Abstract
Bis-lentivirus lytic protein 1 (Bis-LLP1) and polymyxin B exhibited similar killing activities against Serratia marcescens. By electron microscopy, bis-LLP1 interacted with the outer and cytoplasmic bacterial membranes, while polymyxin B affected only the outer membrane. The results of standard biochemical probes supported the findings of the electron microscopy studies, suggesting that these antimicrobial peptides have different mechanisms of action.
Vertebrate-derived antimicrobial peptides have been recognized as important components of the innate immune response (4). Appreciation of their biological activities has enabled their development as anti-infective agents (3). Possible mechanisms of membrane disruption have been proposed to involve outer membrane perturbation, breakdown of the cytoplasmic membrane, or interaction with cytoplasmic targets (13).
The lentivirus lytic peptides (LLPs), encoded by discrete C-terminal sequences of the human immunodeficiency virus type 1 transmembrane protein, demonstrate potent antimicrobial activity (9), even though they have not evolved specifically for host defense against bacterial infection. While the antimicrobial activity of the LLPs is similar to that of other cationic antimicrobial peptides (CAPs) (10), there has been no investigation of its molecular basis of activity. This study compared the mechanisms of action of bis-LLP1, a dimerized and amidated LLP1 derivative, and polymyxin B against S. marcescens by using a standard in vitro broth dilution assay followed by electron microscopy (EM) and biochemical analysis.
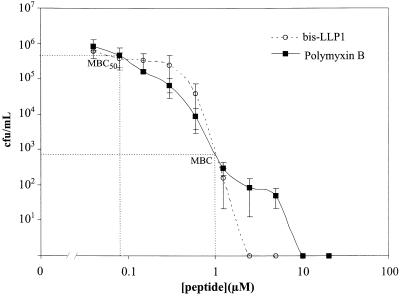
The potencies of bis-LLP1 and polymyxin B for S. marcescens were compared on a molar basis (Fig. 1) with a standard broth dilution assay (8) and found to be equivalent (minimal bactericidal concentration [MBC], 1 μM). Polymyxin B consistently demonstrated a biphasic curve with an inflection between 1 and 10 μM, which was not typical of most antimicrobial peptides. Complete sterilization of a bacterial broth culture was achieved at concentrations greater than 10 μM for polymyxin B, possibly resulting from its organization into micelles. The relevant finding from this experiment was that bis-LLP1 and polymyxin B showed equivalent MBCs against S. marcescens.
FIG. 1.
Susceptibility of S. marcescens to bis-LLP1 and polymyxin B. The bactericidal activities of bis-LLP1 and polymyxin B for S. marcescens were measured with a standard bacterial killing assay (9). In this assay, 106 CFU of bacteria per ml in phosphate buffer were exposed to increasing concentrations of peptides for 1 h. Viable bacteria were diluted and spread onto plates, followed by overnight incubation at 37°C. The averaged data of three separate trials were expressed as log CFU of viable bacteria per milliliter and plotted as a function of peptide concentration. The points of intersection of the MBCs and MBC50s are indicated by the dotted lines intersecting the curves. Based on this analysis, identical values for both the bis-LLP1 and polymyxin B were obtained. (1 μM polymyxin B = 1.2 μg/ml; 1 μM LLP1 = 3.3 μg/ml)
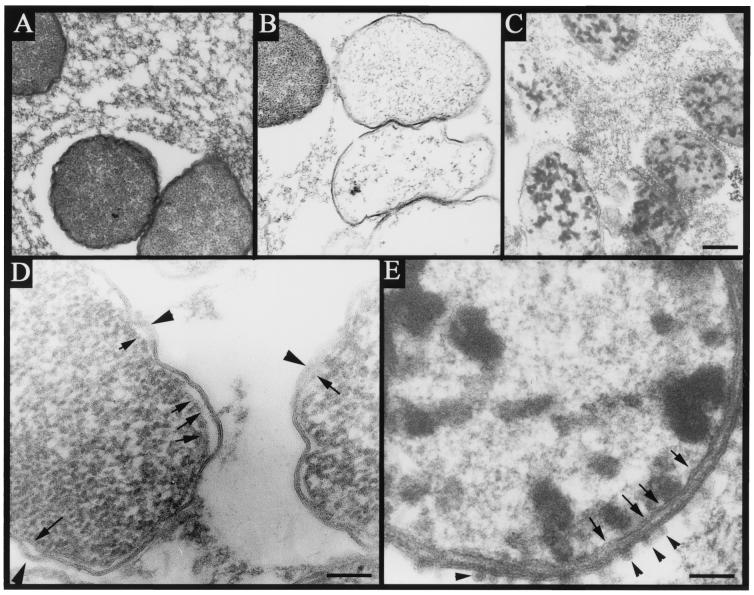
