Abstract
Deferoxamine, a drug used to treat patients with iron overload, has the capacity to promote systemic Y. enterocolitica infections in humans. The aim of this study was to determine whether deferiprone, the only orally active alternative treatment, has the same potential. When Y. enterocolitica IP864 was grown in an iron-poor chemically defined medium, addition of deferoxamine promoted its growth, while various concentrations of deferiprone did not display this activity. Similarly, on iron-poor agar plates, various Y. enterocolitica strains were able to grow around paper disks impregnated with deferoxamine in a dose-dependent manner, while no growth was observed around the deferiprone disks. In a mouse experimental model of infection, the 50% lethal dose (LD50) of strain IP864 was decreased by more than 5 log units in mice pretreated with deferoxamine, while a deferiprone pretreatment did not affect it. Therefore, in contrast to deferoxamine, deferiprone does not enhance growth of pathogenic Y. enterocolitica in vitro and does not have the potential to promote Y. enterocolitica septicemia in a mouse model of infection. Deferiprone may thus represent a useful alternative iron-chelation therapy during invasive Y. enterocolitica infections.
Deferoxamine, a siderophore produced by Streptomyces pilosus (14), is the standard iron chelation agent used for the treatment of patients with iron overload. However, treatment with this drug requires prolonged parenteral infusion, on at least four to five days each week, which renders compliance difficult. Deferoxamine also has some serious side effects, one of which is the occurrence of Yersinia enterocolitica septicemia in patients with severe β-thalassemia (1, 9, 11, 15). A prospective study performed in Italy reported a frequency of Y. enterocolitica infection as high as 10% in 144 thalassemic patients monitored over a 1-year period (9). A recent Canadian survey performed with a series of 177 β-thalassemic patients over 15 years indicated that their risk of developing invasive Y. enterocolitica infections is 5,000-fold greater than that of the general population (1).
After considerable efforts devoted to seeking an orally active alternative treatment, only one drug, deferiprone, has succeeded in entering clinical trials and has recently been approved for human use (13). Whether deferiprone could also favor Y. enterocolitica sepsis remained unclear. Synthetic iron chelators belonging to the L1 (deferiprone) and L4 series were shown to have no Y. enterocolitica growth-enhancing effect after a 3-h incubation period in human serum (7). However, occurrence of septicemia in a thalassemic patient undergoing deferiprone therapy was recently reported (1). This sepsis might have been the result of either the underlying iron overload status of the patient or the enhancing effect of deferiprone.
The aim of the present study was to evaluate the potential of deferiprone to promote the growth of pathogenic Y. enterocolitica under laboratory conditions and to increase the virulence of this organism in a mouse experimental model of infection.
MATERIALS AND METHODS
Strains and growth conditions.
The Y. enterocolitica strains used in this study (Table 1) were taken from the strain collection of the French Reference Laboratory and WHO Collaborating Center for Yersinia (Institut Pasteur, Paris, France). Y. enterocolitica IP864 was used as the reference strain. All strains were grown at 28°C for 24 to 48 h.
TABLE 1.
Y. enterocolitica strains used in this study
| Strain | Biotype | Serotype | Phage type | Origin | Site | Country |
|---|---|---|---|---|---|---|
| IP864 | 4 | O:3 | VIII | Human | Stool | Belgium |
| IP8944 | 4 | O:3 | VIII | Human | Blood | Greece |
| IP383 | 2 | O:9 | X3 | Human | Stool | Belgium |
| IP22981 | 2 | O:5,27 | Xz | Human | Blood | France |
| IP885 | 2 | O:5,27 | Xz | Dog | Stool | Great Britain |
Media and chemicals.
The media used in this study were Luria Bertani (LB) broth, LB plates (LB supplemented with 7.5% agar), top agar (LB containing 0.6% agar), Trypticase soy agar (TSA) plates, and the chemically defined liquid medium (CDLM) prepared with highly pure components containing minimal traces of iron, as described in reference 8. Iron-poor conditions were obtained by addition of the iron chelator α,α′-dipyridyl (Sigma). Concentrations of stock solutions were 15 mM for α,α′-dipyridyl, 10 mM for deferoxamine (Desferal; Novartis, Rueil-Malmaison, France), and 20 mM for deferiprone (Ferriprox; Apotex, Toronto, Canada).
