Abstract
Seven peptidyl proteasome inhibitors were tested for in vitro activity against Trypanosoma brucei bloodstream forms. Two compounds showed activity in the low nanomolar range. In general, trypanosomes were more susceptible to the compounds than were human HL-60 cells. The data support the potential of proteasome inhibitors for rational antitrypanosomal drug development.
Trypanosoma brucei is the causative agent of human sleeping sickness in Africa. Over 60 million persons are at risk of acquiring the infection, and approximately 50,000 new cases are reported annually (21). If left untreated, the disease is fatal. For chemotherapy of sleeping sickness only four drugs, with serious side effects, are available (5). Therefore, the identification of novel targets for chemotherapy is urgently required if new treatments of the disease are to be developed.
The proteasome is a multicatalytic proteinase complex which plays a critical role in intracellular protein degradation (4). Inhibitors of the proteasome have recently received attention as a novel class of anticancer drugs (2, 8, 12, 15, 17). However, drugs developed as potential antitumor agents could also be of use against sleeping sickness, as has been shown for the ornithine decarboxylase inhibitor d,l-α-difluoromethylornithine (3). We therefore investigated the trypanocidal activities of different peptidyl proteasome inhibitors against in vitro-cultured bloodstream form T. brucei.
Proteasome inhibitors.
N-Acetyl-leucyl-leucyl-norleucinal (Ac-Leu-Leu-Nle-CHO), N-acetyl-leucyl-leucyl-methional (Ac-Leu-Leu-Met-CHO), N-benzyloxycarbonyl-isoleucyl-γ-t-butyl-glutamyl-alanyl-leucinal [Z-Ile-Glu(OtBu)-Ala-Leu-CHO],N-benzyloxycarbonyl-leucyl-leucyl-leucinal (Z-Leu-Leu-Leu-CHO), and N-benzyloxycarbonyl-leucyl-leucyl-tyrosyl α-keto aldehyde (Z-Leu-Leu-Tyr-COCHO) were purchased from Bachem Biochemica GmbH (Heidelberg, Germany), and N-benzyloxycarbonyl-leucyl-leucyl-phenylalanal (Z-Leu-Leu-Phe-CHO) and N-benzyloxycarbonyl-leucyl-leucyl-leucyl boronic acid [Z-Leu-Leu-Leu-B(OH)2] were purchased from Alexis Deutschland GmbH (Grünberg, Germany). All compounds have been shown previously to be inhibitors of the proteasome (8-10, 16, 17).
Assays.
Bloodstream form T. brucei (TC221) and human myeloid leukemia HL-60 cells were grown axenically as described previously (11).
For toxicity tests, cells were seeded into 24-well plates at appropriate densities (104 trypanosomes/ml; 5 × 104 HL-60 cells/ml) in 1 ml of medium containing various concentrations (10−4 to 10−12 M) of proteasome inhibitors dissolved in 100% dimethyl sulfoxide (DMSO). The controls contained DMSO alone. In all experiments, the final DMSO concentration was 1%, which had no effect on the cell growth (11). After 48 h of incubation, living cells were counted with a Neubauer hemocytometer. The control cell counts were 106 trypanosomes/ml and 5 × 105 HL-60 cells/ml. Each experiment was set up in duplicate and repeated three times.
For detection of apoptosis, cells were exposed to proteasome inhibitors at various concentrations for 24 h, harvested by centrifugation, and fixed overnight with 70% ethanol at −20°C. Then, cells were washed twice with HBSS (Hanks balanced salt solution) and 5 min with 900 μl of HBSS plus 300 μl of 200 mM Na2HPO4-100 mM citrate (pH 7.8) and stained with 200 μl of HBSS containing 0.5 mg of RNase per ml and 0.5 μg of propidium iodide per ml. After incubation for 30 min at room temperature, the DNA content of propidium iodide-stained cells was analyzed with a FACScan analytical cytometer using CellQuest Software (Becton Dickinson, Heidelberg, Germany). Cells from the sub-G0/G1 peak were counted as apoptotic cells (14).
Antitrypanosomal activities of proteasome inhibitors.
