Abstract
Intravascular catheter-associated bloodstream infections significantly increase rates of morbidity and hospital costs. Microbial colonization and development of biofilms, which are known to be recalcitrant to antibiotic therapy, often lead to the loss of otherwise patent vascular access systems. We evaluated a new taurolidine- and citrate-based catheter lock solution (Neutrolin; Biolink Corporation, Norwell, Mass.) for its activity against planktonic microbes, antimicrobial activity in a catheter model, and biofilm eradication activity. In studies of planktonic microbes, after 24 h of contact, 675 mg of taurolidine-citrate solution per liter caused >99% reductions in the initial counts of Staphylococcus aureus, Staphylococcus epidermidis, Pseudomonas aeruginosa, and Entercoccus faecalis. A solution of 13,500 mg/liter was cidal for Candida albicans. Ports and attached catheters inoculated with 50 to 600 CFU of these bloodstream isolates per ml were locked with heparin or the taurolidine-citrate solution. After 72 h, there was no growth in the taurolidine-citrate-treated devices but the heparin-treated devices exhibited growth in the range of 6 × 102 to 5 × 106 CFU/ml. Biofilms were developed on silicone disks in modified Robbins devices with broth containing 6% serum (initial counts, 106 to 108 CFU/cm2). The axenic biofilms were treated for 24 h with taurolidine-citrate or heparin. Taurolidine-citrate exposure resulted in a median reduction of 4.8 logs, whereas heparin treatment resulted in a median reduction of 1.7 logs (P < 0.01). No significant differences in the effects of the two treatments against P. aeruginosa and C. albicans were observed. These findings suggest that taurolidine-citrate is a promising combination agent for the prevention and treatment of intravascular catheter-related infections.
Intravascular catheter-related bloodstream infections (CRBSIs) lead to increased rates of morbidity, independently prolong hospital stays, and increase medical costs (13, 20, 24; L. A. Mermel, Letter, Ann. Intern. Med. 133:395, 2000). Insertion site colonization is an important source of microbes that cause CRBSIs; however, intraluminal catheter colonization may be a more important source of CRBSIs in patients with long-term catheterization (17, 21).
Bacterial and fungal adherence to catheters and/or host-derived proteins on the catheter surface is a prerequisite for implant-associated infections (7). The organisms within biofilms on a catheter surface possess significantly increased levels of resistance to antimicrobial agents and undergo lower levels of phagocytosis relative to the levels of resistance and phagocytosis for their planktonic counterparts (7). In addition, supraphysiologic concentrations of antibiotics may be required to eradicate microbial biofilms (5). The technique of filling indwelling catheters with an intraluminal antimicrobial agent combined with an anticoagulant solution may prevent catheter-related infections, as high concentrations of active ingredients can be maintained in direct contact with the internal surface of the device for prolonged periods of time (16).
The current standard of care in many institutions is to use heparin as a catheter lock solution to prevent thrombosis. Bacteria such as staphylococci can survive and grow in heparin-locked catheters (4) since heparin has limited intrinsic antimicrobial activity (12). However, preservatives can confer some antimicrobial activity to commercially available solutions (8). Potential complications of heparin use in catheters exist, such as heparin-induced thrombocytopenia and thrombosis (1). The use of therapeutic catheter lock solutions containing antimicrobial agents used for systemic therapy, either alone or in combination with anticoagulants, has been described previously (10, 16). The drawback of such an approach is that it may lead to the emergence of antibiotic resistance (22). Taurolidine [2 H-1,2,4-thiadiazine-4,4′-methylenebis(tetrahydro-1,1,1′,1′-tetraoxide)] is a derivative of aminosulfonamide-taurinamide. It is a unique, nontoxic substance with antiadherence and immunomodulatory properties (9, 28). Taurolidine has antimicrobial activity against a broad range of bacteria and fungi (25, 26; L. A. Mermel, N. Magill, and S. Zinner, Abstr. 38th Intersci. Conf. Antimicrob. Agents Chemother., abstr. F-190 and F-191, 1998). It also inhibits Staphylococcus coagulase, a clotting activator not inhibited by heparin, hirudin, or antithrombin (19), and inhibits other clotting pathways (18). In solution, taurolidine is present with active equilibrium products and metabolites which act as methylol transfer agents. It is hypothesized that these methylol groups bind irreversibly to bacterial or fungal cell wall constituents and exert their cidal actions (27). There was no evidence of resistance to taurolidine when it was tested against a broad range of microbial pathogens (25). Citrate solutions have been demonstrated to maintain patency by preventing blood coagulation and platelet aggregation (2, 3). Thus, an antimicrobial-anticoagulant catheter lock solution that consists of taurolidine and citrate (23) may possess the desirable attributes of an effective strategy to prevent CRBSIs.