Determination of the minimal concentration of α,α′-dipyridyl necessary to inhibit growth of Y. enterocolitica. (i) Growth in CDLM.
Y. enterocolitica IP864 was grown for 24 h at 28°C (optimal in vitro growth temperature) with shaking in 10 ml of iron-poor LB broth (supplemented with 0.2 mM α,α′-dipyridyl), washed twice in H2O, and inoculated at a concentration of 103 CFU/ml into CDLM alone or CDLM supplemented with 150 μM FeCl3 (iron rich) or with 0.1, 0.2, 0.3, or 0.4 mM α,α′-dipyridyl. The tubes were incubated at 28°C with shaking. At various time points after inoculation (0, 4, 8, 24, 30, and 48 h), aliquots of the cultures were taken, and tenfold dilutions in H2O were streaked in duplicate onto TSA plates. Bacterial colonies were counted after 48 h.
(ii) Growth on agar plates.
IP864 was grown overnight in 10 ml of iron-poor LB broth. 108 bacteria were mixed with 5 ml of melted top agar (0.6% agar) containing various concentrations of α,α′-dipyridyl (0, 0.1, 0.2, 0.3, 0.35, 0.4, or 0.45 mM) and poured on LB agar plates containing the same amounts of α,α′-dipyridyl. Plates were incubated at 28°C, and bacterial growth was monitored after 24 h. The minimal concentrations of α,α′-dipyridyl necessary to inhibit bacterial growth in CDLM or on agar plates were recorded and used for further experiments.
Evaluation of the iron-chelation capacity of deferiprone under the conditions of the experiments. (i) Growth in CDLM.
Y. enterocolitica IP864 was pregrown in iron-poor LB broth and inoculated at a concentration of 103 CFU/ml into CDLM alone; CDLM with 0.3 mM α,α′-dipyridyl; CDLM with 0.3, 0.5, 0.7, 1.5, or 2.3 mM deferiprone; or CDLM with these various deferiprone concentrations and 150 or 300 μM FeCl3. At time points 0, 4, 24, 32, and 48 h postinoculation, aliquots of the cultures were taken and their optical density at 600 nm was measured.
(ii) Growth on agar plates.
IP864 (108 CFU) pregrown in iron-poor LB broth was mixed with 5 ml of melted top agar containing a subinhibitory concentration of α,α′-dipyridyl (0.3 mM). Six-millimeter-diameter filter paper disks soaked in 50, 100, or 150 mM deferiprone solutions were placed on the solidified top agar. Plates were incubated at 28°C for 24 h, and the diameter of the zone of growth inhibition surrounding each disk was recorded after 24 h. The minimal concentration of deferiprone necessary to inhibit bacterial growth around the paper disk was then mixed with various concentrations of FeCl3 (0.15, 0.5, 1, 10, or 100 mM) and deposited on the disks placed on the top agar. The diameter of the inhibitory zone around each disk was evaluated after 24 h.
Kinetics of growth of Y. enterocolitica IP864 in the presence of deferiprone or deferoxamine.
Five milliliters of CDLM alone (control for bacterial growth), or iron-poor CDLM supplemented with 0, 10, 50, 100, or 150 μM deferiprone or deferoxamine was inoculated with an overnight iron-depleted culture of Y. enterocolitica IP864 as described above. These concentrations of the two iron chelators were chosen in order to cover the range of concentrations achieved in human serum (7 to 10 μM for deferoxamine and 70 μM for deferiprone). At 0 to 48 h postinoculation, 10-fold dilutions of the bacterial cultures were streaked in duplicate onto TSA plates, and CFU were counted after 48 h. Two independent experiments were performed.
Growth of various strains of Y. enterocolitica around paper disks soaked into deferiprone or deferoxamine.