Peptide aldehydes are the best-characterized inhibitors of the proteasome. These agents are substrate analogues, and cells are permeable to them. Previous studies have revealed that peptide aldehydes of the scaffold R-Leu-Leu-Xaa-CHO are the most potent inhibitors of the proteasome (1). Therefore, Ac-Leu-Leu-Nle-CHO, Ac-Leu-Leu-Met-CHO, Z-Leu-Leu-Leu-CHO, Z-Leu-Leu-Phe-CHO, and Z-Leu-Leu-Tyr-COCHO were chosen to have their antitrypanosomal activities tested. Since peptide boronates are much more potent inhibitors of the proteasome (1, 8), the trypanocidal activity of the boronate analogue of Z-Leu-Leu-Leu-CHO, Z-Leu-Leu-Leu-B(OH)2, was also investigated. Z-Ile-Glu(OtBu)-Ala-Leu-CHO was included in this study because this compound has been shown to inhibit the proteasome purified from T. brucei (13). All seven compounds exhibited antitrypanosomal activities against bloodstream form T. brucei, with 50% effective doses (ED50s) and MICs varying 10,000-fold (Table 1). The most trypanocidal proteasome inhibitors were Z-Ile-Glu(OtBu)-Ala-Leu-CHO and Z-Leu-Leu-Leu-B(OH)2, with ED50s of 0.086 and 0.32 nM and MICs of 40 and 10 nM, respectively. Thus, the antitrypanosomal activities of these two proteasome inhibitors are comparable to that of pentamidine isethionate, which is used to treat sleeping sickness (ED50 range, 1 to 2.5 nM; MIC range, 5.8 to 35 nM [11]).
TABLE 1.
ED50s and MICs of proteasome inhibitors for bloodstream form T. brucei and human HL-60 cellsa
| Compound |
T. brucei
|
HL-60
|
||
|---|---|---|---|---|
| ED50 (nM) | MIC (nM) | ED50 (nM) | MIC (nM) | |
| Ac-Leu-Leu-Nle-CHO | 45 | 100,000 | 4,600 | >100,000 |
| Ac-Leu-Leu-Met-CHO | 1,600 | 100,000 | 20,000 | >100,000 |
| Z-Ile-Glu(OtBu)-Ala- Leu-CHO | 0.086 | 40 | 36 | 100,000 |
| Z-Leu-Leu-Leu-CHO | 5.5 | 4,000 | 73 | >100,000 |
| Z-Leu-Leu-Phe-CHO | 150 | 7,000 | 120 | 100,000 |
| Z-Leu-Leu-Tyr-COCHO | 720 | 100,000 | 3,000 | >100,000 |
| Z-Leu-Leu-Leu-B(OH)2 | 0.32 | 10 | 1.1 | 1,000 |
Values are the means of three independent experiments. The standard error never exceeded 30%.
The general cytotoxicities of the proteasome inhibitors were assayed with HL-60 cells (Table 1). All compounds were also active against the human cells, with ED50s ranging from 1.1 nM to 20 μM. Encouragingly, the MICs of six compounds were ≥100 μM, the highest concentration tested; only Z-Leu-Leu-Leu-B(OH)2 had a MIC of 1 μM. However, the ED50 and MIC ratios of cytotoxic to trypanocidal activities were found to be in a modest range for nearly all proteasome inhibitors (Table 2). Only Z-Ile-Glu(OtBu)-Ala-Leu-CHO gave substantial ED50 and MIC ratios, with values of 400 and 2,500, respectively. For comparison, anti-sleeping sickness drugs generally have higher ED50 and MIC ratios (11).
TABLE 2.
ED50 and MIC ratios of cytotoxic to trypanocidal activities of proteasome inhibitorsa
| Compound | ED50 (HL-60)/ED50 (T. brucei) | MIC (HL-60)/MIC (T. brucei) |
|---|---|---|
| AC-Leu-Leu-Nle-CHO | 102 | >1 |
| AC-Leu-Leu-Met-CHO | 13 | >1 |
| Z-Ile-Glu(OtBu)-Ala-Leu-CHO | 419 | 2,500 |
| Z-Leu-Leu-Leu-CHO | 13 | >25 |
| Z-Leu-Leu-Phe-CHO | 0.8 | 14 |
| Z-Leu-Leu-Tyr-COCHO | 4 | >1 |
| Z-Leu-Leu-Leu-B(OH)2 | 3 | 100 |
ED50 and MIC ratios were calculated from ED50s and MICs shown in Table 1.