Both bis-LLP1 and polymyxin B have been proposed to act via bacterial membrane perturbation. Because of the well-described morphological effects of polymyxin B on the outer membrane of S. marcescens, (11, 12) thin-section transmission EM was employed to compare ultrastructural changes imposed by bis-LLP1 and polymyxin B. Equimolar peptide concentrations corresponding to the MBC at which 50% of strains tested are killed (MBC50) for the index strain were used (Fig. 1). These concentrations were used because at higher concentrations bacterial structures could not be pelleted. Treatment of S. marcescens with bis-LLP1 at its MBC50 resulted in significant changes in bacterial membrane structure (Fig. 2B and D). The outer and cytoplasmic membranes showed membrane fusion alternating with regions of low density. Visual inspection indicated clearing of electron-dense cytoplasmic material, suggesting dissipation of cellular contents following bacterial membrane disruption. In untreated control cells (Fig. 2A), membrane architecture remained intact, and the cytoplasm maintained a normal distribution of electron density.
FIG. 2.
Bacterial ultrastructure following treatment with bis-LLP1 or polymyxin. Cultures of S. marcescens were treated with no peptide (A), 0.5 μM (3.3 μg/ml) bis-LLP1 (B), or 0.5 μM (0.6 μg/ml) polymyxin B (C) for 30 min at 37°C and then fixed in 2.5% glutaraldehyde. Treatment with either agent caused marked destruction of bacteria compared to controls. Inspection of membranes from bis-LLP1-treated bacteria at higher magnification (D) indicated areas in which both cytoplasmic (arrows) and outer (arrowheads) membranes were disrupted by thinning and dissolution. Polymyxin B-treated bacteria (E) showed nearly intact cytoplasmic membranes (arrows) and outer membrane evaginations (arrowheads) characteristic of this treatment. Size bars: panel C (representing panels A to C), 200 nm; panel D, 100 nm; panel E, 50 nm.
Polymyxin B-treated organisms demonstrated evaginations of the outer membrane (Fig. 2C and E), a membrane effect reported for S. marcescens (11), Escherichia coli, Salmonella enterica serovar Typhimurium, and Pseudomonas aeruginosa (5, 6, 13). Coalescence of cytoplasmic contents by polymyxin B was observed in treated bacteria, an effect previously described for polymyxin B-treated P. aeruginosa (2).
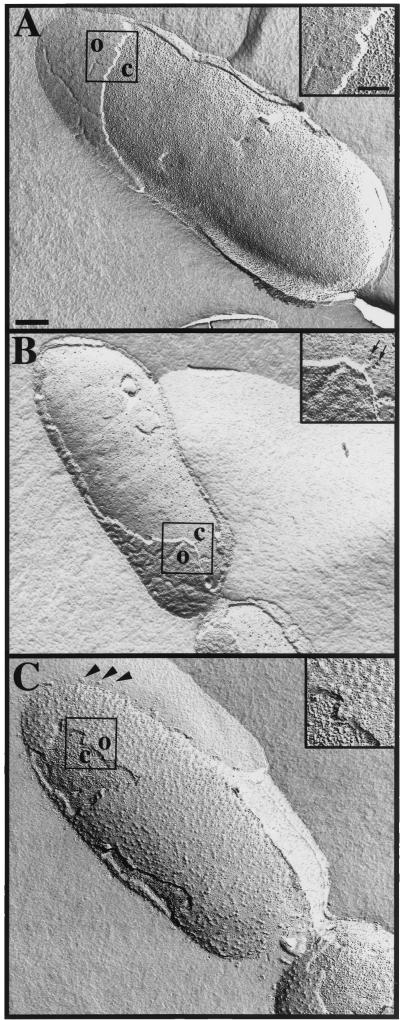
Freeze fracture analysis, a technique that places more emphasis on membrane integrity than thin-section EM, was used to assess the effects of bis-LLP1 and polymyxin B on membrane perturbation. Bis-LLP1-treated S. marcescens demonstrated outer membrane convolution with deep grooves between points of membrane attachment (Fig. 3B ). In comparison, untreated bacterial cells (Fig. 3A) revealed relatively smooth surfaces. Freeze fracture processing consistently produced fractures of both membranes in the same regions in treated bacteria. Untreated bacteria showed outer membrane fracture with preservation of the cytoplasmic membrane. Freeze fracture analysis of S. marcescens treated with polymyxin B (Fig. 3C) revealed different surface membrane alterations, with many round protrusions extending from discrete portions of the outer membrane instead of ridge-like patterns. Organelle-free membrane vesicles were also observed around the bacterial cell perimeter.