The various Y. enterocolitica strains listed in Table 1 were grown overnight, mixed with top agar and poured onto iron-poor LB plates as described above. Filter paper disks soaked in various concentrations (0, 0.1, 0.5, 1, 2.5, 5, 10, 50, 100, or 150 μM) of deferoxamine or deferiprone, were placed on the solidified top agar. Plates were incubated at 28°C for 24 h, and the diameter of the zone of bacterial growth surrounding each disk was recorded. The experiments were repeated twice.
Evaluation of the virulence-enhancing effects of deferoxamine and deferiprone in the Y. enterocolitica IP864 mouse experimental model of infection.
The promoting effect of deferoxamine on Y. enterocolitica septicemia has been previously established in a mouse model of infection (17). We thus used the same experimental model to compare the effects of deferoxamine and deferiprone to cause systemic dissemination of Y. enterocolitica IP864 and mouse death. Since a single intraperitoneal injection of deferoxamine to mice was previously found to be as efficient as doses divided over a 3-day period (17), we adopted the same single-dose regimen to compare the effects of the two drugs in vivo. The dose of deferiprone used in mice was chosen in order to be proportional to the doses of deferiprone and deferoxamine used during human therapy. Daily doses of 60 mg of deferoxamine/kg of body weight and 75 mg of deferiprone/g are used in humans (2, 12), and thus, based on the same ratio, a dose of 5 mg of deferoxamine in mice corresponded to 6.25 mg of deferiprone. These doses were inoculated intraperitoneally to 5-week-old OF1 female mice (Iffa Credo, L’Arbresle, France). Twenty-four hours later, serial dilutions of IP864 suspensions in saline were inoculated intraperitoneally to groups of five animals. Infected mice were monitored daily for 3 weeks, and the 50% lethal doses (LD50) were calculated (16).
RESULTS
Minimal concentration of α,α′-dipyridyl necessary to inhibit growth of Y. enterocolitica. (i) Growth in CDLM.
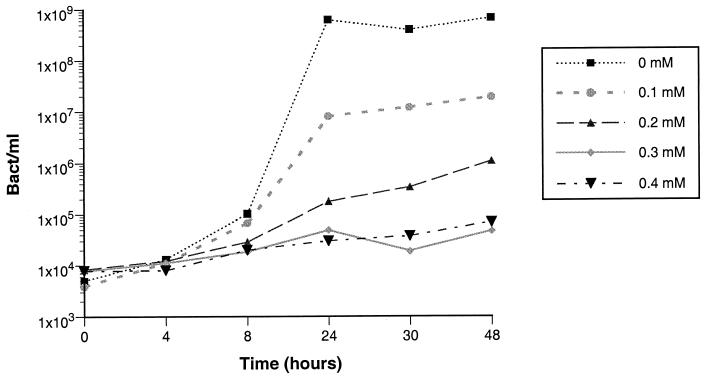
As previously observed (8), the amount of iron present in CDLM, although limited, was still sufficient to promote efficient bacterial growth. In order to obtain iron depletion conditions, various concentrations of the iron chelator α,α′-dipyridyl were added to the medium, and growth of strain IP864 was monitored over time. Bacterial growth was inversely proportional to the amount of α,α′-dipyridyl added. The minimal concentration that inhibited bacterial growth was 0.3 mM (Fig. 1) and this concentration was subsequently used for further experiments.
FIG. 1.
Kinetics of growth of Y. enterocolitica IP864 in the iron-poor defined medium in the presence of various concentrations (0, 0.1, 0.2, 0.3, or 0.4 mM) of α,α′-dipyridyl.
(ii) Growth on agar plates.
Growth of Y. enterocolitica IP864 was visible on agar plates containing 0.1, 0.2, 0.3, and 0.35 mM α,α′-dipyridyl. The concentration of 0.4 mM α,α′-dipyridyl was the lowest that inhibited bacterial growth (data not shown), and this concentration was subsequently used.
Iron chelation capacity of deferiprone.
