Abstract
BAY 57-1293 belongs to a new class of antiviral compounds and inhibits replication of herpes simplex virus (HSV) type 1 and type 2 in the nanomolar range in vitro by abrogating the enzymatic activity of the viral primase-helicase complex. In various rodent models of HSV infection the antiviral activity of BAY 57-1293 in vivo was found to be superior compared to all compounds currently used to treat HSV infections. The compound shows profound antiviral activity in murine and rat lethal challenge models of disseminated herpes, in a murine zosteriform spread model of cutaneous disease, and in a murine ocular herpes model. It is active in parenteral, oral, and topical formulations. BAY 57-1293 continued to demonstrate efficacy when the onset of treatment was initiated after symptoms of herpetic disease were already apparent.
During the last 50 years, the treatment of herpesvirus infections has been continuously refined. Following the discovery of iodoxuridine in the mid-1950s and its successful demonstration as a topical therapeutic agent for herpes simplex virus (HSV) keratoconjunctivitis, vidarabine was licensed for systemic use and approved for the treatment of HSV encephalitis in 1978. Since it was first approved in 1981, the guanosine analogue acyclovir and later its l-valyl ester prodrug valacyclovir have been widely used in the treatment of HSV infections. Additional compounds used to treat HSV infections are famciclovir, the prodrug of penciclovir; ganciclovir; foscarnet; and cidofovir.
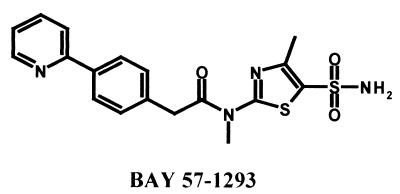
Nevertheless, a high medical need exists for improved antiherpetic drugs for the treatment of severe disease. Encephalitis in newborns, for example, results in 15% mortality, and only 29% of survivors develop normally after acyclovir therapy (22). Also, for patients with less severe disease, an agent that will achieve a better reduction of lesion duration with episodic treatment beyond the 1 to 2 days' reduction achieved with current medications is urgently required (16). Furthermore, a drug which continues to show profound efficacy when given at later stages of herpetic disease would be a new and highly desired standard in the treatment of herpes (10). BAY 57-1293(N-[5-(aminosulfonyl)-4-methyl-1,3-thiazol-2-yl]-N-methyl-2-[4-(2-pyridinyl)phenyl]acetamide) (Fig. 1) is a member of the thiazolylsulfonamides, a recently discovered class of nonnucleosidic compounds with potent antiherpetic activity in vitro and in vivo, based on a novel mechanism of action (11a). It inhibited the replication of HSV type 1 (HSV-1) and HSV-2 in Vero cells with a 50% inhibitory concentration (IC50) of 20 nM and a selectivity index of 2,500 and had about equal potencies against different strains and clinical isolates, while under the same assay conditions, acyclovir exhibited an IC50 of 1 μM and a selectivity index of 250. BAY 57-1293 targets the viral primase-helicase complex and inhibits its ATPase activity in a dose-dependent manner with an IC50 of 30 nM. Resistant viral mutants exhibited amino acid substitutions in viral UL5 and/or UL52, which code for components of the viral primase-helicase complex. Accordingly, BAY 57-1293 also is active against acyclovir-resistant mutant strains which carry mutations in the tk or DNA pol genes. The compound showed favorable pharmacokinetics in all species investigated (mouse, rat, and dog), with an oral bioavailability of >60% and an elimination half-life of >6 h. In the study described here we have examined the activities of BAY 57-1293 in various rodent animal models of herpetic disease.
FIG. 1.
Structure of the thiazolylsulfonamide BAY 57-1293 (N-[5-(aminosulfonyl)-4-methyl-1,3-thiazol-2-yl]-N-methyl-2-[4-(2-pyridinyl)phenyl]acetamide).
MATERIALS AND METHODS
Virus strains, tissue culture, and animals.
The viral strains used in this study were HSV-1F (ATCC VR-733), HSV-1walki (laboratory stock), HSV-2MS (ATCC VR-540), and HSV-2G (ATCC VR-734). Virus stocks were grown on Vero (ATCC CCL-81) cells. After a cytopathic effect was evident, the cells were frozen-thawed several times; the cell debris was removed by centrifugation; and the supernatant was aliquoted, titrated, and kept at −80°C. Mice and rats were purchased from a commercial supplier (M&B A/S, Bomholtvej, Denmark) and kept under standard conditions.
Lethal challenge model in mice and rats.
A total of 50 μl of virus suspension (HSV-1walki or HSV-2MS) in ice-cold phosphate-buffered saline (PBS; ∼5 × 104 PFU) was applied to the nares of BALB/cABom female mice (weight, 19 g; age, 7 weeks) lightly anesthetized with ether. The infection resulted in a mortality rate of 90 to 100% due to disseminated disease after 6 to 10 days. Ten mice from each group were used. LEW/Mol female rats (weight, 150 to 200 g) were used for the experiments with rats. A total of 200 μl of virus suspension (HSV-1walki) in ice-cold PBS (∼2 × 105 PFU) was applied to the nares of rats lightly anesthetized with ether. The infection resulted in a mortality rate of 90 to 100% after 6 to 10 days. Five rats from each group were used.