We have performed several in vitro studies to determine the potential utility of using a catheter lock solution (Neutrolin; Biolink Corporation, Norwell, Mass.) containing taurolidine (1.35% [wt/vol]) and citrate (2.61% [wt/vol] as citric acid) to prevent intravascular catheter-related infections. Here, we describe in vitro studies that evaluated the taurolidine-citrate solution for its activity against planktonic microbes, antimicrobial activity in a catheter model, and biofilm eradication activity.
(This work was presented in part at the 101st General Meeting of the American Society for Microbiology, Orlando, Fla., May 2001, and at the 4th Decennial International Conference on Nosocomial and Healthcare-Associated Infections, Atlanta, Ga., March 2000.)
MATERIALS AND METHODS
Activity against planktonic microbes. (i) Test organisms.
Staphylococcus aureus (ATCC 700788), Staphylococcus epidermidis (ATCC 27626), Enterococcus faecalis (ATCC 700802), Pseudomonas aeruginosa (ATCC 27313), and Candida albicans (ATCC 90029) were used in the experiments evaluating the taurolidine-citrate solution for its activity against planktonic microbes.
(ii) Test organism preparation.
S. aureus, S. epidermidis, and P. aeruginosa were grown on Trypticase soy agar, E. faecalis was grown on brain heart infusion agar, and C. albicans was grown on Sabouraud dextrose agar plates. The isolated colonies were transferred to 5 ml of sterile 0.9% sodium chloride. The inoculum concentration was adjusted to approximately 5 × 107 CFU/ml by dilution in NaCl. This study was performed by the use of modified methods of NCCLS (15).
(iii) Antimicrobial activity tests.
Taurolidine-citrate solution (1.35% taurolidine, 2.61% citric acid) was serially diluted in Mueller-Hinton broth. Each dilution was tested in triplicate and was inoculated with the challenge organisms at final concentrations of 5 × 105 CFU/ml. The ratio of the taurolidine-citrate solution to the inoculum was 1.0 ml:0.01 ml (by volume). After a 24-h contact period with the microbial inoculum, the number of viable bacteria in each sample was determined by the spread plate method. Serial dilutions were performed (if necessary) in AOAC Letheen broth, and 0.1 ml was transferred to the surfaces of the agar plates. The detection limit was 10 CFU/ml. Inoculated Mueller-Hinton broth to which the test article was not added served as the control.
(iv) Data analysis.
Statistical analyses (analysis of variance [ANOVA]) were performed with STATGRAPHICS PLUS software (Professional version, Windows 4.0; Statistical Graphics Corporation, Englewood Cliffs, N.J.).
Antimicrobial activity in a catheter model. (i) Test organisms.
S. aureus, Staphylococcus hominis, P. aeruginosa, Pantoea agglomerans, E. faecalis, and C. albicans were used to evaluate the taurolidine-citrate solution for its antimicrobial activity in a catheter model. These isolates were from patients with primary bloodstream infections.
(ii) Test organism culture.
The test organisms were grown in Trypticase soy broth (TSB; Difco Laboratories, Detroit, Mich.) and were then diluted to between 5 and 60 CFU/0.1 ml in TSB.
(iii) Test device.
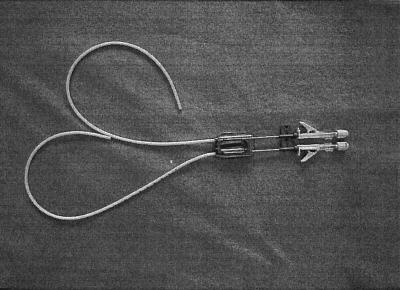
Study of the antimicrobial activity of the taurolidine-citrate solution in a catheter model was performed with the Dialock Hemodialysis Access system (Biolink Corporation), which consists of a titanium access port and two silicone catheters (Fig. 1).
FIG. 1.
Dialock access port, catheters, and needles.
(iv) Device inoculation and treatment.