To demonstrate that, under our experimental conditions, deferiprone had the ability to chelate iron, strain IP864 was grown in CDLM in the presence of various concentrations of deferiprone. Inhibition of bacterial growth was observed at deferiprone concentrations of 0.5 mM and higher. When the CDLM containing the MICs of deferiprone (0.5 mM) was supplemented with 150 μM iron, this growth inhibition effect disappeared (data not shown). Similarly, paper disks impregnated with 100 mM deferiprone or higher concentrations were surrounded by a halo of growth inhibition, while addition of 10 mM FeCl3 to the deferiprone solution abolished the inhibitory effect of deferiprone. These results demonstrate that deferiprone does chelate iron under our experimental conditions and that the bacteriostatic effect observed is the result of iron deprivation.
Kinetics of growth of Y. enterocolitica IP864 in the presence of deferoxamine or deferiprone.
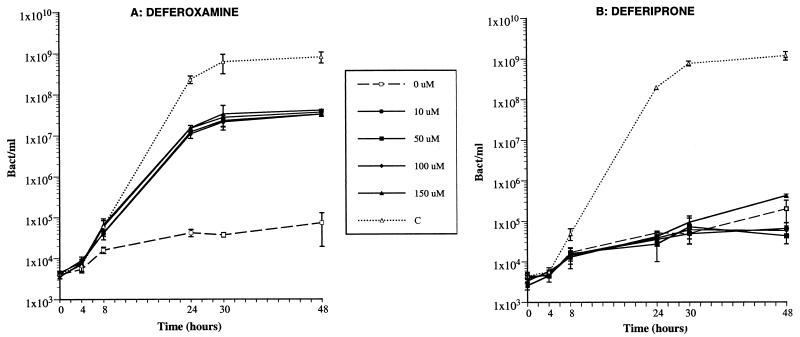
Deferoxamine was used in this study as a control for a drug able to promote growth of iron-starved Y. enterocolitica. As shown on Fig. 2A, a concentration of deferoxamine as low as 10 μM was sufficient to promote a 1,000-fold increase in the growth of Y. enterocolitica. Higher concentrations of this drug did not significantly enhance bacterial growth. The potential of deferiprone to promote growth of Y. enterocolitica was investigated under conditions identical to those used with deferoxamine. In contrast to deferoxamine, various concentrations of deferiprone were unable to eliminate the iron-limiting conditions of the medium, even for concentrations as high as 150 μM (Fig. 2B). Therefore, deferiprone did not display the growth-promoting effect seen with deferoxamine in the iron-deprived CDLM.
FIG. 2.
Kinetics of growth of Y. enterocolitica IP864 in the iron-poor defined medium in the presence of various concentrations (0, 10, 50, 100, or 150 μM) of deferoxamine (A), or deferiprone (B). C, control growth of IP864 in the non-iron depleted define medium (i.e., with no α,α′-dipyridyl added). Vertical bars represent the standard deviation from two independent experiments.
Promoting effect of deferoxamine or deferiprone on Y. enterocolitica IP864 growth on agar plates.
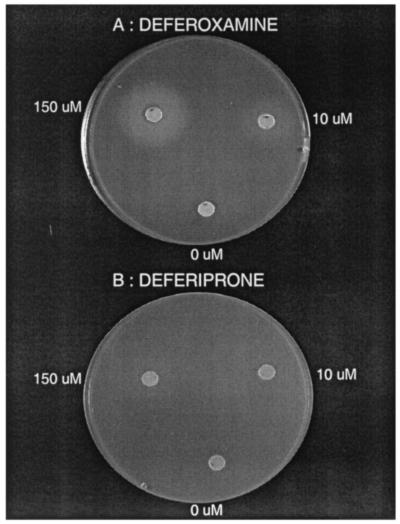
The potential of deferoxamine and deferiprone to promote growth of Y. enterocolitica was also investigated by measuring the halo of bacterial growth around paper disks impregnated with various concentrations of each drug and placed on iron-restricted agar plates. When strain IP864 was grown on agar plates containing 0.4 mM α,α′-dipyridyl, no growth was visible around paper disks soaked in solutions containing 0, 0.1, 0.5, or 1 μM deferoxamine (Table 2). However a zone of bacterial growth started to be visible at a drug concentration of 2.5 μM. The diameter of the zone was proportional to the concentration of deferoxamine added to the disk and was reproducible during different experiments (Table 2). An example of bacterial growth around deferoxamine disks is shown on Fig. 3A. In contrast, concentrations of deferiprone identical to those used for deferoxamine (ranging from 0.1 to 150 μM) were unable to promote visible bacterial growth around the disks (Table 2 and Fig. 3B).