In all cases, the infected animals were inspected daily for signs of disease (encephalitis, paralysis), and moribund animals were euthanized. Mortality was recorded over a period of 21 days postinfection. All of the compounds tested have sufficient enteral bioavailabilities in mice and rats (5, 11a).
Zosteriform spread model.
C3H/TifBom-hr female mice (weight, 18 g; age, 7 weeks) were anesthetized with ether, and the lateral side of the body was scratched 10 times in a crossed-hatch pattern with a 27-gauge needle. A total of 10 μl of virus suspension (1 × 106 PFU of HSV-2G) was applied to the scarified area and rubbed in with a pipette tip. The mice were inspected daily; and disease severity was determined with the following scoring system: 0, no signs of infection visible; 1, vesicle formation; 2, slight zoster spread; 3, formation of large patches of zoster; 4, confluent zoster band; 5, hind limb paralysis; and 6, death. Moribund animals (e.g., those with a score of 5) were euthanized.
Ocular herpes model.
BALB/cABom female mice (weight, 19 g; age, 7 weeks) were purchased (M&B A/S) and inoculated 1 week later. The mice were anesthetized with ether, and the right cornea was scratched three times vertically and three times horizontally with a sterile 30-gauge needle. A total of 5 μl of virus suspension (7.5 × 105 PFU of HSV-1walki or 5 × 105 PFU of HSV-2G) was applied to the scarified cornea. The mice were inspected daily for signs of herpes infection (blepharitis, keratitis, encephalitis). Moribund animals were euthanized.
Antiviral compounds and treatment regimen.
BAY 57-1293 was synthesized at Bayer AG (Leverkusen, Germany) and micronized with a conventional air-jet mill. Acyclovir was purchased as Zovirax injection flasks (GlaxoWellcome), valacyclovir was purchased as Valtrex film tablets (GlaxoSmithKline), famciclovir was purchased as Famvir (Novartis Pharma), ganciclovir was purchased as Cymeven injection flasks (Roche), and brivudine was purchased as Helpin tablets (Berlin-Chemie). For some experiments valacyclovir which had previously been extracted and purified from Valtrex tablets was used. For oral treatment, the compounds were suspended in 0.5% tylose (MH 4000 P2; Clariant) in PBS (Gibco) via ultrasonication, and the suspension was subsequently administered via oral gavage in a total volume of 200 μl/dose for mice and in a total volume of 1 ml/dose for rats. For topical treatment, the compounds were suspended in 30% isopropylmyristate-70% ethanol via ultrasonication, and subsequently, 10 μl of the suspension was applied to the infected skin. For ocular treatment, the compounds were suspended in Liquifilm eyedrops (Allergan, Ettlingen, Germany) via ultrasonication, and 5 μl of the resulting suspension was applied to the infected eye. For the various formulations, the active compound content was calculated by extrapolation from the drug content in the tablet stated by the manufacturer. For purified compounds, the concentrations were verified by high-pressure liquid chromatography analysis.
Titration of virus in tissue samples.
Tissue samples (lung, brain cortex, trigeminal ganglia) were ground in glass homogenizers in 300 μl of Dulbecco modified Eagle medium without fetal calf serum on ice and frozen-thawed three times. The debris was removed by centrifugation, and the supernatant was applied in serial dilutions to Vero cell monolayers in 24-well plates and covered with medium containing 0.5% methylcellulose. Virus plaques were counted after 72 h of incubation.
Detection of HSV via Southern blotting.
Total DNA was prepared from tissue samples as described previously (1a). Southern blotting was performed with the DIG system (Roche) according to the instructions of the manufacturer. The digoxigenin (DIG)-labeled probe was amplified from purified HSV-1 DNA with the help of the PCR DIG probe synthesis kit (Roche) with the primers 5′-AAG AGG CCT TGT TCC GCT TC-3′ and 5′-TTC TGT GGT GAT GCG GAG AG-3′. These primers amplify a fragment of ∼650 bp lying in the junction region of the HSV-1 genome (6). The probe hybridizes to the junctional and terminal BamHI fragments of HSV-1 DNA.
Measurement of neutralizing anti-HSV antibodies.
Fifty-microliter serial dilutions of mouse serum in cell culture medium were pipetted into a 96-well plate. A total of 50 μl of medium containing 500 PFU of HSV-1 was added to each well. The plate was sealed and incubated for 24 h at 4°C. After the addition of 50 μl of diluted guinea pig complement (Calbiochem) (1 part complement, 3 parts PBS), the plates were resealed and incubated for 1 h at 37°C, and then 104 Vero cells in 50 μl of medium were added. After 3 days of incubation, the medium was removed, and the cells were washed once with PBS and finally incubated for 30 min at room temperature with 10 μg of fluorescein diacetate per ml in PBS. Only viable cells hydrolyze the dye to fluorescein (17). Fluorescence was measured with a 485-nm excitation wavelength and a 538-nm emission wavelength. The percentage of antiviral protection provided by the serum compared to that provided by an uninfected cell control was calculated.
RESULTS
Activities of BAY 57-1293 in a murine lethal challenge model.
