Abstract
Enterococcus faecalis isolates are resistant to clindamycin (CLI) and quinupristin-dalfopristin (Q-D), and this is thought to be a species characteristic. Disruption of a gene (abc-23, now designated lsa, for “lincosamide and streptogramin A resistance”) of E. faecalis was associated with a ≥40-fold decrease in MICs of Q-D (to 0.75 μg/ml), CLI (to 0.12 to 0.5 μg/ml), and dalfopristin (DAL) (to 4 to 8 μg/ml) for the wild-type E. faecalis parental strain (Q-D MIC, 32 μg/ml; CLI MIC, 32 to 48 μg/ml; DAL MIC, 512 μg/ml). Complementation of the disruption mutant with lsa on a shuttle plasmid resulted in restoration of the MICs of CLI, Q-D, and DAL to wild-type levels. Under high-stringency conditions, lsa was found in 180 of 180 isolates of E. faecalis but in none of 189 other enterococci. Among 19 erm(B)-lacking Enterococcus faecium strains, 9 (47%) were highly susceptible to CLI (MIC, 0.06 to 0.25 μg/ml) and had DAL MICs of 4 to 16 μg/ml; for the remaining erm(B)-lacking E. faecium strains, the CLI and DAL MICs were 4 to >256 and 2 to >128 μg/ml, respectively. In contrast, none of 32 erm(B)-lacking E. faecalis strains were susceptible (CLI MIC range, 16 to 32 μg/ml; DAL MIC range, ≥32 μg/ml). When lsa was introduced into an E. faecium strain initially susceptible to CLI, the MICs of CLI and DAL increased ≥60-fold and that of Q-D increased 6-fold (to 3 to 6 μg/ml). Introduction of lsa into two DAL-resistant (MICs, >128 μg/ml), Q-D-susceptible (MICs, 0.5 and 1.5 μg/ml) E. faecium strains (CLI MICs, 12 and >256 μg/ml) resulted in an increase in the Q-D MICs from 3- to 10-fold (to 8 and >32 μg/ml), respectively. Although efflux was not studied, the similarity (41 to 64%) of the predicted Lsa protein to ABC proteins such as Vga(A), Vga(B), and Msr(A) of Staphylococcus aureus and YjcA of Lactococcus lactis and the presence of Walker A and B ATP-binding motifs suggest that this resistance may be related to efflux of these antibiotics. In conclusion, lsa appears to be an intrinsic gene of E. faecalis that explains the characteristic resistance of this species to CLI and Q-D.
Over the past few years, enterococci have emerged as important bacterial pathogens in nosocomial infections (12, 25-27, 40). These organisms have acquired and/or intrinsic resistance to many different antibiotics (18, 19), which poses a serious problem for the treatment of patients infected with these organisms. Studies have shown that Enterococcus faecalis, unlike E. faecium, is usually resistant to quinupristin-dalfopristin (Q-D), with MICs of 4 to ≥32 μg/ml (3, 10, 12, 35), and that both species are typically resistant to clindamycin (CLI) (12). Acquired resistance to Q-D in E. faecium has also been described, and contributing mechanisms include drug inactivation by enzymes, structural or conformational alterations in ribosomal target binding sites, and efflux of antibiotic out of cells (3, 10). In E. faecalis, however, the mechanism of resistance to Q-D has not been well studied.
We recently investigated the presence of putative transporters in E. faecalis, identified 34 possible transporter homologs, and made disruption mutants of 31 of these (8). Among these mutants were ones with increased susceptibility to novobiocin, pentamidine, daunorubicin, and norfloxacin and one, whose disrupted gene was originally designated abc-23, with reduced susceptibility to Q-D and CLI; the MICs of ∼20 other compounds were similar to those for wild-type OG1RF (8). In the present study, we have further studied this gene and its effect by comparing it with known ABC transporters, by complementing the disruption mutant and introducing the gene into E. faecium strains on a shuttle vector, and by determining its distribution among Enterococcus spp. Based on these results, we have renamed this gene lsa in recognition of its apparent role in the intrinsic resistance of E. faecalis to lincosamides (CLI) and streptogramin A (dalfopristin [DAL]).
MATERIALS AND METHODS
Bacterial strains and MIC studies.