TABLE 2.
Growth of various pathogenic strains of Y. enterocolitica around paper disks impregnated with deferoxamine or deferiprone
| Strain | Iron chelatora | Diam (cm) of growth around paper disk with indicated chelator concnb
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 μM | 1 μM | 2.5 μM | 5 μM | 10 μM | 50 μM | 100 μM | 150 μM | ||
| IP864 | DFO | 0 | 0 | 1.65 (0.07) | 1.80 (0.14) | 2.00 (0.00) | 2.55 (0.07) | 2.65 (0.21) | 2.90 (0.14) |
| DFP | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| IP8944 | DFO | 0 | 0 | 0 | 1.55 (0.07) | 1.95 (0.07) | 2.40 (0.14) | 2.45 (0.07) | 2.80 (0.00) |
| DFP | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| IP383 | DFO | 0 | 0 | 0 | 0 | 0 | 2.50 (0.07) | 2.6 (0.07) | 2.7 (0.00) |
| DFP | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| IP22981 | DFO | 0 | 0 | 0 | 1.55 (0.35) | 1.85 (0.21) | 2.45 (0.07) | 2.50 (0.00) | 2.60 (0.00) |
| DFP | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| IP885 | DFO | 0 | 0 | 1.50 (0.00) | 1.75 (0.07) | 1.90 (0.07) | 2.70 (0.14) | 2.60 (0.00) | 2.70 (0.00) |
| DFP | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
DFO, deferoxamine; DFP, deferiprone.
Data shown are means from two experiments (standard deviations shown in parentheses).
FIG. 3.
Representative growth of Y. enterocolitica IP864 on iron-poor agar plates, around paper disks impregnated with 0, 10, and 150 μM deferoxamine (A) or deferiprone (B).
Promoting effect of deferoxamine or deferiprone on the growth of various strains of Y. enterocolitica on agar plates.
In order to determine whether the results obtained with strain IP864 were applicable to other strains of Y. enterocolitica, four additional isolates (Table 1) which are representatives of the bioserotypes of Y. enterocolitica most commonly responsible for human infections worldwide, were tested for their ability to grow on iron-poor agar plates around disks impregnated with various concentrations of deferoxamine or deferiprone. As shown in Table 2, the minimal concentration of deferoxamine required to promote bacterial growth ranged from 2.5 to 50 μM, depending on the isolates. All five strains grew around disks impregnated with 50, 100, and 150 μM deferoxamine. The diameter of bacterial growth was proportional to the amount of deferoxamine present in the disk and was reproducible for a given isolate. In contrast to deferoxamine, no visible growth around paper disks impregnated with deferiprone was observed with the five strains tested, even at the highest concentration of 150 μM (Table 2).
Virulence-enhancing effect of deferoxamine or deferiprone in a mouse model of Y. enterocolitica IP864 infection.
The onset of Y. enterocolitica septicemia in iron overload patients treated with deferoxamine has been extensively documented (1, 9, 11, 15). The experimental demonstration that the systemic spread of this organism was not only the consequence of the iron overload but was also promoted by the drug itself has been borne out in the mouse experimental model (17). We used the same experimental model and conditions to compare the potential of deferoxamine and deferiprone to cause systemic dissemination of Y. enterocolitica IP864 and subsequently mouse death. In control mice that received intraperitoneal injections of saline prior to the bacterial challenge, the LD50 of strain IP864 was 6.8 × 108 bacteria. In animals pretreated with deferoxamine, the LD50 of this strain dropped to 103 bacteria, whereas prior injection of deferiprone did not modify the LD50 (4.2 × 108 bacteria). Therefore, deferiprone does not have the virulence-enhancing effect observed with deferoxamine during experimental Y. enterocolitica infection in mice.