The activities of orally administered BAY 57-1293 for the treatment of acute HSV-1 and HSV-2 infections were assessed in a murine lethal challenge model of disseminated herpes, including herpes encephalitis. This model is widely used for the evaluation of antiviral agents (4, 9). Mice were infected intranasally and were treated 6 h later with the compounds under evaluation for 5 consecutive days three times a day (t.i.d.). Survival curves were recorded over a period of 3 weeks. BAY 57-1293 and several compounds currently used for the treatment of herpes infections in humans were tested in escalating doses, and the individual dose at which 50% of the infected animals survived (ED50) was calculated by nonlinear regression. The results are summarized in Table 1. With an ED50 of 0.5 mg/kg of body weight t.i.d. against HSV-1 and HSV-2, BAY 57-1293 was the most potent compound tested. No toxic side effects of BAY 57-1293 treatment were apparent in the mice upon gross inspection, and the highest dose tested (60 mg/kg t.i.d.) appeared to be well tolerated.
TABLE 1.
Activity of BAY 57-1293 versus those of current drugs in the murine lethal challenge model
| Compound | ED50 (mg/kg)
|
|
|---|---|---|
| HSV-1 | HSV-2 | |
| BAY 57-1293 | 0.5 | 0.5 |
| Ganciclovir | 2.5 | 1.0 |
| Valacyclovir | 17 | 15 |
| Famciclovir | 17 | 24 |
| Acyclovir | 22 | 16 |
| Brivudin | 37 | >60 |
Effects of BAY 57-1293 treatment on viral burden in infected animals.
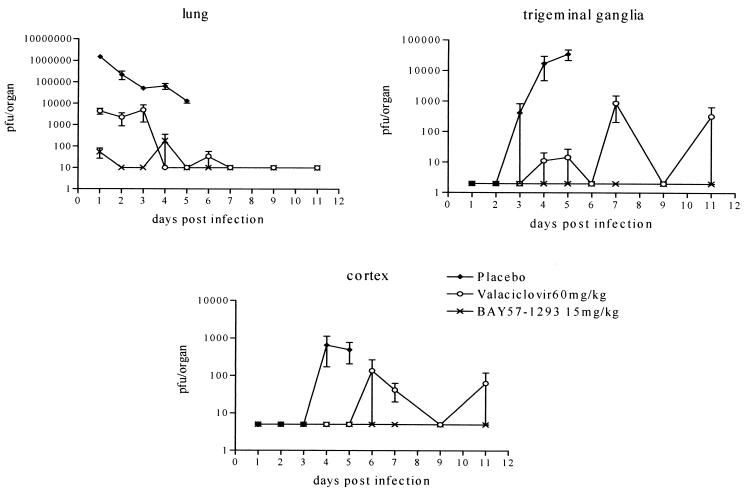
In addition to monitoring the survival of infected animals in the lethal challenge model, a quantitative study of virus replication in the lungs, brain cortex, and trigeminal ganglia of untreated, valacyclovir-treated (60 mg/kg t.i.d.), and BAY 57-1293-treated (15 mg/kg t.i.d.) mice was conducted. The mean viral titers per group are depicted in Fig. 2 for various organs and various time points postinfection. In all organs and at all time points tested, viral titers were strongly reduced in the BAY 57-1293-treated group compared to the titers in the placebo-treated group. Most importantly, in contrast to the findings for the valacyclovir-treated animals, animals treated with BAY 57-1293 did not show a recurrence of infectious virus in the trigeminal ganglia or brain cortex after the cessation of antiviral therapy (Fig. 2).
FIG. 2.
Viral titers in various organs of HSV-infected animals. Mice were infected intranasally with HSV-1walki and treated via oral gavage with placebo, valacyclovir (60 mg/kg), or BAY 57-1293 (15 mg/kg) t.i.d. from day 0 to day 4 postinfection. At the indicated time points, four animals from each group were killed, their organs were prepared, and the number of infectious HSV particles (PFU) was quantitated by titration on Vero cell monolayers. After day 5, no samples were available from placebo-treated animals, as they had all died by that time.
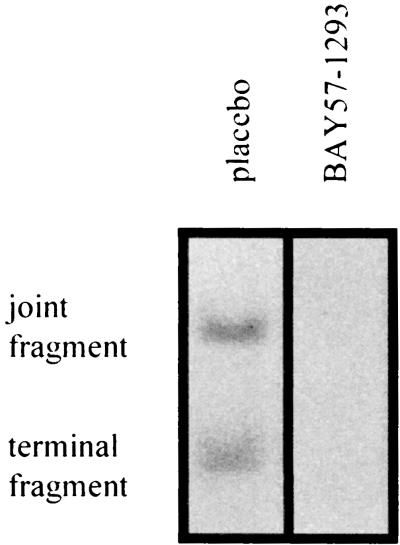
At day 5 postinfection, BAY 57-1293 (60 mg/kg t.i.d.)-treated and placebo-treated animals were killed and total DNA was prepared from their trigeminal ganglia. As shown in Fig. 3, HSV DNA could be detected by Southern blotting analysis in samples derived from placebo-treated animals. In contrast, no viral DNA could be detected by this method in animals treated with BAY 57-1293. By PCR and reverse transcription-PCR, however, it was possible to detect trace amounts of viral DNA and RNA (data not shown).