Approximately 0.15 ml of a test organism in broth was added to each lumen of an access device. Each inoculated device was sealed and incubated overnight at 37°C. After incubation, sterile silicone catheters were connected to each lumen of the device and a sterile needle set was inserted into each lumen of the port. Approximately 2 ml of taurolidine-citrate solution (sufficient to fill the needle set, access port, and catheter) was slowly injected into each access port lumen with a sterile syringe attached to the hub of the needle sets. Catheters were clamped at the distal end as soon as the lock solution filled each lumen, and the needle sets were then removed. The procedure described above was repeated with a separate Dialock set with heparin sodium solution (10,000 U/ml preserved with 0.15% methylparaben and 0.015% propylparaben [Fujisawa USA, Inc., Deerfield, Ill.] was diluted 1:1 with sterile 0.9% saline solution [Abbott Laboratories, Chicago, Ill.] to 5,000 U/ml). Each test system was maintained in a horizontal orientation and incubated at 37°C for 72 h. Each organism was tested in duplicate.
(v) Recovery of treated organisms.
After 72 h, each access device and clamped catheter system was removed from the incubator. Prior to detachment of the catheters, all external surfaces of the system were thoroughly wiped with alcohol and allowed to dry completely. Each catheter was separated from the device and supported vertically near the clamp. The catheter fluid was collected by opening the clamps, which allowed fluid to drain from the opposite catheter end into a sterile test tube. A 100-μl volume of this fluid from each catheter was transferred to plates containing Trypticase soy agar with 5% sheep blood (BBL Microbiology Systems, Cockeysville, Md.), and the plates were incubated overnight at 37°C. A 5-cm segment was cut from the midsection of each detached catheter, placed in a sterile test tube filled with 3 ml of TSB, sonicated for 1 min, and then vortexed for 15 s. A 100-μl volume of the sonicated broth was transferred to blood agar plates, and the plates were incubated at 37°C. A new sterile Dialock needle was introduced into the open lumen of the access port to the docked position and was retracted approximately 5 mm. One milliliter of broth was flushed through the port lumen and collected in a test tube. A 100-μl volume of broth was transferred to blood agar plates, and the plates were incubated at 37°C. The broth flushed through each device, the solution drained from each catheter, and the broth of the sonicated catheter segments were serially diluted 10-fold. The detection limits for the cultures of samples from the access port and lumen were 10 CFU/ml; the detection limit for the cultures with sonicated broth of the catheter segment was 30 CFU/ml.
(vi) Data analysis.
Statistical analyses (ANOVA) were performed with STATGRAPHICS PLUS software (Professional version, Windows 4.0; Statistical Graphics Corporation).
Biofilm eradication activity. (i) Test organisms.
Isolates were obtained from the following sites: soft tissue (S. aureus, E. faecalis), blood (P. aeruginosa), respiratory tract infection (C. albicans), and central venous catheter (S. epidermidis). The isolates were kindly provided by Robert Arbeit of the Boston Veterans Affairs Hospital and Boston University and were different from those used in the catheter model study.
(ii) Test device preparation .
The study of biofilm eradication activity involved biofilm colonization of silicone coupon surfaces (diameter, 0.8 cm; thickness, 1 mm; NuSil 4970) in modified Robbins device flow cells. Detailed information on the Robbins device can be found elsewhere (6). Silicone coupons were sealed into coupon housings with sealant (Dow Corning 732 silicone; Dow Corning, Midland, Mich.). The coupons were allowed to set for 24 h before they were swabbed with 70% isopropyl alcohol. The completed Robbins devices were sterilized with ethylene oxide.
(iii) Continuous culture of challenge organisms .
All isolates were grown on Mueller-Hinton agar (Gibson Laboratories, Lexington, Ky.) for 72 h at 35°C before a single colony was transferred to 20 ml of Mueller-Hinton broth containing 6% (vol/vol) newborn calf serum (Sigma Chemical Co., St. Louis, Mo.). Suspensions of the challenge organisms were washed twice in 100 mM phosphate-buffered saline (PBS) and inoculated into 300 ml of Mueller-Hinton broth with 6% (vol/vol) newborn calf serum in a bioreactor to achieve a final concentration of 1.0 × 107 CFU/ml. The bioreactor was maintained at 35°C with an agitation rate of approximately 60 rpm for 24 h. At 24 h, a continuous culture was started with a dilution rate of 15 ml/h. After approximately 12 h, recirculation of the cultures was initiated through each of two separate Robbins devices at a flow rate of 1 ml/min for 72 h in order to establish a biofilm layer on the test coupons.
(iv) Device treatment and posttreatment analysis.