DISCUSSION
Clinical reports of deferoxamine-treated thalassemic patients that developed fulminant Y. enterocolitica septicemia are numerous (1, 9, 11, 15). This virulence-enhancing effect of deferoxamine has at least two causes. One is the synthesis by Y. enterocolitica of the outer membrane protein FoxA that acts as a receptor for the exogenous siderophore (6). The other is the modulation and/or abolition of the action of specific and nonspecific immune cells and the inhibition of cytokine production by macrophages, leading to partial immunosuppression of the host (3, 4). Thus, low-pathogenicity Y. enterocolitica strains usually restricted to the digestive tract can use deferoxamine to obtain limiting iron molecules and benefit from the induced immune deficiency to disseminate in their host and cause systemic infections.
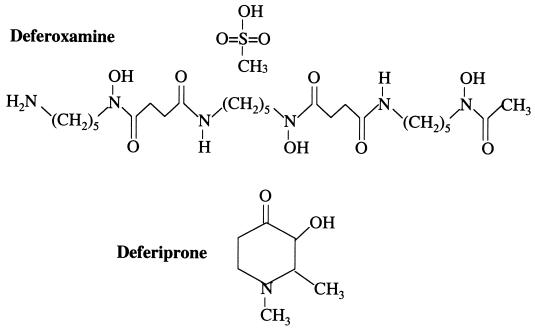
Deferiprone (1,2-dimethyl-3-hydroxypyridin-4-one) is a synthetic iron chelator whose chemical structure (Fig. 4) is completely different from that of deferoxamine (C25H48N6O8 ·CH4O3S). It was thus unlikely that this new drug could have the same enhancing capacities on Y. enterocolitica growth and virulence as deferoxamine. However, this remained to be clearly demonstrated. In the present study we show that deferiprone is unable to promote growth of Y. enterocolitica in vitro, even after prolonged contact (48 h) or when concentrations of the drug as high as 150 μM are used. This absence of in vitro growth-promoting effect of deferiprone is not restricted to one specific bioserotype but is extendable to the three bioserotypes of Y. enterocolitica most commonly isolated from patients worldwide (4/O:3, 2/O:9, and 2/O:5,27). Furthermore, in contrast to deferoxamine, deferiprone does not have the potential to promote Y. enterocolitica septicemia, as shown in our mouse experimental model of infection.
FIG. 4.
Chemical structures of the iron chelators deferoxamine mesylate and deferiprone.
Neither deferiprone nor deferoxamine represents a perfect therapy for patients with iron-overload from various clinical disorders, most importantly thalassemia major, because both drugs can be associated with minor and major side effects (5, 10). During invasive Y. enterocolitica infections in these patients, an immediate discontinuation of deferoxamine treatment is necessary. Based on the results of this study, the use of deferiprone, instead of deferoxamine, in these infected patients may be valuable to avoid interruption of the iron chelation therapy.
Acknowledgments
This work was partly financed by Apotex Research Laboratories (Toronto, Canada).
We thank Daniel Dykhuizen for his helpful corrections of the manuscript.
