Abstract
Three isolates of zygomycetes belonging to three different genera (Rhizopus microsporus, Absidia corymbifera, and Apophysomyces elegans) were used to produce a disseminated infection in nonimmunocompromised mice. The therapeutic efficacy of amphotericin B, given intraperitoneally at doses ranging from 0.5 to 4.5 mg/kg of body weight/day, oral itraconazole at 100 mg/kg/day, and oral terbinafine at 150 mg/kg/day was evaluated in this model. The markers of antifungal efficacy were the median survival time, the mortality rate, and the percentage of infected organs. Organ culture was performed along with microscopic direct examinations of tissues to assess the presence of an active infection. An acute and lethal infection was obtained in untreated mice challenged with each of the three strains. The data obtained for direct examinations and qualitative cultures indicate that, due to the nonseptate nature of the hyphae, each technique gives different information and should be used together with the others. Against all three strains, amphotericin B yielded a 90 to 100% survival rate. Itraconazole was inactive against R. microsporus but significantly reduced mortality in mice infected with A. corymbifera or A. elegans. Terbinafine had no beneficial effects against R. microsporus and A. corymbifera despite documented absorption of the drug. Overall, only limited correlations were observed between MICs determined in vitro and in vivo efficacy of the drugs. The efficacy of itraconazole in these models of zygomycosis suggests that this drug, as well as the new azole compounds presently under development, warrants close evaluation.
Zygomycetes are filamentous fungi capable of causing fatal infection, mostly in immunocompromised patients. These infections develop in patients with diabetic ketoacidosis, neutropenia, or iron overload treated with deferoxamine and in patients receiving corticosteroids (22). The major forms of zygomycosis include rhinocerebral, pulmonary, cutaneous, gastrointestinal, and disseminated diseases. Rhizopus, Mucor, Rhizomucor, and Absidia are the most common organisms that cause zygomycosis in humans. Apophysomyces elegans, a newly described species (15), has also been reported as a causative agent of zygomycosis, especially in immunocompetent patients (22).
Intravenous amphotericin B remains the drug of choice for treatment of these life-threatening infections. Nevertheless, the use of amphotericin B is limited by its narrow therapeutic index and new drugs that may have a role in the management of severe fungal infections are needed. Despite the use of the new lipid formulations of amphotericin B that allow for higher doses to be administered, mortality remains high, particularly in disseminated zygomycosis (22). Triazoles and allylamines are synthetic antifungal drugs characterized by their action as sterol biosynthesis inhibitors. Although it is generally assumed that there is no indication for the use of azole drugs in treating zygomycosis (13, 28), in vitro and in vivo susceptibility studies are very scarce. Nevertheless, it has been shown that azole compounds alone (7) or in combination (30) had beneficial effects in animal models of Rhizopus infection. Moreover, the efficacy of itraconazole for the treatment of Absidia infection in mice has been recently evaluated (16). Although low MICs of terbinafine against some zygomycete strains have been reported (9, 24) the potential of this drug for treatment of zygomycosis is largely unknown.
Animal models are useful to test the relevance of in vitro antifungal susceptibilities, particularly for opportunistic mold infections because of their low incidence in patients. The most commonly used criteria for evaluating the efficacy of antifungal therapy in animal models of acute infections are the mortality rate, the median survival time, and the results of qualitative or quantitative culture of target organs. One of the problems in the determination of the organ fungal load is that zygomycetes are difficult to culture from infected tissue in experimental infections (1) as well as in infected patients (29).
The aim of this study was to compare, in a murine model of disseminated zygomycosis, the in vivo efficacies of amphotericin B, itraconazole, and terbinafine against three strains of zygomycetes that had different susceptibility profiles.
MATERIALS AND METHODS
Organisms.
Three strains, chosen based on their in vitro susceptibility profiles, were used in this study: Rhizopus microsporus var. rhizopodiformis (AZN 1185) isolated from an invasive infection, Absidia corymbifera (AZN 4095 [CBS 271.65]) from unknown origin, and Apophysomyces elegans (AZN 1829 [CBS 658.93 and ATCC 90757]) isolated from a patient with osteomyelitis (14). Isolates were stored as conidial suspensions at −80°C in 10% glycerol until used.
In vitro susceptibility testing.