Following the 72-h biofilm colonization period, four silicone disks were removed from the device and processed as described below. Each Robbins device was then flushed with 1 void volume of either taurolidine-citrate solution or preservative-containing heparin solution (5,000 U/ml; Elkins-Sinn, Cherry Hill, N.J.) and incubated under static conditions at 35°C for 24 h. Four silicone coupons were then aseptically removed from the Robbins devices. Excess liquid was removed by aseptically blotting the edges of the three coupons onto sterile petri dishes. The coupons were then placed in sterile 5-ml tubes with PBS (which had been filtered through a 0.45-μm-pore size filter) and 3-mm-diameter glass beads. Adherent biofilm bacteria were recovered by low-power sonication of the PBS-coupon tubes in an ice-cold bath five times at 3-s intervals before they were vortexed for 1 min. This procedure has been shown to recover approximately 99% of adherent bacteria from the coupon surfaces (14). Viable spread plate assays were performed in duplicate with the appropriate dilutions of PBS on Mueller-Hinton agar (Sabouraud dextrose agar was used for C. albicans), with incubation at 35°C for 48 h. Data were normalized to the numbers of viable cells per square centimeter as a function of the treatment solution type and the challenge organism. The minimum detection limit was 100 CFU/cm2.
(v) Data analysis.
Statistical analyses (ANOVA) were performed with InStat software (version 3.00 for Windows 95; GraphPad Software, San Diego, Calif.).
RESULTS
Activity against planktonic microbes.
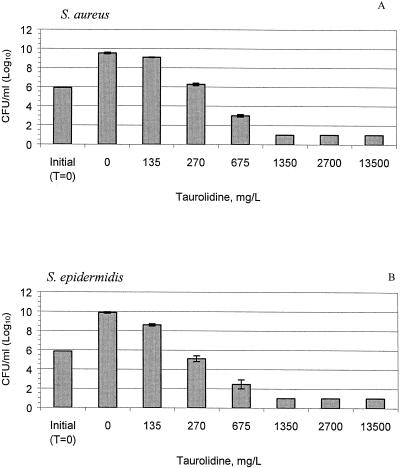
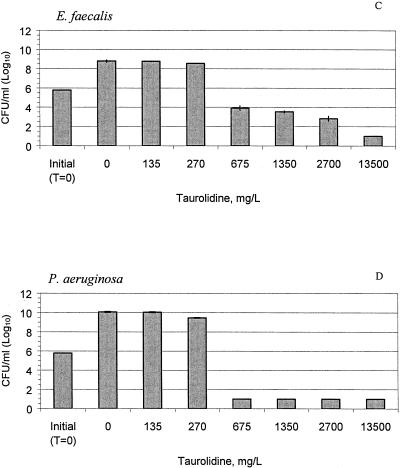
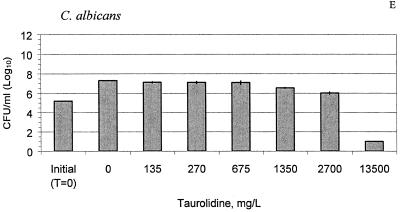
The MICs and cidal concentrations of the taurolidine-citrate solution are presented in Table 1. After 24 h, the concentrations of all test organisms in the control solution increased 2 logs from the initial counts (P < 0.01), as shown in Fig. 2A to E. Treatment with 675 mg of taurolidine-citrate solution per liter led to 2- to 5-log reductions of S. aureus, S. epidermidis, P. aeruginosa, and E. faecalis compared with the counts for the controls (P < 0.05). Against C. albicans, only the undiluted taurolidine-citrate solution (13,500 mg/liter) was effective and resulted in a 4.2-log reduction compared with the counts for the controls (P < 0.001). At concentrations as low as 135 mg/liter, the taurolidine-citrate solution significantly inhibited the growth of S. aureus, S. epidermidis, and C. albicans compared to the growth of the controls (P < 0.05).
TABLE 1.
MIC and MBC ranges of taurolidine-citrate solution
| Organism | MIC range (mg/liter) | MBC range (mg/liter) | MBC/MIC range |
|---|---|---|---|
| S. aureus | 270-675 | 270-675 | 0.4-2.5 |
| S. epidermidis | 135-270 | 270-675 | 1-5 |
| E. faecalis | 270-675 | 1,350-2,700 | 2-10 |
| P. aeruginosa | 270-675 | 270-675 | 0.4-2.5 |
| C. albicans | >2,700 | >2,700 |
FIG. 2.
Taurolidine-citrate activity against S. aureus (A), S. epidermidis (B), E. faecalis (C), P. aeruginosa (D), and C. albicans (E) at different concentrations at 24 h. The bars labeled “initial” indicate the organism counts in broth at time zero (T = 0 h). The control bars (0 mg/liter) indicate no treatment with taurolidine-citrate. The results are expressed as means ± standard deviations (the standard deviations are indicated by error bars).