FIG. 3.
Detection of viral DNA by Southern blotting analysis. Animals were infected intranasally with HSV-1walki and treated via oral gavage with placebo or BAY 57-1293 (60 mg/kg) t.i.d. from day 0 to day 4 postinfection. At day 5 postinfection the animals were killed and total DNA was prepared from trigeminal ganglia and analyzed by Southern blotting hybridization (6). Equal amounts of DNA were loaded into each lane. The results of a typical experiment are shown.
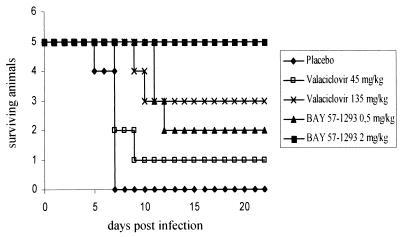
Effects of BAY 57-1293 in a rat lethal challenge model.
In order to ensure that the superiority of BAY 57-1293 is not restricted to the mouse, we established a rat lethal challenge model using Lewis rats and HSV-1walki. The results of a typical experiment are shown in Fig. 4. BAY 57-1293 exhibited profound antiviral activity in this model as well, and no toxic effects of BAY 57-1293 were apparent upon gross inspection. While the ED50 of BAY 57-1293 was similar in the rat and in the mouse, valacyclovir showed decreased activity in the rat (Fig. 4).
FIG. 4.
Rat lethal challenge model. Lewis rats were inoculated with HSV-1walki intranasally and were treated via oral gavage t.i.d. from day 0 to day 4 postinfection, as described in Materials and Methods. Five animals from each group were used. Treatment with 2 mg of BAY 57-1293 per kg t.i.d. is significantly better than treatment with 45 mg of valacyclovir per kg t.i.d. (P = 0.016 by the unpaired two-tailed t test).
Activities of BAY 57-1293 with once-daily dosing.
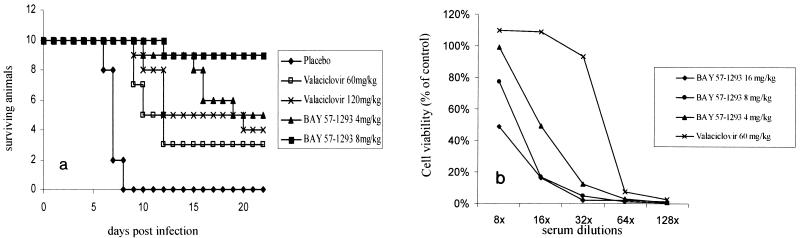
Once-daily dosing of valacyclovir is successfully used as treatment for the suppression of genital herpes (15). We investigated whether once-daily dosing of BAY 57-1293 would suffice to protect animals in the HSV-2 murine lethal challenge model in comparison to the activity of valacyclovir. For both BAY 57-1293 and valacyclovir, the ED50s increased by approximately a factor of 6 with the once-daily dosing regimen compared to that with the t.i.d. dosing regimen. Accordingly, in the once-daily dosing regimen, BAY 57-1293 clearly retained its superior activity compared to the activity of valacyclovir (Fig. 5a).
FIG. 5.
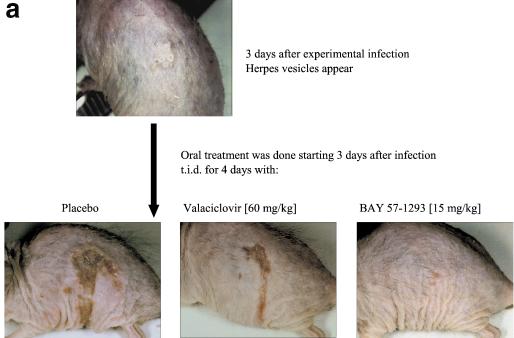
(a) Comparison of BAY 57-1293 with valacyclovir in the murine lethal challenge model using once-daily dosing. Mice were infected intranasally with a potentially lethal dose of HSV-2MS and were treated orally with BAY 57-1293 or valacyclovir once daily from day 0 to day 4 postinfection at the indicated doses. Ten animals from each group were used. Infected mice were inspected daily, and a survival curve was recorded. Treatment with 8 mg of BAY 57-1293 per kg was significantly superior to treatment with 120 mg of valacyclovir per kg under once-daily-dosing conditions (P = 0.02 by the unpaired two-tailed t test). (b) Neutralizing anti-HSV antibody titers. Animals treated with the indicated doses as described above for panel a were killed 4 weeks after infection, and their serum was analyzed for HSV-neutralizing activity, as described in Materials and Methods. Antibody production was decreased in BAY 57-1293-treated animals compared with that in valacyclovir-treated animals. Average values for three to six animals per group are shown.