The bacteria used in this study were obtained from the collection of our laboratory, which was compiled over the past 20 years. The recipient strains and plasmids used in the study are listed in Table 1. These include E. faecalis strain OG1RF (29) and E. faecium isolates SE34 (TX1330; recovered from feces of a healthy community volunteer [7]), TX2466 (a clinical isolate [23]), and D344-S (36); the E. faecium strains were chosen because of their differing susceptibilities to CLI (MICs of 8 to 16, <0.25, and >256 μg/ml, respectively). A total of 492 isolates of enterococci, including 257 of E. faecalis, 216 of E. faecium, 6 of E. hirae, 5 of E. durans, 2 of E. casseliflavus, 2 of E. mundtii, 1 of E. gallinarum, 2 of E. solitarius, and 1 of E. raffinosus (12, 44), were used for lsa probing and/or susceptibility testing. MICs were determined by agar dilution (30, 31) or by the E test (PDM Epsilometer test; AB BIODISK North America, Inc., Piscataway, N.J.) in accordance with the manufacturer's instructions. Erythromycin (ERY), CLI, kanamycin (KAN), and chloramphenicol (CHL) were purchased from Sigma Chemical Co., St. Louis, Mo., and quinupristin, DAL, and Q-D were provided by Aventis Pharma S.A., Vitry-sur-Seine Cedex, France.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Purpose and relevant characteristic(s) | Reference(s) or source |
|---|---|---|
| Strains | ||
| E. faecalis OG1RF | Rifr Fusr; used for lsa insertional mutagenesis | 29 |
| E. faecalis V583 | TIGR sequenced strain; used to amplify lsa for disruption and complementation experiments | 40 |
| E. faecium TX2466 | Used as recipient strain; CLI MIC, 0.19 μg/ml | 23 |
| E. faecium TX1330 | Used as recipient strain; CLI MIC, 12-24 μg/ml | 7 |
| E. faecium D344-S | Used as recipient strain; CLI MIC, >256 μg/ml; erm(B)+ | 36 |
| TX5332 | lsa gene disruption mutant (OG1RF lsa::pTEX4577); Kanr | 8 |
| TX5333 | Complemented lsa gene disruption mutant [TX5332(pWM401::lsa)]; Kanr Chlr | This study |
| Plasmids | ||
| pTEX4577 | pBluescript SK (−) with aph(3')-IIIa inserted into ScaI site; Kanr; used for lsa insertion mutagenesis | 13, 43 |
| pWM401 | Shuttle vector; Chlr Tetr | 47 |
| pCR2.1 vector | PCR product cloning vector | Invitrogen |
| pTEX5333 | pWM401::lsa; Chlr; used for complementation of TX5332 | This study |
DNA extraction, PCR, sequencing, and cloning.
DNA extraction (46) and PCRs were performed with the PCR Optimizer kit (Invitrogen, San Diego, Calif.); PCR products were analyzed by automated DNA sequencing at the Microbiology and Molecular Genetics core facility, University of Texas Medical School, Houston, Tex. Sequence analysis was done by using the BLAST network service of the National Center for Biotechnology Information. The Genetics Computer Group software package (Genetics Computer Group, Madison, Wis.) was used to compare similarities among other sequences. ClustalW, at the Baylor College of Medicine website, was used, and the GeneDoc software was used for editing and shading of sequences. Cloning was done with standard methods (42).
Disruption mutation in lsa (abc-23) of E. faecalis.
The disruption mutation in strain OG1RF was created previously (8). Briefly, the disruption mutant was created by using a PCR-amplified ∼700-bp intragenic DNA fragment from E. faecalis strain V583 inserted into previously described pBluescript derivative pTEX4577 containing aph(3′)-IIIa (13, 43), resulting in pTEX5332, which was electroporated into competent cells of OG1RF and selected with KAN at 2,000 μg/ml. The resulting mutant was previously shown by PCR to have the targeted insertion of the plasmid (8). In the present study, the insertion was also confirmed by hybridizing EcoRI digests of genomic DNAs of wild-type E. faecalis OG1RF and the lsa disruption mutant with an intragenic lsa DNA probe under high-stringency conditions. In addition, the susceptibility of this mutant to quinupristin, DAL, ampicillin, tetracycline, and ERY was determined by the E test. Recombinant colonies of TX5332 (lsa disruption mutant) were further analyzed by pulsed-field gel electrophoresis (28) of SmaI-digested genomic DNA by comparison with wild-type OG1RF to confirm the host background.
Complementation of TX5332 (lsa disruption mutant).
An ∼2-kb fragment (the 1,497-bp lsa ORF, ∼300 bp upstream and ∼200 bp downstream obtained from the V583 E. faecalis TIGR database) was PCR amplified from wild-type E. faecalis V583 and was first cloned into the pCR2.1 vector of the TA cloning kit. This PCR fragment was excised from vector pCR2.1 by digestion with restriction enzymes XbaI and BamHI and then recloned into shuttle vector pWM401 (47), resulting in pTEX5333. Plasmid pTEX5333 DNA was electroporated into competent cells of TX5332 (lsa disruption mutant), and selection was made on Todd-Hewitt agar (Becton Dickinson, Cockeysville, Md.) supplemented with 0.25 M sucrose, KAN at 2,000 μg/ml and CHL at 8 μg/ml. The resulting colonies were restreaked on KAN-CHL plates and analyzed for resistance to CLI, Q-D, and DAL by the E-test or agar dilution method.