FIG.3.
Membrane ultrastructure examined by freeze fracture analysis. Cultures of S. marcescens were treated with no peptide (A), 0.5 μM (3.3 μg/ml) bis-LLP1 (B), or 0.5 μM (0.6 μg/ml) polymyxin B (C) for 30 min at 37°C and then processed. The untreated control showed a relatively smooth outer membrane (O) and an evenly stippled cytoplasmic (C) membrane. Bis-LLP1-treated bacteria demonstrated smoother cytoplasmic membrane surfaces unevenly marked by deep pitting (insert, arrows). Outer membrane roughness was evident when compared to the texture of control bacterial membrane. Polymyxin B-treated bacteria revealed outer membrane evaginations (arrowheads) and cytoplasmic membranes with morphological features similar to those of control bacteria. Size bars: panel A (representing panels A to C), 200 nm; top insert (representing all inserts), 100 nm.
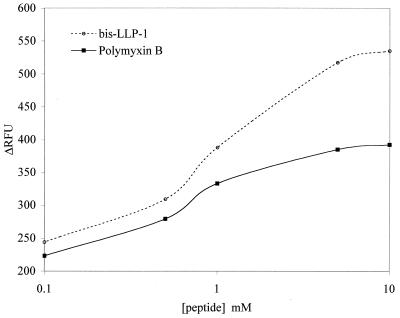
To support the observations of the ultrastructural analysis and to further define membrane activity, a well-described assay for outer membrane permeability was performed (1). N-Phenyl-naphthylamine (NPN) is an uncharged hydrophobic probe that fluoresces weakly in an aqueous environment. Upon exposure to a cell membrane via permeabilization by a membrane-active peptide, NPN partitions into the hydrophobic membrane, increasing its fluorescence. Concentration-dependent increases in fluorescence are shown (Fig. 4) for both polymyxin B and bis-LLP1. These effects were more pronounced on a molar basis at concentrations above the MBC and were consistent with the ultrastructural findings showing that peptide treatment influenced membrane permeability.
FIG. 4.
Comparison of bis-LLP1 with polymyxin B permeabilization of the S. marcescens outer membrane according to NPN uptake. Mid-log-phase S. marcescens cells were collected and incubated with bis-LLP1 or polymyxin B at different concentrations in the presence of NPN. NPN uptake was measured by the increase in relative fluorescence units (ΔRFU). Consistent with the EM data, both peptides appeared to act on the outer membrane. On a per mole basis, bis-LLP1 induced a greater level of outer membrane permeabilization than polymyxin B.
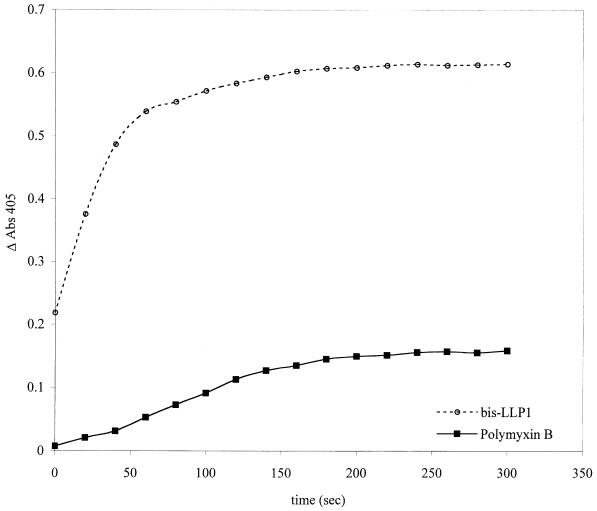
Permeabilization of the S. marcescens cytoplasmic membrane by bis-LLP1 or polymyxin B was determined by an established technique that follows cytosolic β-galactosidase activity resulting either from bacterial enzyme liberation or from the uptake of o-nitrophenyl-β-d-galactopyranoside (ONPG), a membrane-impermeable chromogenic substrate, into the cytosol (5). ONPG hydrolysis was measured as a function of time following addition of peptide (Fig. 5). Bis-LLP1 efficiently permeabilized the cytoplasmic membrane of S. marcescens in a dose-dependent manner at concentrations above 1 μM. In contrast, polymyxin B was ineffective at permeabilizing the cytoplasmic membrane until peptide concentrations were greater than 5 μM.