The detection in serum of antibodies which recognize a given pathogen is widely used as a means of diagnosis of a history of infection with that pathogen. Furthermore, it has previously been shown that treatment with acyclovir reduces anti-HSV antibody titers in serum (1). Therefore, at 4 weeks after infection we assessed the HSV-neutralizing activity in the serum of surviving mice that had been treated once daily from day 0 to day 4 postinfection with either 60 mg of valacyclovir per kg or escalating doses of BAY 57-1293. Figure 5b demonstrates that neutralizing anti-HSV antibody titers were higher in animals treated with 60 mg of valacyclovir per kg than in animals treated with 4 mg of BAY 57-1293 per kg. This is in accordance with the finding that valacyclovir-treated animals suffered from a higher HSV burden than the BAY 57-1293-treated animals.
Activities of BAY 57-1293 in the murine zosteriform spread model mimicking recurrent cutaneous herpetic disease.
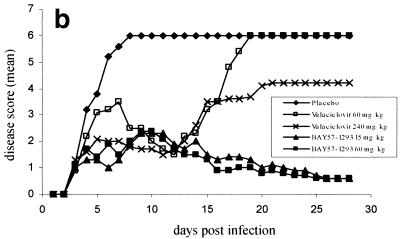
Intradermal infection of mice at the flank leads to local virus replication followed by the entry of virus into the nerves innervating the skin and spread to the ganglion. After replication in the ganglion, the virus disseminates along the sensory nerves to cause infection 5 to 10 days later in the whole innervated dermatome, thereby generating a zosteriform lesion (18). This spread from the sensory ganglia to the innervated skin is a model for recrudescent disease. In order to more closely mimic the clinical situation, in which a patient usually sees a doctor only when the first disease symptoms are already apparent, we used a regimen in which we initiated treatment at day 3 postinfection, i.e., when herpes vesicles had already developed (Fig. 6a).
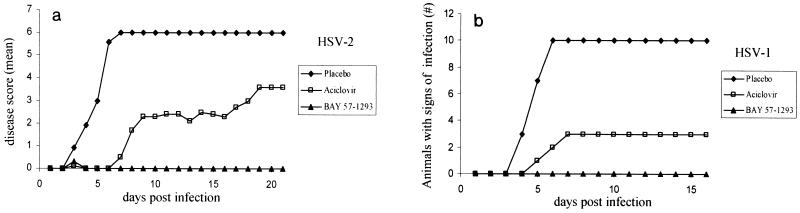
FIG. 6.
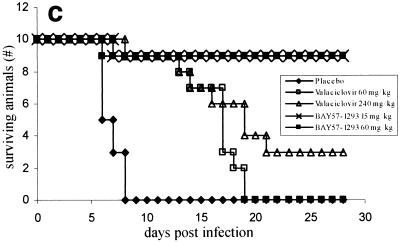
Zosteriform spread model. Comparison of BAY 57-1293 with valacyclovir in the zosteriform spread model. C3H/TifBom-hr female mice were dermally infected with 106 PFU of HSV-2G, and the disease score (the mean for 10 animals per group) was subsequently recorded on a daily basis by use of the scoring system described in Materials and Methods. Delayed oral treatment (t.i.d.) with 15 and 60 mg of BAY 57-1293 per kg and 60 and 240 mg of valacyclovir per kg was commenced on day 3 postinfection and continued until day 7 postinfection. (a) Zosteriform lesions for typical animals at the start of treatment (day 3 postinfection) and after 4 days of treatment (day 6 postinfection) with placebo, valacyclovir, or BAY 57-1293. Ten animals from each group were used. (b) The mean disease score for each treatment group was calculated at different times postinfection and plotted versus the time postinfection. The cumulative disease scores were calculated and compared between treatment groups by one-way analysis of variance. In order to account for multiple tests, the resulting P values were adjusted by the Bonferroni-Holm method. Treatment with 15 mg of BAY 57-1293 per kg t.i.d. was more effective than treatment with 240 mg of valacyclovir per kg t.i.d. (P < 0.011). (c) Survival curves for the various treatment groups.
Animals infected with HSV-2 by dermal scarification developed progressive zoster lesions starting 3 to 4 days postinfection and later succumbed to a lethal encephalitis, such that all placebo-treated animals died by day 8 postinfection (Fig. 6b and c). Animals treated orally with 60 mg of valacyclovir per kg t.i.d. from day 3 to day 7 postinfection showed a healing of lesions, leading to a minimal disease score at day 12, after which, however, the animals started to show symptoms of encephalitis, and all animals died by day 19 postinfection. Such a rebound infection was also apparent in animals treated with 240 mg of valacyclovir per kg t.i.d., although to a lesser extent (three animals survived) (Fig. 6b and c). In marked contrast to the results obtained with valacyclovir, treatment with either 15 or 60 mg of BAY 57-1293 per kg t.i.d. resulted in a sustained therapeutic effect. After treatment was stopped, only one animal developed encephalitis and 90% of the animals survived (Fig. 6b and c).
Activities of BAY 57-1293 for topical treatment of mucocutaneous and ocular herpes.
Treatment of recurrent mucocutaneous HSV infections in immunocompetent patients with topical antiviral formulations is widespread, although it is of only limited clinical benefit (13). In order to test for the topical effectiveness of BAY 57-1293, we assessed its activity in the murine zosteriform spread model using topical treatment with an ethanol-based formulation. Topical 2% BAY 57-1293 was compared with 2% acyclovir. The results are depicted in Fig. 7a. Topical BAY 57-1293 was highly effective and appeared to be more active than topical acyclovir.