Determination of the stability of the components of TX5333.
Growth curves comparing wild-type OG1RF, TX5332 (the lsa disruption mutant), and TX5333 (the complemented lsa disruption mutant) were determined with and without antibiotics selective for the chromosomal disruption and/or the shuttle plasmid. Observations were made by measuring optical density at 600 nm hourly, and CFU determinations at 24 h were made on brain heart infusion (BHI) agar (Difco Laboratories, Detroit, Mich.) with and without antibiotics. BHI broth was used to grow wild-type OG1RF; BHI and BHI-KAN were used to grow TX5332; and BHI, BHI-KAN, and BHI-KAN-CHL were used to grow TX5333.
Effect of lsa on E. faecium antibiotic resistance .
Electrocompetent cells of E. faecium strains TX1330, TX2466, and D344-S were prepared as previously described (14, 20). Following electroporation of pTEX5333, selection was made on Todd-Hewitt agar supplemented with 0.25 M sucrose and CHL at 8 μg/ml. The resulting colonies were restreaked onto BHI agar-CHL plates and tested for susceptibility to CLI, Q-D, DAL, and quinupristin by the E-test or agar dilution method and also by pulsed-field gel electrophoresis (28) of SmaI-digested genomic DNA and compared to each parental E. faecium strain.
Distribution of lsa among Enterococcus spp.
Three hundred sixty-nine enterococcal isolates were tested for the presence of lsa by colony lysate hybridization under high-stringency conditions with an lsa intragenic DNA probe as previously described (43). The erm(B) DNA gene probe was PCR amplified as previously described (44) and used for hybridization under high-stringency conditions. The DNA gene probes and hybridization conditions for efaAfs and aac(6′)-Iifm were the same as those used in a previous study (12).
RESULTS AND DISCUSSION
Determination of stability of components of the E. faecalis lsa disruption mutant by complementation and susceptibility testing.
Testing of the complemented lsa disruption mutant (TX5333) showed that when TX5333 was grown in BHI broth or in BHI with CHL at 8 μg/ml, there was an ∼3-log reduction in the number of CFU of Kanr colonies per ml versus when this strain was grown in the presence of KAN at 2,000 μg/ml-CHL at 8 μg/ml. Because of the apparent instability of the chromosomal disruption (Kanr), the mutant was subsequently tested for susceptibility in the presence of KAN at 2,000 μg/ml in Mueller-Hinton II agar (Becton Dickinson) for TX5332 to maintain the chromosomal disruption and in Mueller-Hinton II agar with KAN at 2,000 μg/ml and CHL at 8 μg/ml to maintain the chromosomal disruption and shuttle plasmid.
The MICs determined for wild-type E. faecalis OG1RF, TX5332, and TX5333 are presented in Table 2; the MICs of ampicillin, tetracycline (data not shown), norfloxacin, ciprofloxacin, ethidium bromide, and other compounds previously tested (8) showed no difference among these strains. The lsa mutant was tested on multiple occasions and showed a >40-fold decrease in the MIC of Q-D (0.75 μg/ml) and a ≥64-fold decrease in the MICs of CLI (0.12 to 0.5 μg/ml) and DAL (4 to 8 μg/ml) versus the wild-type E. faecalis parental strain (Q-D MIC, 32 μg/ml; CLI MIC, 32 to 48 μg/ml; DAL MIC, 512 μg/ml). This indicates that lsa or some downstream function is necessary for resistance to CLI and DAL in E. faecalis. Complementation of the disruption mutant with lsa (and ∼300 bp of the upstream sequence and ∼200 bp of the downstream sequence) on a shuttle plasmid resulted in restoration of the MICs of CLI (from 0.12 to 0.5 μg/ml to 32 to 48 μg/ml), Q-D (from 0.75 μg/ml to 32 μg/ml), and DAL (from 4 to 8 μg/ml to 512 μg/ml) (Table 2). This confirms the importance of lsa, as opposed to a possibly cotranscribed downstream gene. The MIC of quinupristin was 16 μg/ml for OG1RF and the two derivatives, and the ERY MIC was 1 μg/ml. The increase in the MICs of CLI (a lincosamide) and DAL (streptogramin A) and the lack of a change in the MICs of macrolides (ERY) or quinupristin (streptogramin B) correspond to the LSA phenotype (5), and the data presented here clearly indicate the involvement of lsa in this phenotype in E. faecalis.
TABLE 2.