Antimicrobial activity in a catheter model.
The results obtained for postincubation cultures of broth flushed through the access port, sonicated catheter segment broth, and the contents drained from the catheter lumen are presented in Table 2. Cultures of samples from taurolidine-citrate-treated devices showed no growth, and the concentrations were found to be below the detection limit (P < 0.001 versus the initial counts). Conversely, the cultures of samples from the heparin-treated devices had growth in the range of 6 × 102 to 5 × 106 CFU/ml (P < 0.05 versus the initial counts).The culture of one sample of broth flushed through a taurolidine-citrate-treated access port exhibited 102 CFU of P. aeruginosa per ml. The final counts of P. agglomerans in cultures of two samples from taurolidine-citrate-treated catheter lumens and one sample from a heparin-treated catheter lumen could not be quantified because the respective cultures dried during the experiments. The counts of S. hominis in the culture of one sample from a heparin catheter lumen could not be quantified for the same reason.
TABLE 2.
Anticolonizing activities of taurolidine-citrate and heparin lock solutions
| Culture and organism (inoculum [CFU/ml]) | Growth (CFU/ml)a
|
|
|---|---|---|
| Heparin lock solution | T/C lock solution | |
| Broth flushed from access port | ||
| S. aureus (160) | 8.5 × 103 | No growth |
| S. hominis (600) | 2.2 × 104 | No growth |
| E. faecalis (350) | 2.6 × 104 | No growth |
| P. agglomerans (100) | 6.0 × 102b | No growth |
| P. aeruginosa (50) | 8.0 × 105 | 1.0 × 102b |
| C. albicans (500) | 1.9 × 104 | No growth |
| Broth of sonicated segment | ||
| S. aureus (160) | 1.7 × 104 | No growth |
| S. hominis (600) | 3.0 × 104 | No growth |
| E. faecalis (350) | 7.5 × 104 | No growth |
| P. agglomerans (100) | 1.5 × 103 | No growth |
| P. aeruginosa (50) | 3.5 × 105 | No growth |
| C. albicans (500) | 1.2 × 103 | No growth |
| Catheter lumen flush solution | ||
| S. aureus (160) | 7.5.0 × 104 | No growth |
| S. hominis (600) | 1.0 × 104b | No growth |
| E. faecalis (350) | 2.5 × 104 | No growth |
| P. agglomerans (100) | 3.0 × 104b | |
| P. aeruginosa (50) | 4.25 × 106 | No growth |
| C. albicans (500) | 2.25 × 104 | No growth |
The data represent the averages of two replicates, unless noted otherwise.
The second replicate culture dried during the experiment.
Biofilm eradication activity.
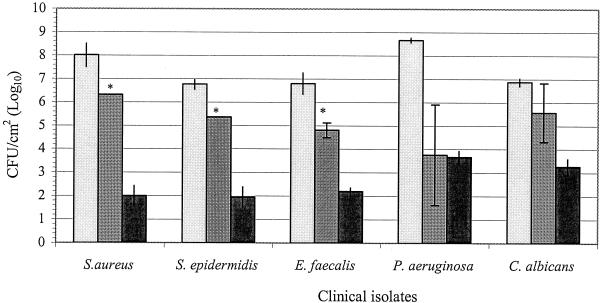
The viable counts of organisms within biofilms prior to exposure to either taurolidine-citrate or heparin solutions ranged from 106 to 108 CFU/cm2, as shown in Fig. 3. The viability of each test organism in biofilms following 24 h of exposure to the two test solutions is also shown in Fig. 3. Taurolidine-citrate was significantly more effective than heparin in eradicating S. aureus, E. faecalis, and S. epidermidis within biofilms (P < 0.001). After a 24-h exposure to taurolidine-citrate, the counts of the S. aureus, E. faecalis, and S. epidermidis organisms within biofilms decreased 6.0, 4.6, and 4.8 logs from the initial viable counts, respectively (P < 0.001). The viable counts of these organisms within biofilms were reduced 1.7, 2.0, and 1.4 logs from the initial viable counts, respectively, after 24 h of contact with heparin (P < 0.01). For C. albicans, neither taurolidine-citrate nor heparin caused a significant reduction in the viable counts compared with the initial viable counts (P > 0.05). The two treatments led to average reductions of 3.6 and 1.3 logs from the initial viable counts, respectively (P < 0.01). The taurolidine-citrate and heparin solutions caused 5.0- and 4.9-log reductions in the initial viable counts of P. aeruginosa, respectively (P < 0.05). For P. aeruginosa, there was no significant difference between the effectiveness of either taurolidine-citrate or heparin treatment (P > 0.05). The biofilm experiments with P. aeruginosa were repeated five times. The taurolidine-citrate and heparin treatments led to 4.7- and 4.8-log reductions in the number of viable P. aeruginosa organisms, respectively.