FIG. 7.
Topical treatment with BAY 57-1293. (a) Animals were infected dermally with HSV-2G on the right flank, as described in Materials and Methods (zosteriform spread model). Treatment commenced at 6 h postinfection twice a day up to day 4 postinfection. BAY 57-1293 (2% [wt/vol]) and acyclovir (2% [wt/vol]) resuspended in 30% isopropylmyristate-70% ethanol were topically applied to the infected site, and the average disease score was recorded daily. Ten animals from each group were used. (b) Animals were infected with HSV-1walki via the scarified cornea, as described in Materials and Methods. Treatment was given starting 6 h after infection five times daily for a period of 5 days and consisted of 5 μl of eyedrops with 2% (wt/vol) BAY 57-1293 or acyclovir applied onto the infected eye. The number of animals showing signs of a herpes infection (blepharitis, keratitis, encephalitis) was recorded. Ten animals from each group were used.
The efficacy of BAY 57-1293 as a topical treatment for ocular HSV infections was assessed with an ophthalmic formulation. Animals were infected (Fig. 7b) via the scarified cornea and treated with eyedrops. All placebo-treated animals exhibited signs of disease (blepharitis, keratitis) at day 4 postinfection, developed encephalitis, and died by day 7 postinfection. Treatment with 2% topical acyclovir suppressed disease progression during treatment. When treatment was terminated, however, signs of HSV infection became apparent and 3 of 10 animals (Fig. 7b) succumbed to infection. In contrast, treatment with 2% topical BAY 57-1293 prevented herpetic disease in all animals for the duration of the experiment (16 days). Among the animals infected with HSV-2, 9 of 10 animals treated with acyclovir died, while only 1 of 10 animals treated with BAY 57-1293 succumbed to infection (not shown).
Pathogenicities of HSV strains resistant to BAY 57-1293.
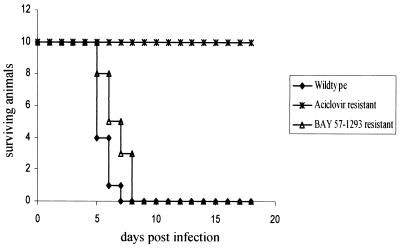
Acyclovir resistance in HSV in most cases is associated with a deficiency in viral thymidine kinase (7). Such thymidine kinase-deficient HSV strains have been shown to be nonpathogenic in animal models (14). Mutant HSV strains, resistant to BAY 57-1293, exhibit mutations in UL5 and UL52, which code for components of the viral primase-helicase complex (11a). In order to study whether a BAY 57-1293-resistant viral mutant also exhibits altered pathogenicity in vivo, BALB/c mice were infected intranasally with 3 × 105 PFU of HSV-1F, HSV-1F resistant to acyclovir, and HSV-1F resistant to BAY 57-1293 (mutation in UL5 K356N). As shown in Fig. 8, wild-type HSV-1F and the BAY 57-1293-resistant mutant exhibited almost equal pathogenicities, while the acyclovir-resistant mutant was nonpathogenic, as judged by animal survival after infection. Other BAY 57-1293-resistant mutants might behave differently.
FIG. 8.
Pathogenicity of a BAY 57-1293-resistant HSV-1 mutant. Mice were infected intranasally with 3 × 105 PFU of HSV-1F, HSV-1F resistant to acyclovir (acyclovir IC50, 125 μM), and HSV-1F resistant to BAY 57-1293 (BAY 57-1293 IC50, >125 μM). While acyclovir-resistant HSV-1 was totally apathogenic, BAY 57-1293-resistant virus was almost as pathogenic as the wild-type strain. Ten animals from each group were used.
DISCUSSION
BAY 57-1293 showed excellent antiherpetic potency in a murine model of disseminated herpes, with an ED50 of 0.5 mg/kg t.i.d. Five other antiviral compounds currently used for the treatment of HSV infections exhibited markedly lower activities in this murine model. The rank order of the potencies was BAY 57-1293 > ganciclovir > valacyclovir > famciclovir > acyclovir > brivudine. The ED50s published in the literature vary according to the model and treatment regimen used. The superiority of ganciclovir over acyclovir, as evident from our work, is well documented in various murine models in the literature (3, 9, 11). The activity of valacyclovir versus that of famciclovir has been a matter of debate (21), with a superiority of famciclovir being seen by some (20) but not by others (12). The reduced efficacy of famciclovir against HSV-2 compared with that against HSV-1, as also apparent from our data, however, is in accordance with results obtained in in vitro plaque reduction assays (8). The poor oral activity of brivudine, despite its strong in vitro activity against HSV-1, has also been described previously (9). The failure of brivudine in the HSV-2 lethal challenge model is reflected by its poor in vitro activity against HSV-2. These comparisons provide support for our model and the apparent superiority of BAY 57-1293 against HSV-1 and HSV-2 in vivo.
BAY 57-1293 was also markedly more active than valacyclovir (on a weight basis) in the rat (Fig. 4). Our ED50 of ∼100 mg/kg t.i.d. for valacyclovir is in good accordance with the findings presented in a recent report in which only acyclovir doses greater than 100 mg/kg were able to reduce mortality in a rat model of HSV infection (2).