MICs for E. faecalis OG1RF, TX5332 (lsa disruption mutant), and TX5333 (complemented mutant)
| Organism | MIC (μg/ml)
|
||||
|---|---|---|---|---|---|
| CLIa | Q-Da | DALb | Quinupristinb | ERYa | |
| OG1RF | 32-48 | 32 | 512 | 16 | 1 |
| TX5332 (OG1RF lsa::pTEX4577)c | 0.12-0.5 | 0.75 | 4-8 | 16 | 1 |
| TX5333 [TX5332(pWM401::lsa)]d | 32-48 | 32 | 512 | 16 | 1 |
E-test MICs (determined multiple times, resulting in a range of concentrations).
Agar dilution MICs.
Determined in the presence of KAN at 2,000 μg/ml in Mueller-Hinton agar to maintain the chromosomal disruption.
Determined in the presence of KAN at 2,000 μg/ml and CHL at 8 μg/ml in Mueller-Hinton agar to maintain the chromosomal disruption and the shuttle plasmid.
Effect of lsa on the antibiotic resistance of E. faecium.
To determine if the 2-kb lsa region could function in a heterologous host, we selected strains of E. faecium to serve as recipients of the lsa gene by evaluating 68 E. faecium strains for susceptibility to CLI and for the presence of erm(B), since the latter influences CLI susceptibility (Table 3). While most of the isolates were erm(B)+, an interesting observation was the bimodal distribution of CLI MICs among the erm(B)-lacking E. faecium isolates. Of 19 erm(B)-lacking E. faecium isolates, 10 showed high CLI MICs (MIC, 4 to >256 μg/ml) and 9 showed low CLI MICs (MIC, 0.06 to 0.25 μg/ml). In contrast, none of the 32 erm(B)-lacking E. faecalis isolates showed low CLI MICs (MIC, 16 to 32 μg/ml). We chose three E. faecium strains (Tables 1 and 4) with different CLI and DAL susceptibilities [one of which is erm(B)+] and introduced the lsa gene on a shuttle plasmid. For the most susceptible strain (TX2466 [CLI MIC, 0.19 μg/ml; DAL MIC, 2 μg/ml; Q-D MIC, 0.5 μg/ml]), there was a marked increase in the MICs of CLI (12 μg/ml) and DAL (>128 μg/ml) and an increase to 3 to 6 μg/ml in the Q-D MIC. The ranges of the MICs of Q-D and the other agents were derived by testing on three or more different occasions. For the highly DAL-resistant (MIC, >128 μg/ml) and moderately CLI-resistant (MIC, 12 to 24 μg/ml) recipient TX1330, the MICs of DAL and CLI changed very little, if at all, after lsa was introduced but the Q-D MIC increased from 0.5 μg/ml to 6 to 8 μg/ml. The most pronounced increase in the Q-D MIC (from 3 to ≥32 μg/ml) was seen in strain D344 erm(B)+, which was initially highly DAL resistant (MIC, >128 μg/ml) and CLI resistant (MIC, >256 μg/ml). These data show the interspecies function of lsa and show a marked increase in the MICs of CLI and DAL and a moderate increase in the MIC of Q-D when it is introduced into E. faecium strains susceptible to these antibiotics. Bozdogan and Leclercq (5) also noted the influence of an LSA phenotype in E. faecium on Q-D MICs, where introduction of the sat(A) or vgb gene into a Q-D-susceptible E. faecium strain with the LSA phenotype conferred resistance to Q-D while, in contrast, introduction of these genes into another E. faecium strain susceptible to lincosamide, streptogramin A, and streptogramin B resulted in a 1- or 2-dilution increase in the MIC of Q-D (5).
TABLE 3.
Susceptibility of E. faecalis and E. faecium strains to CLI and streptogramins
| Organism (no. of isolates) | MIC (μg/ml) range (agar dilution)
|
|||
|---|---|---|---|---|
| CLI | Q-D | DAL | Quinupristin | |
| E. faecium (68) | ||||
| erm(B)+ (49) | >256 | 1-16 | 8-≥32a | ≥64a |
| erm(B) lacking (19) | ||||
| Clirb (10) | 4−>256 | 1-16 | 2->128 | 8->32 |
| Clis (9) | 0.06−0.25 | 0.5-1 | 4-16 | 8-32 |
| E. faecalis (95) | ||||
| erm(B)+ (63) | 128−>256 | 2-64 | ≥32c | ≥64c |
| erm(B) lacking (32) | 16−32 | 2-64 | ≥32d | 8-≥64d |
Only 18 isolates were tested.
Determined by using NCCLS breakpoints for S. aureus (susceptible, ≤0.5 μg/ml; intermediate, 1-2 μg/ml, resistant, ≥4 μg/ml).
Only nine isolates were tested.
Only 10 isolates were tested.
TABLE 4.
