivity of Encephalitozoon cuniculi spores suspended in water. Folia Parasitol. (Prague) 46:171-174. [PubMed] [Google Scholar]
- 7.Kucerova-Pospisilova, Z., D. Carr, G. Leitch, M. Scanlon, and G. S. Visvesvara. 1999. Environmental resistance of Encephalitozoon spores. J. Eukaryot. Microbiol. 46:11S-13S. [PubMed] [Google Scholar]
- 8.Shadduck, J. A., and M. B. Polley. 1978. Some factors influencing the in vitro infectivity and replication on Encephalitozoon cuniculi. Protozoology 25:491-496. [DOI] [PubMed] [Google Scholar]
- 9.Sparfel, J. M., C. Sarfati, O. Liguory, et al. 1997. Detection of microsporidia and identification of Enterocytozoon bieneusi in surface water by filtration followed by specific PCR. J. Eukaryot. Microbiol. 6:78S. [DOI] [PubMed] [Google Scholar]
- 10.Waller, T. 1980. Sensitivity of Encephalitozoon cuniculi to various temperatures, disinfectants and drugs. Lab. Anim. Sci. 13:277-285. [DOI] [PubMed] [Google Scholar]
- 11.Weber, R., R. T. Bryan, D. A. Swartz, and R. L. Owen. 1994. Human microsporidian infections. Clin. Microbiol. Rev. 7:426-461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wolk, D. M., C. H. Johnson, E. W. Rice, N. H. Marshall, K. F. Grahm, C. B. Plummer, and C. R. Sterling. 2000. A spore counting method and cell culture model for chlorine disinfection studies of Encephalitozoon syn. Septata intestinalis. Appl. Environ. Microbiol. 66:1266-1273. [DOI] [PMC free article] [PubMed] [Google Scholar]