FIG. 3.
Comparison of the efficacies of taurolidine-citrate and heparin against biofilm organisms. The results are expressed as means ± standard deviations (the standard deviations are indicated by error bars). The detection limit was 100 CFU/cm2 (2 logs). Bars with light shading, initial viable counts; bars with medium shading, viable counts for heparin-treated cultures; solid bars, viable counts for taurolidine-citrate-treated cultures; ∗, significant difference (P < 0.05) between taurolidine-citrate and heparin.
DISCUSSION
A lock solution that prevents colonization and that inhibits the growth of biofilm-containing organisms in a catheter lumen may be an important addition to our armamentarium for the prevention of CRBSIs, especially if it is effective against staphylococci. Although a number of strategies aimed at the prevention of CRBSIs exist, approximately 80,000 central venous catheter-related bloodstream infections occur yearly in U.S. intensive care unit patients (13; Mermel, letter). Most of the interventions subjected to clinical trials have focused on these short-term catheterizations. However, many patients receive long-term intravascular catheters for hemodialysis, cancer chemotherapy, and nutritional support. These catheters may be more likely to become infected by the intraluminal route with microbes colonizing the catheter hubs or infusate (17, 21), and new strategies must focus on preventing infections emanating from these sources. The use of antimicrobial agents to fill the lumens of catheters has been successful in reducing the risk of CRBSIs (10, 16). However, the potential for the development of resistance as a result of the widespread use of therapeutic agents may limit the use of systemic antimicrobials in this fashion (22). Taurolidine is not effective systemically as an antimicrobial agent; however, preliminary clinical studies of its use as a catheter lock solution have been promising (11, 23). As a lock solution, taurolidine would be expected to prevent colonization of a catheter lumen and inhibit the growth of biofilm-containing organisms in a catheter lumen. In a taurolidine-citrate solution, broad-spectrum antimicrobial activity predominantly resides in the taurolidine component. Experiments with metabolites of taurolidine have revealed that the citrate component in a taurolidine-citrate solution does not contribute to antimicrobial activity (data not shown).
The addition of citrate is expected to reduce the likelihood of catheter lumen occlusion by blood (2). Taurolidine-citrate solution contains an equivalent of 4% trisodium citrate, nearly 10-fold less than the 46% trisodium citrate which has been associated reported to cause severe adverse events, and the Food and Drug Administration has issued a warning against its use (information can be found at the Food and Drug Administration website [http://www.fda.gov/bbs/topics/ANSWERS/ANS01009.html]). The use of 4% citrate in the taurolidine-citrate solution has not been associated with adverse events (23).
The results of our in vitro susceptibility studies confirm the observations of previous investigators (25, 26, 27).
We studied the in vitro activity of a taurolidine-citrate solution and compared it with that of preservative-containing heparin, which is used to flush intravascular catheters. In the studies with catheters, the ports and catheters were inoculated with the test organisms at concentrations (50 to 600 CFU/ml) similar to those that would be expected if contamination of the device occurred from a breach in aseptic technique during its use in human subjects. The taurolidine-citrate solution was effective in preventing the growth of the inoculated organisms. However, the organisms grew in the heparin-treated devices.
The taurolidine-citrate solution was effective at killing a diverse group of bacteria within biofilms, as well as C. albicans, within 24 h. The biofilms were developed under in vitro conditions designed to simulate the in situ catheter environment. The heparin treatment was significantly less effective than the taurolidine-citrate treatment against most of the challenge organisms with the exception of P. aeruginosa and C. albicans organisms within biofilms. The antimicrobial activity of heparin found in the biofilm study may be attributed, in part, to the preservatives contained in the formulation (8). At concentrations below 6,000 U/ml, preservative-free heparin lacks antimicrobial properties (4). The commercial formulations of heparin contain preservatives such as methylparaben, propylparaben, or benzyl alcohol. These preservatives may impart antimicrobial action, depending on their concentrations. In the studies with catheters, a commercial formulation of 10,000 U of heparin per ml was diluted to 5,000 U/ml with 0.9% saline. This dilution also caused a 50% reduction in the concentrations of preservatives that may have otherwise reduced the antimicrobial activity of the heparin solution used in experiments with contaminated ports and catheters (data not shown). In contrast to our catheter studies, the biofilm study used a commercial formulation of 5,000 U of heparin per ml without dilution. This undiluted preservative-containing heparin inhibited the growth of the organisms used in our biofilm model. Some of our preliminary unpublished data suggest that heparin flush solutions have reduced antimicrobial activities. Therefore, dilution of solutions of 10,000 U of heparin per ml may result in increased rates of catheter-related infections. This issue warrants further investigation.