MICs for E. faecium strains with and without lsa
| Strain | MICs (μg/ml)a
|
||||
|---|---|---|---|---|---|
| CLIb | Q-Db | DALc | Quinupristinc | ERYb | |
| TX2466 | 0.19 | 0.5 | 2 | 8-16 | 1.5-2 |
| TX2466(pWM401) | 0.064 | 0.5 | 4 | 16 | 0.75 |
| TX2466(pWM401::lsa) | 12 | 3-6 | >128 | 16 | 1-1.5 |
| TX1330 | 12-24 | 0.5 | >128 | 16 | 0.25 |
| TX1330(pWM401) | 12 | 1.5 | >128 | 16 | 0.125 |
| TX1330(pWM401::lsa) | 16-24 | 6-8 | >128 | 16 | 0.125-0.25 |
| D344-S [erm(B+)]d | >256 | 1.5-3 | >128 | 16 | >256 |
| D344-S(pWM401) | >256 | 3 | >128 | 16 | >256 |
| D344-S(pWM401::lsa) | >256 | ≥32 | >128 | 16 | >256 |
MICs for E. faecium containing shuttle vector pWM401 or the shuttle vector with lsa were determined in the presence of CHL at 8 μg/ml in the medium.
E-test MICs.
Agar dilution MICs.
Slow-growing strain. MIC data were obtained on BHI agar.
Distribution of lsa among Enterococcus spp.
Under high-stringency conditions, hybridization of colony lysates of 369 enterococci showed that lsa was present in all 180 E. faecalis isolates but not in 189 other enterococcal isolates (data not shown). Although most of our isolates were of human origin and animal isolates may differ, these results suggest that lsa is species specific for E. faecalis and may be an intrinsic gene of this species.
Characterization of lsa.
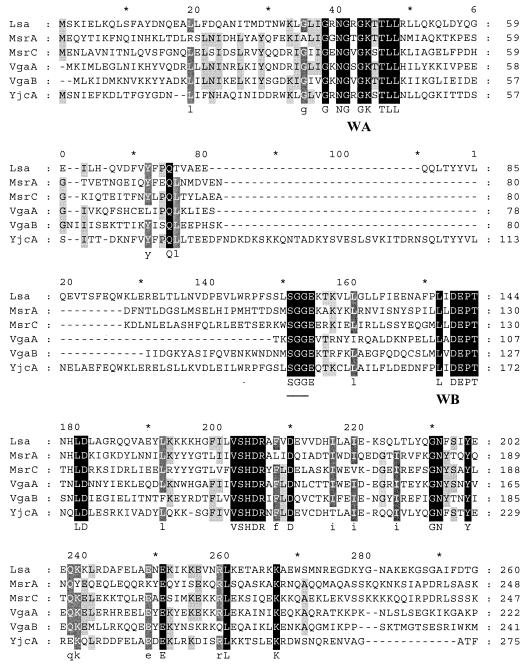
The 2-kb region used for complementation, consisting of the 1,497-bp ORF of lsa, ∼300 bp upstream, and ∼200 bp downstream, was analyzed. The predicted Lsa protein (498 amino acids [aa]) showed similarities to known or postulated ABC proteins of other gram-positive bacteria [64% similarity to YjcA (513 aa) of Lactococcus lactis (4), 42% similarity to MsrC (493 aa) of E. faecium (44), and ca. 41% similarity to Vga(A) (523 aa) (1), Vga(B) (2), and Msr(A) (38) of Staphylococcus aureus (Fig. 1 )]. ABC transporters usually contain four single or joined components that are arranged into two homologous halves, each containing an ATP-binding domain and a membrane-spanning domain composed of several (usually six) putative α-helical transmembrane segments (9, 16, 17, 41). In the case of Msr(A), the two ATP-binding regions are fused into a single protein with internally homologous domains while in other instances, the ATP-binding regions are monomeric and likely form dimers in vivo (9, 22, 24, 41). ABC-type ATPase characteristic features, including a putative ABC signature sequence and the Walker A and B motifs, as reported in the literature for Msr(A), Vga(A), and Vga(B) (1, 2, 37), were identified in the corresponding regions of Lsa (Fig. 1). Hydropathy analysis of Lsa with the TMAP and Tmpred website programs revealed no transmembrane helix or a single strong transmembrane helix, respectively. This is similar to Msr(A), which contains no hydrophobic stretches that might be potential membrane-spanning domains, and it remains unclear for Msr(A) whether it utilizes hydrophobic proteins encoded by the genes smpA, smpB, and smpC mapping on the staphylococcal chromosome (37). Genes encoding other ABC proteins that contain two ATP-binding domains but no hydrophobic domain in gram-positive organisms include lmrC, a lincomycin resistance gene from Streptomyces lincolnensis (33); oleB, an oleandomycin resistance gene from S. antibioticus (32); srmB, a spiramycin resistance gene from S. ambofaciens (15); and a tylosin resistance gene from S. fradiae (39). A 45-aa putative peptide was also identified preceding the lsa start codon; the presence of this sequence seems to be important for the expression of drug resistance, as attempts to complement the disruption mutant with only cloned lsa failed to restore resistance to CLI, Q-D, and DAL. The presence of leader peptide sequences for msr(A), erm(A), erm(B), and erm(C) has been reported or postulated to be involved in posttranscriptional regulation of the expression of these resistance genes (6, 11, 21, 24, 34, 38, 45).
