Abstract
Twelve volunteers completed a two-sequence, three-way crossover study of a single 900-mg aspirin dose and multiple doses of 75 mg of oseltamivir in the absence and presence of 900 mg of aspirin. The plasma and urine results demonstrated no pharmacokinetic interaction between oseltamivir and aspirin.
Oseltamivir is a highly bioavailable (80%) ester prodrug of its active metabolite oseltamivir carboxylate, which is a potent and selective inhibitor of the neuraminidase enzyme of influenza A and B viruses (2). Oseltamivir is approved in the United States and Japan for the treatment of influenza A and B infection in adults and children ≥1 year old. Oseltamivir is metabolized to the carboxylate by high-capacity carboxylesterase in the liver (2, 6). After reaching a maximum, concentrations of oseltamivir in plasma quickly decline (half-life of 1 to 3 h), while levels of the carboxylate decline more slowly (half-life of 6 to 10 h). Approximately 60 to 80% of an oral dose of oseltamivir is excreted in the urine as the carboxylate. Once absorbed, aspirin is rapidly hydrolyzed by esterases in the gut wall, liver, plasma, and red blood cells to form salicylic acid, with a half-life of only 15 to 30 min (5). Salicylic acid is predominantly eliminated by competing hepatic conjugative and oxidative metabolic processes, while one of its metabolites, salicyluric acid, is secreted into the urine (4, 5). Because of the shared route of metabolism and excretion, drug interaction between oseltamivir and aspirin is theoretically possible. The purpose of this study was to evaluate whether such an interaction exists.
Twelve healthy subjects (eight males and four females) with a mean age of 36 ± 9 years, mean body weight of 73 ± 15 kg, and mean calculated creatinine clearance of 96 ± 19 ml/min participated in a three-way crossover study. The subjects were randomized into one of two sequence groups, ABC or BCA, and all subjects received two doses of 900 mg of aspirin and nine doses of 75 mg of oseltamivir (twice daily for 4 days plus a single dose the subsequent day) as shown in Table 1. On days 1, 8, and 9 for group ABC and days 4, 5, and 9 for group BCA, a 5- to 7.5-ml blood sample was collected from each subject predose and at 0.25, 0.5, 0.75, 1.0, 1.5, 2, 3, 4, 5, 6, 8, 10, 12, 18, and 24 h postdose. Urine samples were collected on the same days predose and 0 to 4, 4 to 8, 8 to 12, and 12 to 24 h after drug administration.
TABLE 1.
Overall study design
| Group (n)a | Day(s) | Step |
|---|---|---|
| ABC (6) | −28 to −1 | Screen |
| 1 | Aspirin, 900 mg | |
| 2 to 4 | Washout | |
| 5 to 8 | Oseltamivir, 75 mg b.i.d. | |
| 9 | Oseltamivir, 75 mg, + aspirin, 900 mg | |
| 13 to 19 | Follow-up | |
| BCA (6) | −28 to −1 | Screen |
| 1 to 4 | Oseltamivir, 75 mg b.i.d. | |
| 5 | Oseltamivir, 75 mg, + aspirin, 900 mg | |
| 6 to 8 | Washout | |
| 9 | Aspirin, 900 mg | |
| 13 to 19 | Follow-up |
A, single 900-mg dose of aspirin; B, 75 mg of oseltamivir twice a day (b.i.d.) for 4 days (eight doses); C, single doses of 900 mg of aspirin and 75 mg of oseltamivir. 24-h blood and urine samples were obtained following the morning dose on days 1, 8, and 9 for the ABC group and on days 4, 5, and 9 for the BCA group.
The assay for concentrations of oseltamivir and the carboxylate in plasma and urine has been reported previously (7). Plasma aspirin, salicylic acid, and salicyluric acid and urine salicylic acid and salicyluric acid were isolated by liquid-liquid extraction. One third of samples taken for the aspirin analysis contained additional decomposition peaks that interfered with quantitation. The quality of the determinations for plasma and urine salicylic acid and salicyluric acid was satisfactory, and the coefficients of variation were ≤10%. Pharmacokinetic parameters were derived from the resulting data by using noncompartmental methods. For aspirin and its metabolites, an analysis of variance appropriate for this design was performed, including terms for sequence, subjects within sequence, period, and regimen. For oseltamivir and the carboxylate, there was no term for period since period and regimen were completely confounded. Area under the plasma concentration-time curve (AUC) and maximum concentration (Cmax) of the major metabolites, carboxylate and salicylic acid, were considered the primary parameters, and analysis was performed with natural logarithm-transformed data.