Our findings suggest that taurolidine-citrate solution may prevent colonization of catheter surfaces by a broad range of microbial pathogens and may prevent life-threatening CRBSIs during clinical use.
Acknowledgments
This work was supported by Biolink Corporation.
We acknowledge the assistance of Harry Martins of Biolink Corporation in the design of the experiments and critical review of the manuscript.
REFERENCES
- 1.Arepally, G., and D. B. Cines. 1998. Heparin-induced throbocytopenia and thrombosis. Clin. Rev. Allergy Immunol. 16:237-247. [DOI] [PubMed] [Google Scholar]
- 2.Branson, P. K., R. A. McCoy, B. A. Phillips, and G. D. Clifton. 1993. Efficacy of 1.4 percent sodium citrate in maintaining arterial catheter patency in patients in a medical ICU. Chest 103:882-885. [DOI] [PubMed] [Google Scholar]
- 3.Buturovic, J., R. Ponikvar, A. Kandus, M. Boh, J. Klinkmann, and P. Ivanovich. 1998. Filling hemodialysis catheters in the interdialytic period: heparin versus citrate versus polygeline: a prospective randomized study. Artif. Organs 22:945-947. [DOI] [PubMed] [Google Scholar]
- 4.Capdevila, J. A., J. Gavalda, J. Fortea, P. Lopez, M. T. Martin, X. Gomis, and A. Pahissa. 2001. Lack of antimicrobial activity of sodium heparin for treating experimental catheter-related infection due to Staphylococcus aureus using antibiotic-lock technique. Clin. Microbiol. Infect. 7:206-212. [DOI] [PubMed] [Google Scholar]
- 5.Ceri, H., M. E. Olson, C. Stremick, R. R. Read, D. Morck, and A. Buret. 1999. The Calgary biofilm device: new technology for rapid determination of antibiotic susceptibilities of bacterial biofilms. J. Clin. Microbiol. 37:1771-1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dasgupta, M. K., K. Ward, P. A. Noble, M. Larabie, and J. W. Costerton. 1994. Development of bacterial biofilms on silastic catheter materials in peritoneal dialysis fluid. Am. J. Kidney Dis. 23:709-716. [DOI] [PubMed] [Google Scholar]
- 7.Eiff, C. V., C. Heilmann, and G. Peters. 1999. New aspects in the molecular basis of polymer-associated infections due to staphylococci. Eur. J. Clin. Microbiol. Infect. Dis. 18:843-846. [DOI] [PubMed] [Google Scholar]
- 8.Elliott, T. S. J., and A. Curran. 1989. Effects of heparin and chlorbutol on bacterial colonization of intravascular cannulae in an in vitro model. J. Hosp. Infect. 14:193-200. [DOI] [PubMed] [Google Scholar]
- 9.Gorman, S. P., D. F. McCafferty, A. D. Woolfson, and D. S. Jones 1987. A comparative study of the microbial antiadherence capacities of three antimicrobial agents. J. Clin. Pharmacol. Ther. 12:393-399. [DOI] [PubMed] [Google Scholar]
- 10.Henrickson, K. J., R. A. Axtell, S. M. Hoover, S. M. Kuhn, J. Pritchett, S. C. Kehl, and J. P. Klein. 2000. Prevention of central venous catheter-related infections and thrombotic events in immunocompromised children by the use of vancomycin/ciprofloxacin/heparin flush solution: a randomized, multicenter, double blind trial. J. Clin. Oncol. 18:1269-1278. [DOI] [PubMed] [Google Scholar]
- 11.Jurewitsch, B., T. Lee, J. Park, and K. Jeejeebhoy. 1998. Taurolidine 2% as an antimicrobial lock solution for prevention of recurrent catheter-related bloodstream infections. J. Parenter. Enteral Nutr. 22:242-244. [DOI] [PubMed] [Google Scholar]
- 12.Mermel, L. A., S. M. Stolz, and D. G. Maki. 1993. Surface antimicrobial activity of heparin-bonded and antiseptic-impregnated vascular catheters. J. Infect. Dis. 167:920-924. [DOI] [PubMed] [Google Scholar]
- 13.Mermel, L. A. 2000. Prevention of intravascular catheter-related infections. Ann. Intern. Med. 132:391-402. [DOI] [PubMed] [Google Scholar]