FIG. 5.
Comparison of bis-LLP1 with polymyxin B permeabilization of the S. marcescens cytoplasmic membrane by the β-galactosidase liberation assay. Mid-log-phase S. marcescens cells (108 bacteria/ml) were collected and incubated with bis-LLP1 or polymyxin B at 10 μM in the presence of ONPG. The rate of ONPG hydrolysis was monitored by a change in A405 and plotted as a function of time. As suggested by the EM data, cytoplasmic membrane compromise was most efficiently accomplished by bis-LLP1.
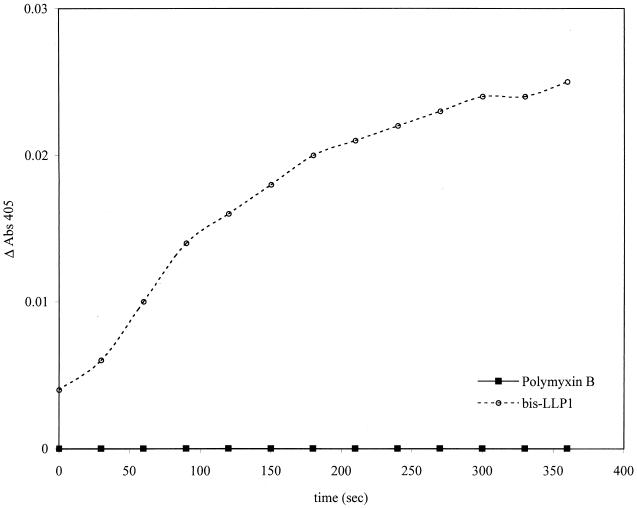
The standard cytoplasmic membrane perturbation assay was modified by diluting bacteria to a suspension of 106 CFU/ml, giving a peptide/bacterium ratio identical to that at the bacterial MBC. Bis-LLP1 and polymyxin B (MBC, 1 μM) were used. Figure 6 demonstrates that under these conditions, bis-LLP1 revealed cytosolic β-galactosidase activity, whereas treatment with polymyxin B did not. This result supported the EM findings that polymyxin B acted only on the outer membrane of these bacteria, while bis-LLP1 permeabilized both the outer and the cytoplasmic membranes.
FIG. 6.
Comparison of bis-LLP1 with polymyxin B permeabilization of the S. marcescens cytoplasmic membrane at the MBC. Mid-log-phase S. marcescens cells (106 bacteria/ml) were collected and incubated with bis-LLP1 or polymyxin B at their MBCs in the presence of ONPG. The rate of ONPG hydrolysis was monitored by a change in A405. Consistent with the EM results was the finding that no hydrolysis was observed with polymyxin B-treated bacteria, while there was discernible hydrolysis with bis-LLP1-treated bacteria.
Although both bis-LLP1 and polymyxin B are cationic antimicrobial peptides demonstrating similar antimicrobial potencies, they function via different mechanisms of action. By EM, bis-LLP1 acted at the outer and cytoplasmic membranes by causing the formation of ridges and valleys on the outer membrane, leading to fusion and dissolution of the outer and cytoplasmic membranes. Its activity appears to be similar to that of gramicidin, an antimicrobial peptide known to interact with both the outer and cytoplasmic bacterial membranes (7). In contrast, polymyxin B produced characteristic changes in the outer membrane by thin-section EM and freeze fracture analysis. This effect has been reported in other studies, suggesting severe alteration of outer membrane curvature via intercalation into its outer leaflet. A less rigorous survey (i.e., treatment of bacteria well below the MBC) of the effect of other CAPs by EM analysis observed that other antimicrobial peptides such as lactoferricin and tritryptocin were similar to polymyxin B, while magainin-2 more resembled bis-LLP1. Bacterial membrane disruption at an achievable MBC is relevant to the design of antimicrobial agents. The findings from this study suggest that bis-LLP1 acts in synergy with other conventional antimicrobial agents through physical disruption of the outer and cytoplasmic membranes.