FIG. 1.
Multiple-sequence alignment of Lsa, Msr(A), Msr(C), Vga(A), Vga(B), and YjcA. ClustalW, at the Baylor College of Medicine website, was used, and the GeneDoc software was used for editing and shading. Shown are the two ATP-binding domains, consisting of Walker A and B motifs (underlined) and an SGG sequence (underlined). The bottom line shows the consensus sequence. Regions in which the six proteins are 100% identical are marked in solid black, dark gray shows regions >80% identical, and light gray shows regions with >60% identical amino acids.
In conclusion, we have shown the importance of the lsa gene of E. faecalis for the intrinsic LSA phenotype (CLI and DAL resistance) and Q-D resistance of this species. The apparent species specificity of lsa also suggests that it may be useful for the identification of E. faecalis isolates. We did not study efflux, but Lsa showed sequence similarities to known and postulated ABC transporters, including Msr(A), Vga(A), and Vga(B), suggesting that the protection of E. faecalis against CLI and DAL may be related to ATP-energized efflux of these antibiotics. We have also shown that lsa is functional in isolates of E. faecium and increases the MICs of CLI, DAL, and/or Q-D to various degrees, depending on the initial host's level of susceptibility to these agents.
Acknowledgments
This work was supported, in part, by USPHS grant AI47923 to Barbara E. Murray from the Division of Microbiology and Infectious Diseases of the National Institutes of Health.
REFERENCES
- 1.Allignet, J., V. Loncle, and N. El Sohl. 1992. Sequence of a staphylococcal plasmid gene, vga, encoding a putative ATP-binding protein involved in resistance to virginiamycin A-like antibiotics. Gene 117:45-51. [DOI] [PubMed] [Google Scholar]
- 2.Allignet, J., and N. El Sohl. 1997. Characterization of a new staphylococcal gene, vgaB, encoding a putative ABC transporter conferring resistance to streptogramin A and related compounds. Gene 202:133-138. [DOI] [PubMed] [Google Scholar]
- 3.Allington, D. R., and M. P. Rivey. 2001. Quinupristin/dalfopristin: a therapeutic review. Clin. Ther 23:24-44. [DOI] [PubMed] [Google Scholar]
- 4.Bolotin, A., P. Wincker, S. Mauger, O. Jaillon, K. Malarme, J. Weissenbach, S. D. Ehrlich, and A. Sorokin. 2001. The complete genome sequence of the lactic acid bacterium Lactococcus lactis ssp. lactis IL-1403. Genome Res. 11:731-753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bozdogan, B., and R. Leclercq. 1999. Effects of genes encoding resistance to streptogramins A and B on the activity of quinupristin-dalfopristin against Enterococcus faecium. Antimicrob. Agents Chemother. 43:2720-2725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brisson-Noel, A., M. Arthur, and P. Courvalin. 1988. Evidence for natural gene transfer from gram-positive cocci to Escherichia coli. J. Bacteriol. 170:1739-1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coque, T. M., J. F. Tomayko, S. C. Ricke, P. O. Okhuysen, and B. E. Murray. 1996. Vancomycin-resistant enterococci from nosocomial, community, and animal sources in the United States. Antimicrob. Agents Chemother. 40:2605-2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davis, D. R., J. B. McAlpine, C. J. Pazoles, M. K. Talbot, E. A. Alder, C. White, B. M. Jonas, B. E. Murray, G. M. Weinstock, and B. L. Rogers. 2001. Enterococcus faecalis multi-drug resistance transporters: application for antibiotic discovery. J. Mol. Microbiol. Biotechnol. 3:179-184. [PubMed] [Google Scholar]
- 9.Dean, M., and R. Allikmets. 1995. Evolution of ATP-binding cassette transporter genes. Curr. Opin. Genet. Dev. 5:779-785. [DOI] [PubMed] [Google Scholar]
- 10.Delgado, G. J., M. M. Neuhauser, D. T. Bearden, and L. H. Danziger. 2000. Quinupristin-dalfopristin: an overview. Pharmacotherapy 20:1469-1485. [DOI] [PubMed] [Google Scholar]
- 11.Dubnau, D. 1984. Translational attenuation: the regulation of bacterial resistance to the macrolide-lincosamide-streptogramin B antibiotics. Crit. Rev. Biochem. 16:103-132. [DOI] [PubMed] [Google Scholar]
- 12.Duh, R. W., K. V. Singh, K. Malathum, and B. E. Murray. 2001. In vitro activity of 19 antimicrobial agents against enterococci from healthy subjects and hospitalized patients and use of an ace gene probe from Enterococcus faecalis for species identification. Microb. Drug Resist. 7:39-46. [DOI] [PubMed] [Google Scholar]