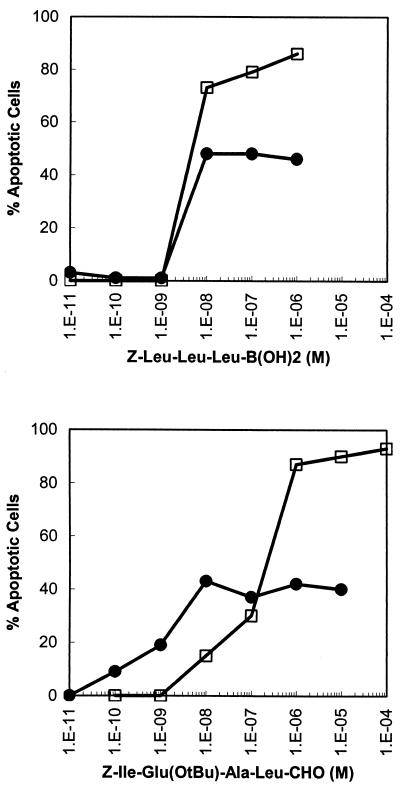
Proteasome inhibitors have been shown to induce apoptosis in many oncogenic cell types (17), and trypanosomes have been reported to be able to undergo apoptosis (18-20). This prompted us to investigate whether the growth-inhibitory effects of Z-Leu-Leu-Leu-B(OH)2 and Z-Ile-Glu(OtBu)-Ala-Leu-CHO, the two most toxic proteasome inhibitors studied (Table 1), can be attributed to induction of apoptosis. Based on hypodiploid DNA content, only 45% of trypanosomes appeared as apoptotic cells compared to 90% of HL-60 cells after exposure to Z-Leu-Leu-Leu-B(OH)2 or Z-Ile-Glu(OtBu)-Ala-Leu-CHO for 24 h (Fig. 1). The latter finding is in agreement with previous observations that treatment of HL-60 cells with proteasome inhibitors results in induction of apoptosis (6). Since only half of the trypanosomes appeared as apoptotic cells after treatment with both compounds, it is questionable whether the trypanocidal effect of proteasome inhibitors can be actually ascribed to induction of programmed cell death in bloodstream form T. brucei. Targets other than, or in addition to, the proteasome may be affected (8, 17).
FIG. 1.
Effects of Z-Leu-Leu-Leu-B(OH)2 and Z-Ile-Glu(OtBu)-Ala-Leu-CHO in inducing apoptosis in T. brucei and human cells. TC221 bloodstream forms (•) and HL-60 cells (□) were incubated with various concentrations of the proteasome inhibitors. After 24 h of culture, cells were stained with propidium iodide and analyzed with a fluorescence-activated cell sorter. Cells from the sub-G0/G1 peak were counted as apoptotic cells. The percentages of apoptotic cells were calculated in relation to the respective controls in the absence of proteasome inhibitor. Data points represent mean values from three independent experiments. The standard errors were typically less than 10%. 1.E-11 to 1.E-04, 10−11 to 10−4.
Although the compounds investigated in this study are not suitable for clinical use because of their cytotoxic effects, the results provide evidence that inhibition of the proteasome represents a new approach for the development of antitrypanosomal drugs. This finding may also be exploited in the future by utilizing the wealth of information currently being generated on proteasome inhibitors as anticancer agents (8, 12, 15, 17). For example, the relatively high trypanocidal activity of Z-Leu-Leu-Leu-B(OH)2 suggests that novel boronic acid proteasome inhibitors currently under clinical evaluation (2) may provide a new class of anti-sleeping sickness drugs in the future. Since trypanosome and mammalian proteasomes differ in terms of their substrate specificities (7), specific and nontoxic proteasome inhibitors are the rational choice for future antitrypanosomal drug development.
Acknowledgments
This work was supported in part by the Deutsche Forschungsgemeinschaft (SFB 544/Kontrolle Tropischer Infektionskrankheiten and Graduierten Kolleg 388/Biotechnologie).
We thank Kathy Andrews for critical reading of the manuscript.