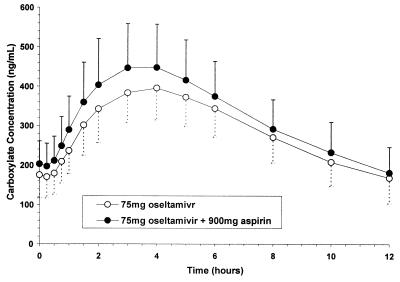
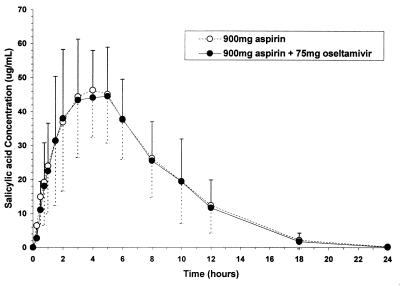
Because of assay interference, pharmacokinetic parameters for aspirin were not determinable. Plasma concentration-time profiles and mean pharmacokinetic parameters of the major metabolites, the carboxylate and salicylic acid, in the absence and presence of concomitant drug are displayed in Tables 2 and 3 and Fig. 1 and 2. The ratio of the means (C/B or C/A) and corresponding 90% confidence intervals for the primary parameters, Cmax and AUC from 0 to 12 h (AUC0-12), of the carboxylate and salicylic acid were within the 80-to-125% bioequivalence range when given separately or in combination, indicating a lack of interaction. Similarly, the AUC0-12 values and 90% confidence intervals of oseltamivir were within the bioequivalence range, while the Cmax of oseltamivir was higher in the presence of aspirin; this is considered likely due to intrasubject variability. The ratio of the means and corresponding 90% confidence intervals for Cmax and AUC0-∞ of salicyluric acid in the absence and presence of oseltamivir were also within the 80-to-125% bioequivalence range. The urinary excretion of oseltamivir, the carboxylate, salicylic acid, and salicyluric acid were comparable in the absence and presence of concomitant drug (Tables 2 and 3). The primary pharmacokinetic parameters of salicylic acid were equivalent in the presence and absence of oseltamivir; thus, it is reasonable to conclude the absence of an interaction between oseltamivir and aspirin in terms of hepatic esterase conversion. This finding is consistent with literature reports, since although metabolic drug interactions involving aspirin hydrolysis are theoretically possible, no studies to date have shown conclusively that aspirin hydrolysis is altered by coadministered drugs (4).
TABLE 2.
Steady-state pharmacokinetic parameters of oseltamivir carboxylate and oseltamivir in the absence and presence of aspirin
| Drug and regimena | Mean value (SD)b
|
||||||
|---|---|---|---|---|---|---|---|
| Cmax (ng/ml) | AUC0-12 (ng · h/ml) | Tmax (h) | t1/2 (h) | Oral CL (ml/min) | % Excreted | Renal CL (ml/min) | |
| Oseltamivir carboxylate | |||||||
| B | 404 (81) | 3,450 (744) | 3.7 (0.7) | 6.0 (2.2) | 345 (73) | 47 (9) | 161 (40) |
| C | 458 (113) | 3,880 (1,000) | 3.3 (0.7) | 5.9 (1.5) | 310 (72) | 52 (13) | 158 (46) |
| Oseltamivir | |||||||
| B | 72 (22) | 156 (51) | 1.0 (0.8) | 2.2 (0.6) | 8,820 (2,940) | 2.8 (1.0) | 238 (79) |
| C | 99 (52) | 165 (61) | 0.7 (0.4) | 2.5 (0.6) | 8,620 (3,460) | 3.1 (1.1) | 241 (63) |
For regimen designations, see Table 1.
Tmax, time to Cmax; t1/2, half-life; CL, clearance.
TABLE 3.