- 13.Fogg, G. C., C. M. Gibson, and M. G. Caparon. 1994. The identification of rofA, a positive-acting regulatory component of prtF expression: use of a mγδ-based shuttle mutagenesis strategy in Streptococcus pyogenes. Mol. Microbiol. 11:671-684. [DOI] [PubMed] [Google Scholar]
- 14.Friesenegger, A., S. Fiedler, L. A. Devriese, and R. Wirth. 1991. Genetic transformation of various species of Enterococcus by electroporation. FEMS Microbiol. Lett. 63:323-327. [DOI] [PubMed] [Google Scholar]
- 15.Geistlich, M., R. Losick, J. R. Turner, and R. N. Rao. 1992. Characterization of a novel regulatory gene governing the expression of a polyketide synthase gene in Streptomyces ambofaciens. Mol. Microbiol. 6:2019-2029. [DOI] [PubMed] [Google Scholar]
- 16.Hendrik, W. V., K. Venema, H. Bolhuis, I. Oussenko, J. Kok, B. Poolman, A. J. M. Driessen, and W. N. Konings. 1996. Multidrug resistance mediated by a bacterial homolog of the human multidrug transporter MDR1. Proc. Natl. Acad. Sci. USA 93:10668-10672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hyde, S. C., P. Emsley, M. J. Hartshorn, M. M. Mimmack, U. Gileadi, S. R. Pearce, M. P. Gallagher, D. R. Gill, R. E. Hubbard, and C. F. Higgins. 1990. Structural model of ATP-binding proteins associated with cystic fibrosis, multidrug resistance and bacterial transport. Nature 346:362-365. [DOI] [PubMed] [Google Scholar]
- 18.Leclercq, R., and P. Courvalin. 1991. Bacterial resistance to macrolide, lincosamide, and streptogramin antibiotics by target modification. Antimicrob. Agents Chemother. 35:1267-1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leclercq, R., and P. Courvalin. 1991. Intrinsic and unusual resistance to macrolide, lincosamide, and streptogramin antibiotics in bacteria. Antimicrob. Agents Chemother. 35:1273-1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li, X., G. M. Weinstock, and B. E. Murray. 1995. Generation of auxotroph mutants of Enterococcus faecalis. J. Bacteriol. 177:6866-6873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matsuoka, M., L. Janosi, K. Endou, and Y. Nakajima. 1999. Cloning and sequences of inducible and constitutive macrolide resistance genes in Staphylococcus aureus that correspond to an ABC transporter. FEMS Microbiol. Lett. 181:91-100. [DOI] [PubMed] [Google Scholar]
- 22.Milton, I. D., C. L. Hewitt, and C. R. Harwood. 1992. Cloning and sequencing of a plasmid-mediated erythromycin resistance determinant from Staphylococcus xylosus. FEMS Microbiol. Lett. 76:141-147. [DOI] [PubMed] [Google Scholar]
- 23.Montecalvo, M. A., H. Horowitz, C. Gedris, C. Carbonaro, F. C. Tenover, A. Issah, P. Cook, and G. P. Wormser. 1994. Outbreak of vancomycin-, ampicillin-, and aminoglycoside-resistant Enterococcus faecium bacteremia in an adult oncology unit. Antimicrob. Agents Chemother. 38:1363-1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murphy, E. 1985. Nucleotide sequence of ermA, a macrolide-lincosamide-streptogramin B determinant in Staphylococcus aureus. J. Bacteriol. 162:633-640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murray, B. E. 1998. Diversity among multidrug-resistant enterococci. Emerg. Infect. Dis. 4:37-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murray, B. E. 1990. The life and times of the enterococcus. Clin. Microbiol. Rev. 3:46-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murray, B. E. 2000. Vancomycin-resistant enterococcal infections. N. Engl. J. Med. 342:710-721. [DOI] [PubMed] [Google Scholar]
- 28.Murray, B. E., K. V. Singh, J. D. Heath, B. Sharma, and G. M. Weinstock. 1990. Comparison of genomic DNAs of different enterococcal isolates using restriction endonucleases with infrequent recognition sites. J. Clin. Microbiol. 28:2059-2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murray, B. E., K. V. Singh, R. P. Ross, J. D. Heath, G. M. Dunny, and G. M. Weinstock. 1993. Generation of restriction map of Enterococcus faecalis strain OG1 and investigation of growth requirements and regions encoding biosynthetic function. J. Bacteriol. 175:5216-5223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.National Committee for Clinical Laboratory Standards. 2000. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically—fifth edition; approved standard. NCCLS document M7-A5. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 31.National Committee for Clinical Laboratory Standards. 2000. Performance standard for antimicrobial susceptibility testing; tenth informational supplement (aerobic dilution). NCCLS document M100-S10. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 32.Olano, C., A. M. Rodriguez, C. Mendez, and J. A. Salas. 1995. A second ABC transporter is involved in oleandomycin resistance and its secretion by Streptomyces antibioticus. FEMS Microbiol. Lett. 16:333-343. [DOI] [PubMed] [Google Scholar]