REFERENCES
- 1.Adamkiewicz, T. V., M. Berkovitch, C. Krishnan, C. Polsinelli, D. Kermack, and N. F. Olivieri. 1998. Infection due to Yersinia enterocolitica in a series of patients with beta-thalassemia: incidence and predisposing factors. Clin. Infect. Dis. 27:1362-1366. [DOI] [PubMed] [Google Scholar]
- 2.Addis, A., R. Loebstein, G. Koren, and T. R. Einarson. 1999. Meta-analytic review of the clinical effectiveness of oral deferiprone (L1). Eur. J. Clin. Pharmacol. 55:1-6. [DOI] [PubMed] [Google Scholar]
- 3.Autenrieth, I. B., E. Bohn, J. H. Ewald, and J. Heesemann. 1995. Deferoxamine B but not deferoxamine G1 inhibits cytokine production in murine bone marrow macrophages. J. Infect. Dis. 172:490-496. [DOI] [PubMed] [Google Scholar]
- 4.Autenrieth, I. B., R. Reissbrodt, E. Saken, R. Berner, U. Vogel, W. Rabsch, and J. Heesemann. 1994. Desferrioxamine-promoted virulence of Yersinia enterocolitica in mice depends on both desferrioxamine type and mouse strain. J. Infect. Dis. 169:562-567. [DOI] [PubMed] [Google Scholar]
- 5.Barman-Balfour, J. A., and R. H. Foster. 1999. Deferiprone: a review of its clinical potential in iron overload in beta-thalassaemia major and other transfusion-dependent diseases. Drugs 58:553-578. [DOI] [PubMed] [Google Scholar]
- 6.Bäumler, A. J., and K. Hantke. 1992. Ferrioxamine uptake in Yersinia enterocolitica: characterization of the receptor protein FoxA. Mol. Microbiol. 6:1309-1321. [DOI] [PubMed] [Google Scholar]
- 7.Brock, J. H., J. Licéaga, and G. J. Kontoghiorghes. 1988. The effect of synthetic iron chelators on bacterial growth in human serum. FEMS Microbiol. Immunol. 47:55-60. [DOI] [PubMed] [Google Scholar]
- 8.Carniel, E., D. Mazigh, and H. H. Mollaret. 1987. Expression of iron-regulated proteins in Yersinia species and their relation to virulence. Infect. Immun. 55:277-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cherchi, G. B., L. Pacifico, S. Cossellu, D. Gallisai, S. Zanetti, G. Fadda, and C. Chiesa. 1995. Prospective study of Yersinia enterocolitica infection in thalassemic patients. Pediatr. Infect. Dis. J. 14:579-584. [DOI] [PubMed] [Google Scholar]
- 10.Cohen, A. R., R. Galanello, A. Piga, A. DiPalma, G. Vullo, and F. Tricta. 2000. Safety profile of the oral iron chelator deferiprone: a multicentre study. Br. J. Haematol. 108:305-312. [DOI] [PubMed] [Google Scholar]
- 11.Hoe, T. S., A. Lammi, and B. Webster. 1994. Homozygous beta-thalassemia: a review of patients who had splenectomy at the Royal Alexandra hospital for children, Sydney. Singapore Med. J. 35:59-61. [PubMed] [Google Scholar]
- 12.Hoffbrand, A. V., and B. Wonke. 1997. Iron chelation therapy. J. Intern. Med. 740(Suppl.):37-41. [PubMed] [Google Scholar]
- 13.Modell, B., M. Khan, and M. Darlison. 2000. Survival in beta-thalassemia major in the UK: data from the UK Thalassemia Register. Lancet 355:2051-2052. [DOI] [PubMed] [Google Scholar]
- 14.Muller, G., and K. N. Raymond. 1984. Specificity and mechanism of ferrioxamine-mediated iron transport in Streptomyces pilosus. J. Bacteriol. 160:304-312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pacifico, L., V. Cianfrano, F. Valentini, A. M. Renzi, and C. Chiesa. 1991. Invasive disease due to Yersinia enterocolitica in children with beta-thalassemia major. Contr. Microbiol. Immunol. 12:286-291. [PubMed] [Google Scholar]
- 16.Reed, L. J., and H. Muench. 1938. A simple method of estimating fifty per cent endpoints. Am. J. Hyg. 27:493-497. [Google Scholar]
- 17.Robins-Browne, R. M., and J. Kaya Prpic. 1985. Effects of iron and desferrioxamine on infections with Yersinia enterocolitica. Infect. Immun. 47:774-779. [DOI] [PMC free article] [PubMed] [Google Scholar]