Single-dose pharmacokinetic parameters of salicylic acid and salicyluric acid in the absence and presence of oseltamivir
| Drug and regimena | Mean value (SD)b
|
||||||
|---|---|---|---|---|---|---|---|
| Cmax (ng/ml) | AUC0-12 (ng · h/ml) | Tmax (h) | t1/2 (h) | Oral CL (ml/min) | % Excreted | Renal CL (ml/min) | |
| Salicylic acid | |||||||
| A | 51 (11) | 413 (153) | 3.8 (1.2) | 2.6 (0.5) | 30.3 (7.8) | 11.3 (5.6) | 3.4 (2.1) |
| C | 52 (14) | 401 (174) | 3.4 (1.4) | 2.6 (0.5) | 32.7 (11.2) | 12.4 (6.2) | 4.1 (2.5) |
| Salicyluric acid | |||||||
| A | 2.0 (0.6) | 23.1 (5.4) | 4.7 (1.7) | 4.2 (1.7) | 800 (181) | 52 (7.0) | 417 (98) |
| C | 1.9 (0.8) | 21.7 (6.6) | 5.3 (2.0) | 4.3 (2.3) | 875 (237) | 54.1 (8.0) | 468 (119) |
For regimen designations, see Table 1.
Tmax, time to Cmax; t1/2, half-life; CL, clearance.
FIG. 1.
Mean (± standard deviation) steady-state plasma concentration-versus-time profiles of oseltamivir carboxylate in the absence and presence of aspirin.
FIG. 2.
Mean (± standard deviation) single-dose plasma concentration-versus-time profiles of salicylic acid in the absence and presence of oseltamivir.
Renal elimination plays a minor role in the elimination of salicylic acid (<16%) (1), and hence the potential for competing with the carboxylate via the renal tubular secretory pathway is low. In contrast, renal excretion plays a significant role in the elimination of salicyluric acid (∼60%) (1). Hence, pharmacokinetics of salicyluric acid has a greater potential to interact with the anionic tubular secretion pathway, which is the major route of elimination of the carboxylate. In this study, the major plasma pharmacokinetic parameters of oseltamivir (AUC only), the carboxylate, salicylic acid, and salicyluric acid were bioequivalent in the absence and presence of concomitant drug, as were the comparability of the urine parameters. These results demonstrate that there was no interaction at the anionic tubular secretion pathway, confirming the low potential for interaction between oseltamivir and other agents at the level of the anionic tubular transporter (3).
REFERENCES
- 1.Dubovska, D., V. K. Piotrovskij, M. Gajdos, Z. Krivosikova, V. Spustova, and T. Trnovec. 1995. Pharmacokinetics of acetylsalicylic acid and its metabolites at low doses: a compartmental modeling. Methods Find. Exp. Clin. Pharmacol. 17:67-77. [PubMed] [Google Scholar]
- 2.He, G., J. Massarella, and P. Ward. 1999. Clinical pharmacokinetics of the prodrug oseltamivir and its active metabolite Ro 64-0802. Clin. Pharmacokinet. 37:471-484. [DOI] [PubMed] [Google Scholar]
- 3.Hill, G., T. Cihlar, C. Oo, E. S. Ho, K. Prior, H. Wiltshire, J. Barrett, B. Liu, and P. Ward. 2002. The anti-influenza drug oseltamivir exhibits low potential to induce pharmacokinetic drug interactions via renal secretion—correlation of in vivo and in vitro studies. Drug Metab. Dispos. 30:13-19. [DOI] [PubMed] [Google Scholar]
- 4.Minors, J. O. 1989. Drug interactions involving aspirin (acetylsalicylic acid) and salicylic acid. Clin. Pharmacokinet. 17:327-344. [DOI] [PubMed] [Google Scholar]
- 5.Needs, C. J., and P. M. Brooks. 1985. Clinical pharmacokinetics of salicylates. Clin. Pharmacokinet. 10:164-177. [DOI] [PubMed] [Google Scholar]
- 6.Oo, C., J. Barrett, G. Hill, J. Mann, A. Dorr, R. Dutkowski, and P. Ward. 2001. Clinical pharmacokinetics and dose recommendation of oseltamivir suspension for the treatment of influenza in children. Paediatr. Drugs 3:229-236. [DOI] [PubMed] [Google Scholar]
- 7.Wiltshire, H., B. Wiltshire, A. Citron, T. Clarke, C. Serpe, D. Gray, and W. Herron. 2000. Development of a high-performance liquid chromatographic-mass spectrometric assay for the specific and sensitive quantification of Ro 64-0802, an anti-influenza drug, and its pro-drug, oseltamivir, in human and animal plasma and urine. J. Chromatogr. 745:373-388. [DOI] [PubMed] [Google Scholar]