BAY 57-1293 was also applied topically with good results (Fig. 7a and b). We expect BAY 57-1293 to show efficacy in clinical trials of topical HSV treatment superior to those of the compounds currently used, whose therapeutic benefits in topical formulations have been questioned (13, 19).
Our data show that HSV infection is very susceptible to treatment with BAY 57-1293. Early treatment of primary herpetic disease with current antivirals in humans does not appear to reduce the level of latent DNA and thereby influence the frequency and severity of subsequent recurrent infections. In this regard, it is especially interesting that in contrast to treatment with valacyclovir, no detectable HSV replication took place in the neuronal tissue of BAY 57-1293-treated animals (Fig. 2). Pharmacokinetic measurements confirmed that the thiazolylsulfonamides are able to pass the blood-brain barrier.
BAY 57-1293 is efficacious even when treatment is initiated after the onset of symptoms in the murine zosteriform spread model of cutaneous infection (Fig. 6). This is of utmost importance for episodic treatment in recurrent herpetic disease when the patient initiates treatment only after first symptoms are apparent. The ability of BAY 57-1293 to be efficacious when treatment is delayed or the viral load is increased is also essential for successful treatment of life-threatening herpes infections like herpes encephalitis and disseminated herpes. In that regard, combination therapy with current nucleosidic DNA polymerase inhibitors and the nonnucleosidic primase-helicase inhibitor BAY 57-1293 is also an option.
The superiority of BAY 57-1293 in vivo can be explained by its inherently strong antiviral activity, with an IC50 in cellular assays of HSV replication of 0.02 μM, compared to an IC50 of 2 μM for acyclovir (the difference was even more pronounced when the multiplicity of infection was increased) and its favorable pharmacokinetic profile (oral bioavailability, >60%; half-life, >6 h) (11a). In addition, its physicochemical parameters (e.g., partition coefficient between egg-lecithin and PBS, 1,590) allow penetration of the blood-brain barrier and its use in topical formulations.
Even high doses of BAY 57-1293 were well tolerated in the animal experiments described here. As a primary sulfonamide, however, BAY 57-1293 is a weak inhibitor of mammalian carbonic anhydrase (IC50, 2 to 5 μM).
In summary, we showed that the thiazolylsulfonamide BAY 57-1293 is highly efficacious in the treatment of HSV-1 and HSV-2 infections in a variety of animal models and is superior to the drugs currently in use. Thiazolylsulfonamides therefore should have the potential to become new standards in the treatment of HSV disease.
Acknowledgments
The contributions of P. Eckenberg, G. Handke, W. Bender, U. Schneider, K. Henninger, A. Jensen, J. Keldenich, J. Baumeister, O. Weber, G. Hewlett, J. Kolb, T. Laich, R. Jäger, U. Bach, A. Popp, R. Schohe-Loop, D. Häbich, H. Heilmann, and T. Schulze to the thiazolylsulfonamide project and the support of S. Wohlfeil, R. Hanko, K. Frobel, J. A. Karlsson, and W. Hartwig are gratefully acknowledged. We thank M. Becka for help with statistical analysis and I. Hulsmann, S. Veldhoen, S. Vogel, S. Schaab, E. Clemente, M. Hucke, K. Ostertag-Palm, M. Werth, J. Wann, and I. Frappa for excellent technical assistance, as well as K. Deres and G. Hewlett for critical reading of the manuscript.
REFERENCES
- 1.Bernstein, D. I., M. A. Lovett, and Y. J. Bryson. 1984. The effects of aciclovir on antibody response to herpes simplex virus in primary genital herpetic infections. J. Infect. Dis. 150:7-13. [DOI] [PubMed] [Google Scholar]
- 1a.Betz, U. A. K., C. A. J. Voβhenrich, K. Rajewsky, and W. Müller. 1996. Bypass of lethality with mosaic mice generated by Cre-loxP-mediated recombination. Curr. Biol. 6:1307-1316. [DOI] [PubMed] [Google Scholar]
- 2.Bidanset, D. J., L. Placidi, R. Rybak, J. Palmer, J.-P. Sommadossi, and E. R. Kern. 2001. Intravenous infusion of cereport increases uptake and efficacy of acyclovir in herpes simplex virus-infected rat brains. Antimicrob. Agents Chemother. 45:2316-2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Collins, P., and N. M. Oliver. 1985. Comparison of the in vitro and in vivo antiherpes virus activities of the acyclic nucleosides, aciclovir (Zovirax) and 9-[(2-hydroxy-1-hydroxymethylethoxy)methyl]guanine (BWB759U) Antivir. Res. 5:145-156. [DOI] [PubMed] [Google Scholar]
- 4.de Clercq, E., and M. Luczac. 1976. Intranasal challenge of mice with herpes simplex virus: an experimental model for the evaluation of the efficacy of antiviral drugs. J. Infect. Dis. 133:A226-A236. [DOI] [PubMed] [Google Scholar]