Acknowledgments
We thank Donald Creighton, Michael Paliotti, and Berthony Deslouches for their roles in this study.
This project was supported in part by seed money from the University of Pittsburgh Cystic Fibrosis Program Project grant FRIZZE97R0 (Ray Frizzell), support from NIH grant no. NIH AR-99-005 no. 1P30 AR47372-01 (T.A.M.), the Alberta Heritage Foundation (H.J.V.), and the Cystic Fibrosis Foundation Clinical Fellowship (S.M.P.), and developmental funds from the Children's Hospital of Pittsburgh (S.M.P.).
REFERENCES
- 1.Fidai, S., S. W. Farmer, and R. E. Hancock. 1997. Interaction of cationic peptides with bacterial membranes. Methods Mol. Biol. 78:187-204. [DOI] [PubMed] [Google Scholar]
- 2.Gilleland, H. E., and L. B. Farley. 1982. Adaptive resistance to polymyxin in Pseudomonas aeruginosa due to an outer membrane impermeability mechanism. Can. J. Microbiol. 28:830-840. [DOI] [PubMed] [Google Scholar]
- 3.Hancock, R. E. 2000. Cationic antimicrobial peptides: towards clinical applications. Expert Opin. Investig. Drugs 9:1723-1729. [DOI] [PubMed] [Google Scholar]
- 4.Huttner, K. M., and C. L. Bevins. 1999. Antimicrobial peptides as mediators of epithelial host defense. Pediatr. Res. 45:785-794. [DOI] [PubMed] [Google Scholar]
- 5.Lehrer, R. I., A. Barton, K. A. Daher, S. S. Harwig, T. Ganz, and M. E. Selsted. 1989. Interaction of human defensins with Escherichia coli. Mechanism of bactericidal activity. J. Clin. Investig. 84:553-561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ohta, M., H. Ito, K. Masuda, S. Tanaka, Y. Arakawa, R. Wacharotayankun, and N. Kato. 1992. Mechanisms of antibacterial action of tachyplesins and polyphemusins, a group of antimicrobial peptides isolated from horseshoe crab hemocytes. Antimicrob. Agents Chemother. 36:1460-1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prenner, E. J., R. N. Lewis, and R. N. McElhaney. 1999. The interaction of the antimicrobial peptide gramicidin S with lipid bilayer model and biological membranes. Biochim. Biophys. Acta 1462:201-221. [DOI] [PubMed] [Google Scholar]
- 8.Tencza, S. B., D. J. Creighton, T. Yuan, H. J. Vogel, R. C. Montelaro, and T. A. Mietzner. 1999. Lentivirus-derived antimicrobial peptides: increased potency by sequence engineering and dimerization. J. Antimicrob. Chemother. 44:33-41. [DOI] [PubMed] [Google Scholar]
- 9.Tencza, S. B., J. P. Douglass, D. J. Creighton, R. C. Montelaro, and T. A. Mietzner. 1997. Novel antimicrobial peptides derived from human immunodeficiency virus type 1 and other lentivirus transmembrane proteins. Antimicrob. Agents Chemother. 41:2394-2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tencza, S. B., T. A. Mietzner, and R. C. Montelaro. 1997. Calmodulin-binding function of LLP segments from the HIV type 1 transmembrane protein is conserved among natural sequence variants. AIDS Res. Hum. Retrovir. 13:263-269. [DOI] [PubMed] [Google Scholar]
- 11.Weber, D. A., M. J. Nadakavukaren, and J. C. Tsang. 1976. Effect of polymyxin B on outer membranes of Serratia marcescens: morphological alterations of the outer membranes and their lipopolysaccharide components. Microbios 17:149-161. [PubMed] [Google Scholar]
- 12.Weber, D. A., M. J. Nadakavukaren, and J. C. Tsang. 1979. Electron microscopic observations of polysaccharide components in polymyxin B treated outer membranes from Serratia marcescens. J. Antibiot. 32:66-72. [DOI] [PubMed] [Google Scholar]
- 13.Zhang, L., P. Dhillon, H. Yan, S. Farmer, and R. E. Hancock. 2000. Interactions of bacterial cationic peptide antibiotics with outer and cytoplasmic membranes of Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 44:3317-3321. [DOI] [PMC free article] [PubMed] [Google Scholar]