- 14.Mittelman, M. W. 1999. Recovery and characterization of biofilm bacteria associated with medical devices. Methods Enzymol. 310:534-551. [DOI] [PubMed] [Google Scholar]
- 15.National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard, 5th ed. NCCLS document M7-A5. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 16.Raad, I., A. Buzaid, J. Rhyme, R. Hachem, R. Darouiche, H. Safar, M. Albitar, and R. J. Sherertz. 1997. Minocycline and ethyelenediaminetetraacetate for the prevention of recurrent vascular catheter infections. Clin. Infect. Dis. 25:149-151. [DOI] [PubMed] [Google Scholar]
- 17.Raad, I., W. Costerton, U. Sabharwal, M. Sacilowski, E. Anaissie, and G. P. Bodey. 1993. Ultrastructural analysis of indwelling vascular catheters: a quantitative relationship between luminal colonization and duration of placement. J. Infect. Dis. 168:400-407. [DOI] [PubMed] [Google Scholar]
- 18.Reinmuller, J. 1999. The influence of taurolidine on physiological and pathological blood coagulation and implications for its use. Zentbl. Chir. 124:13-18. [PubMed] [Google Scholar]
- 19.Reinmüller, J., W. Mutzchler, and H. Meyer. 1999. Hemmung der Staphylokokken-Koagulase durch Taurolin. Hämostaseologie 19:94-97. [Google Scholar]
- 20.Rello, J., A. Ochagavia, E. Sabanes, M. Roque, D. Mariscal, E. Reynaga, and J. Valles. 2000. Evaluation of outcome of intravenous catheter-related infections in critically ill patients. Am. J. Respir. Crit. Care Med. 162:1027-1030. [DOI] [PubMed] [Google Scholar]
- 21.Sherertz, R. J. 1997. Pathogenesis of vascular catheter-related infections, p. 1-30. In H. Seifert, B. Jansen, and B. M. Farr (ed.), Catheter-related infections. Marcel Dekker, Inc. New York, N.Y.
- 22.Sieradzki, K., R. B. Roberts, D. Serur, J. Hargrave, and A. Tomasz. 1998. Recurrent peritonitis in a patient on dialysis and prophylactic vancomycin. Lancet 351:880-881. [DOI] [PubMed] [Google Scholar]
- 23.Sodemann, K., H. D. Polaschegg, and B. Feldmer. 2001. Two years' experience with Dialock and CLS (a new antimicrobial lock solution). Blood Purif. 19:251-254. [DOI] [PubMed] [Google Scholar]
- 24.Soufir, L., J.-F. Timsit, C. Mahe, J. Carlet, B. Regnier, and S. Chevret. 1999. Attributable morbidity and mortality of catheter-related septicemia in critically ill patients: a matched, risk-adjusted cohort study. Infect. Control Hosp. Epidemiol. 20:396-401. [DOI] [PubMed] [Google Scholar]
- 25.Torres-Viera, C., C. Thauvin-Eliopoulos, M. Souli, P. DeGirolami, M. G. Farris, C. B. Wennersten, R. D. Sofia, and G. M. Eliopoulos. 2000. Activities of taurolidine in vitro and in experimental enterococcal endocarditis. Antimicrob. Agents Chemother. 44:1720-1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Traub, W. H., B. Leonhard, and D. Bauer. 1993. Taurolidine: in vitro activity against multiple-antibiotic resistant nosocomially significant clinical isolates of Staphylococcus aureus, Enterococcus faecium, and diverse Enterobacteriaceae. Chemotherapy (Basel) 39:322-330. [DOI] [PubMed] [Google Scholar]
- 27.Waser, P. G., and E. Sibler. 1986. Taurolidine—a new concept in antimicrobial chemotherapy, p. 155-169. In A. F. Harms (ed.), Innovative approaches in drug research. Elsevier Science Publishers B.V., Amsterdam, The Netherlands.
- 28.Watson, R. W., H. P. Redmond, J. McCarthy, and D. Bouchier-Hayes. 1995. Taurolidine, an antilipopolysaccharide agent, has immunoregulatory properties that are mediated by the amino acid taurine. J. Leukoc. Biol. 58:299-306. [DOI] [PubMed] [Google Scholar]