- 5.De Miranda, P., H. C. Krasny, D. A. Page, and G. B. Elion. 1982. Species differences in the disposition of acyclovir. Am. J. Med. 73:31-35. [DOI] [PubMed] [Google Scholar]
- 6.Efstathiou, S., A. C. Minson, H. J. Field, J. R. Anderson, and P. Wildy. 1986. Detection of herpes simplex virus specific DNA sequences in latently infected mice and in humans. J. Virol. 57:446-455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Erlich, K. S., J. Mills, P. Chatis, G. J. Mertz, D. F. Busch, S. E. Follansbee, R. M. Grant, and C. S. Crumpacker. 1989. Aciclovir-resistant herpes simplex virus infections in patients with the acquired immunodeficiency syndrome. N. Engl. J. Med. 320:293-296. [DOI] [PubMed] [Google Scholar]
- 8.Ertl, P., W. Snowden, D. Lowe, W. Miller, P. Collins, and E. Littler. 1995. A comparative study of the in vitro and in vivo antiviral activities of aciclovir and penciclovir. Antivir. Chem. Chemother. 6:89-97. [Google Scholar]
- 9.Field, H. J., J. R. Anderson, and S. Efstathiou. 1984. A quantitative study of the effects of several different nucleoside analogues on established herpes encephalitis in mice. J. Gen. Virol. 65:707-719. [DOI] [PubMed] [Google Scholar]
- 10.Field, H. J., and A. M. Thackray. 1995. The effects of delayed-onset chemotherapy using famciclovir or valaciclovir in a murine immunosuppression model for HSV-1. Antivir. Chem. Chemother. 6:210-216. [Google Scholar]
- 11.Inoue, Y., Y. Ohashi, Y. Shimomura, R. Manabe, M. Yamada, S. Ueda, and S. Kato. 1989. Comparative efficacy of three antiviral drugs in mice herpetic keratitis. Jpn. J. Ophthalmol. 33:125-131. [PubMed] [Google Scholar]
- 11a.Kleymann, G., et al. 2002. New helicase-primase inhibitors as drug candidates for the treatment of herpes simplex disease. Nat. Med. 8:392-398. [DOI] [PubMed] [Google Scholar]
- 12.LeBlanc, R. A., L. Pesnicak, M. Godleski, and S. E. Straus. 1999. The comparative effects of famciclovir and valaciclovir on herpes simplex virus type 1 infection, latency, and reactivation in mice. J. Infect. Dis. 180:594-599. [DOI] [PubMed] [Google Scholar]
- 13.Luby, J. P., J. W. Gnann, J. W. Alexander, V. A. Hatcher, A. E. Friedman-Kein, R. J. Klein, H. Keyserling, A. Nahmias, J. Mills, J. Schachter, J. M. Douglas, L. Corey, and S. L. Sacks. 1984. A collaborative study of patient-initiated treatment of recurrent genital herpes with topical aciclovir or placebo. J. Infect. Dis. 150:1-6. [DOI] [PubMed] [Google Scholar]
- 14.Meignier, B., R. Longnecker, P. Mayromara-Nazos, A. E. Sears, and B. Roizmann. 1988. Virulence and establishment of latency by genetically engineered deletion mutants of herpes simplex virus 1. Virology 162:251-254. [DOI] [PubMed] [Google Scholar]
- 15.Ormrod, D., L. J. Scott, and C. M. Perry. 2000. Valaciclovir: a review of its long term utility in the management of genital herpes simplex virus and cytomegalovirus infections. Drugs 59:839-863. [DOI] [PubMed] [Google Scholar]
- 16.Raborn, G. W., W. T. McGaw, M. Grace, and J. Perca. 1988. Treatment of herpes labialis with aciclovir. Am. J. Med. 85:39.. [PubMed] [Google Scholar]
- 17.Rotmann, B., and B. W. Papermaster. 1966. Membrane properties of living mammalian cells as studied by enzymatic hydrolysis of fluorogenic esters. Proc. Natl. Acad. Sci. USA 55:134.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Simmons, A., and A. A. Nash. 1984. Zosteriform spread of herpes simplex virus as a model of recrudescence and its use to investigate the role of immune cells in prevention of recurrent disease. J. Virol. 52:816-821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sudesh, S., and P. R. Laibson. 1999. The impact of the herpetic eye disease studies on the management of herpes simplex virus ocular infections. Curr. Opin. Ophthalmol. 10:230-233. [DOI] [PubMed] [Google Scholar]
- 20.Thackray, A. M., and H. J. Field. 1996. Differential effects of famciclovir and valaciclovir on the pathogenesis of herpes simplex virus in a murine infection model including reactivation from latency. J. Infect. Dis. 173:291-299. [DOI] [PubMed] [Google Scholar]
- 21.Thackray, A. M., and H. J. Field. 2000. Effects of famciclovir and valaciclovir on herpes simplex virus type 1 infection, latency, and reactivation in mice: how dissimilar are study results? J. Infect. Dis. 181:1517-1518. [DOI] [PubMed] [Google Scholar]
- 22.Whitley, R. J., A. Arvin, C. Prober, et al. 1991. A controlled trial comparing vidarabine with aciclovir in neonatal herpes simplex virus infection. N. Engl. J. Med. 14:444-449. [DOI] [PubMed] [Google Scholar]