REFERENCES
- 1.Adams, J., and R. Stein. 1996. Novel inhibitors of the proteasome and their therapeutic use in inflammation. Annu. Rep. Med. Chem. 31:279-288. [Google Scholar]
- 2.Adams, J., V. J. Palombella, E. A. Sausville, J. Johnson, A. Destree, D. D. Lazarus, J. Maas, C. S. Pien, S. Prakash, and P. J. Elliott. 1999. Proteasome inhibitors: a novel class of potent and effective antitumor agents. Cancer Res. 59:2615-2622. [PubMed] [Google Scholar]
- 3.Barrett, S. V., and M. P. Barrett. 2000. Anti-sleeping sickness drugs and cancer chemotherapy. Parasitol. Today 16:7-9. [DOI] [PubMed] [Google Scholar]
- 4.Coux, O., K. Tanaka, and A. L. Goldberg. 1996. Structure and functions of the 20S and 26S proteasomes. Annu. Rev. Biochem. 65:801-847. [DOI] [PubMed] [Google Scholar]
- 5.Croft, S. L. 1997. The current status of antiparasite chemotherapy. Parasitology 114(Suppl.):S3-S15. [PubMed] [Google Scholar]
- 6.Drexler, H. C. A. 1997. Activation of the cell death program by inhibition of proteasome function. Proc. Natl. Acad. Sci. USA 94:855-860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hua, S.-B., W.-Y. To, T. T. Nguyen, M.-L. Wong, and C. C. Wang. 1996. Purification and characterization of proteasomes from Trypanosoma brucei. Mol. Biochem. Parasitol. 78:33-46. [DOI] [PubMed] [Google Scholar]
- 8.Kisselev, A. F., and A. L. Goldberg. 2001. Proteasome inhibitors: from research tools to drug candidates. Chem. Biol. 8:739-758. [DOI] [PubMed] [Google Scholar]
- 9.Lynas, J. F., P. Harriott, A. Healy, M. A. McKervey, and B. Walker. 1998. Inhibitors of the chymotrypsin-like activity of proteasome based on di- and tri-peptidyl α-keto aldehydes (glyoxals). Bioorg. Med. Chem. Lett. 8:373-378. [DOI] [PubMed] [Google Scholar]
- 10.McCormack, T. A., A. A. Cruikshank, L. Grenier, F. D. Melandri, S. L. Nunes, L. Plamondon, R. L. Stein, and L. R. Dick. 1998. Kinetic studies of the branched chain amino acid preferring peptidase activity of the 20S proteasome: development of a continuous assay and inhibition by tripeptide aldehydes and clasto-lactacystin β-lactone. Biochemistry 37:7792-7800. [DOI] [PubMed] [Google Scholar]
- 11.Merschjohann, K., F. Sporer, D. Steverding, and M. Wink. 2001. In vitro effect of alkaloids on bloodstream forms of Trypanosoma brucei and T. congolense. Planta Med. 67:623-627. [DOI] [PubMed] [Google Scholar]
- 12.Murray, R. Z., and C. Norbury. 2000. Proteasome inhibitors as anti-cancer agents. Anticancer Drugs 11:407-417. [DOI] [PubMed] [Google Scholar]
- 13.Mutomba, M. C., W.-Y. To, W. C. Hyun, and C. C. Wang. 1997. Inhibition of proteasome activity blocks cell cycle progression at specific phase boundaries in African trypanosomes. Mol. Biochem. Parasitol. 90:491-504. [DOI] [PubMed] [Google Scholar]
- 14.Nicoletti, I., G. Migliorati, M. C. Pagliacci, F. Grignani, and C. Riccardi. 1991. A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry. J. Immunol. Methods 139:271-279. [DOI] [PubMed] [Google Scholar]
- 15.Rivett, A. J., and R. C. Gardner. 2000. Proteasome inhibitors: from in vitro uses to clinical trials. J. Pept. Sci. 6:478-488. [DOI] [PubMed] [Google Scholar]
- 16.Rock, K. L., C. Gramm, L. Rothstein, K. Clark, R. Stein, L. Dick, D. Hwang, and A. L. Goldberg. 1994. Inhibitors of the proteasome block the degradation of most cell proteins and the generation of peptides presented on MHC class I molecules. Cell 78:761-771. [DOI] [PubMed] [Google Scholar]
- 17.Shah, S. A., M. W. Potter, and M. P. Callery. 2001. Ubiquitin proteasome pathway: implications and advances in cancer therapy. Surg. Oncol. 10:43-52. [DOI] [PubMed] [Google Scholar]
- 18.Welburn, S. C., C. Dale, D. Ellis, R. Beecroft, and T. W. Pearson. 1996. Apoptosis in procyclic Trypanosoma brucei rhodesiense in vitro. Cell Death Differ. 3:229-236. [PubMed]
- 19.Welburn, S. C., and N. B. Murphy. 1998. Prohibitin and RACK homologues are up-regulated in trypanosomes induced to undergo apoptosis and in naturally occurring terminally differentiated forms. Cell Death Differ. 5:615-622. [DOI] [PubMed]
- 20.Welburn, S. C., S. Lillico, and N. B. Murphy. 1999. Programmed cell death in procyclic form Trypanosoma brucei rhodesiense—identification of differentially expressed genes during Con A induced death. Mem. Inst. Oswaldo Cruz 94:229-234. [DOI] [PubMed] [Google Scholar]
- 21.World Health Organization. March 2001. African trypanosomiasis or sleeping sickness. WHO Fact Sheet 259. [Online.] http://www.who.int/inf-fs/en/fact259.html.