- 33.Peschke, U., H. Schmidt, H. Z. Zhang, and W. Pipersberg. 1995. Molecular characterization of the lincomycin-production gene cluster of Streptomyces lincolnensis 78-11. Mol. Microbiol. 16:1137-1156. [DOI] [PubMed] [Google Scholar]
- 34.Projan, S. J., M. Monod, C. S. Narayanan, and D. Dubnau. 1987. Replication properties of pJM13, a naturally occurring plasmid found in Bacillus subtilis, and of its close relative pE5, a plasmid native to Staphylococcus aureus. J. Bacteriol. 169:5131-5139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rende-Fournier, R., R. Leclercq, M. Galimand, J. Duval, and P. Courvalin. 1993. Identification of the satA gene encoding a streptogramin A acetyltransferase in Enterococcus faecium BM4145. Antimicrob. Agents Chemother. 37:2119-2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rice, L. B., L. L. Carias, H.-T. R., F. Sifaoui, L. Gutmann, and S. D. Rudin. 2001. Penicillin-binding protein 5 and expression of ampicillin resistance in Enterococcus faecium. Antimicrob. Agents Chemother. 45:1480-1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ross, J. I., E. A. Eady, J. H. Cove, and S. Baumberg. 1995. Identification of a chromosomally encoded ABC-transport system with which the staphylococcal erythromycin exporter MsrA may interact. Gene 153:93-98. [DOI] [PubMed] [Google Scholar]
- 38.Ross, J. I., E. A. Eady, J. H. Cove, W. J. Cunliffe, S. Baumberg, and J. C. Wootton. 1990. Inducible erythromycin resistance in staphylococci is encoded by a member of the ATP-binding transport super-gene family. Mol. Microbiol. 4:1207-1214. [DOI] [PubMed] [Google Scholar]
- 39.Rosteck, P. R. J., P. A. Reynolds, and C. L. Hershberger. 1991. Homology between proteins controlling Streptomyces fradiae tylosin resistance and ATP-binding transport. Gene 102:27-32. [DOI] [PubMed] [Google Scholar]
- 40.Sahm, D. F., J. Kissinger, J. S. Gilmore, P. R. Murray, R. Mulder, J. Solliday, and B. Clarke. 1989. In vitro susceptibility studies of vancomycin-resistant Enterococcus faecalis. Antimicrob. Agents Chemother. 33:1588-1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saier, M. H. J., I. T. Paulsen, M. K. Sliwinski, S. S. Pao, R. A. Skurray, and H. Nikaido. 1998. Evolutionary origins of multidrug and drug-specific efflux pumps in bacteria. FASEB J. 12:265-274. [DOI] [PubMed] [Google Scholar]
- 42.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 43.Singh, K. V., T. M. Coque, G. M. Weinstock, and B. E. Murray. 1998. In vivo testing of an Enterococcus faecalis efaA mutant and use of efaA homologs for species identification. FEMS Immunol. Med. Microbiol. 21:323-331. [DOI] [PubMed] [Google Scholar]
- 44.Singh, K. V., K. Malathum, and B. E. Murray. 2001. Disruption of an Enterococcus faecium species-specific gene, a homologue of acquired macrolide resistance genes of staphylococci, is associated with an increase in macrolide susceptibility. Antimicrob. Agents Chemother. 45:263-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weisblum, B. 1985. Inducible resistance to macrolides, lincosamides and streptogramin type B antibiotics: the resistance phenotype, its biological diversity, and structural elements that regulate expression—a review. J. Antimicrob. Chemother. 16(Suppl.A):63-90. [DOI] [PubMed] [Google Scholar]
- 46.Wilson, K. 1994. Preparation of genomic DNA from bacteria, p. 2.4.1-2.4.2. In F. M. Ausubel, R. Brent, R. E. Kingston, D. M. David, J. G. Scidman, J. A. Smith, and K. Struhl (ed.), Current protocols in molecular biology. Green Publishing Associates, Brooklyn, N.Y. [DOI] [PubMed]
- 47.Wirth, R., F. An, and D. B. Clewell. 1987. Highly efficient cloning system for Streptococcus faecalis: protoplast transformation, shuttle vectors, and applications, p. 25-27. In J. J. Ferretti and R. Curtiss III (ed.), Streptococcal genetics. American Society for Microbiology, Washington, D.C.