Susceptibility testing was performed using a National Committee for Clinical Laboratory Standards (NCCLS)-based broth microdilution technique (17). RPMI 1640 (GIBCO BRL, Life Technologies, Woerden, The Netherlands) with l-glutamine but without sodium bicarbonate and buffered at pH 7.0 with 0.165 M morpholinepropanesulfonic acid (MOPS) (Sigma-Aldrich Chemie GmbH, Steinheim, Germany) was used as test medium. Amphotericin B (Bristol-Myers Squibb, Woerden, The Netherlands), itraconazole (Janssen Pharmaceutica, Beerse, Belgium), and terbinafine (Novartis Pharma, Basel, Switzerland) were provided by the manufacturers as powders. Drugs were dissolved in dimethyl sulfoxide to a concentration of 1,600 μg/ml and stored frozen in aliquots at −80°C as stock solutions. The drug dilutions were prepared by following the standard additive twofold drug dilution scheme described in the NCCLS reference method (17). The final drug concentrations were 0.015 to 16 μg/ml for amphotericin B and itraconazole and 0.03 to 32 μg/ml for terbinafine. The isolates were grown on Sabouraud dextrose agar slants at 35°C for 7 days. For A. elegans, sporulation was obtained by culture of the mycelium in sterile distilled water supplemented with 0.1% yeast extract (21) for 10 days at 37°C. The inoculum was prepared by washing the surface of the agar slants with 1 ml of sterile 0.9% saline containing 0.05% of Tween 80. The resulting conidial suspensions were counted with a hemocytometer. Sterile 96-U-shaped-well microplates were used. The conidia were diluted in RPMI medium and each well in rows 1 to 12 was inoculated. The final inoculum concentration was 104 conidia per ml. Row 12 was used as a growth control. Inoculum sizes were checked by quantitative colony counts on Sabouraud dextrose agar. Microplates were incubated at 35°C for 48 h, and the growth in each well was compared with that of the growth control with the aid of a microtiter reading mirror. Each well was then given a numerical score according to the NCCLS guidelines: 4, no reduction in growth; 3, growth reduction of 25%; 2, growth reduction of 50%; 1, growth reduction of 75% or more; and 0, absence of growth (optically clear). MIC endpoints were defined as the lowest drug concentration which had a score of 2 (MIC-2) for itraconazole and terbinafine and the lowest concentration which had a score of 0 (MIC-0) for amphotericin B. The minimum fungicidal concentrations (MFCs) were determined by culture of 100 μl from each well with no visible growth on Sabouraud dextrose agar plates. The plates were incubated at 35°C for 24 h. The MFC was defined as the lowest concentration at which 99.9% of the inocula were killed. The MICs were determined in duplicate, in two different assay runs, with similar results.
In vivo experiments. (i) Mice.
Female CD-1 outbred mice (Harlan, Horst, The Netherlands), 5 to 7 weeks old and weighing 20 to 22 g, were used throughout the experiments. Mice were housed in groups of ten and were given food and water ad libitum. The mice were maintained in a room at 22°C with a 12-h light-dark cycle. Animal studies were conducted in accordance with recommendations of the European Community (Directive 86/609/EEC, 24 November 1986), and all animal research procedures were approved by the institutional animal care and use committee of Nijmegen University.
(ii) Infection.
For R. microsporus and A. corymbifera, the inoculum was prepared by culture of the strains on Sabouraud dextrose agar supplemented with 0.02% chloramphenicol for 7 days at 35°C. Spores were harvested by washing the agar surface with sterile saline containing 0.05% Tween 80. For A. elegans, sporulation was obtained as described above. Spore suspensions were filtered through a nylon filter (pore size, 11 μm), counted in a hemocytometer, and stored at 4°C for no more than 24 h. Viability was determined by plating dilutions prepared in saline with 0.05% Tween 80. Plates were incubated at 35°C, and CFU were counted at 24 h. Viability levels were 90% for R. microsporus and 80% for A. corymbifera and A. elegans. On the day of infection, the spore suspension was adjusted to the required concentration in saline.
Preliminary studies were performed to determine the 90% lethal dose (LD90) for each isolate by testing three to four inoculum sizes. The LD90 levels were 7 × 106, 7.5 × 105, and 3 × 103 CFU/mouse for R. microsporus, A. corymbifera, and A. elegans, respectively. Mice were infected with the LD90 by injection of 0.1 ml of the conidial suspension into a lateral tail vein. After infection, cages were randomized in the different treatment groups.
Drugs and therapy.
Amphotericin B desoxycholate (Fungizone; Bristol-Myers Squibb) was given intraperitoneally in 5% glucose. Itraconazole (Trisporal oral solution; Janssen Cilag) and terbinafine hydrochloride (Lamisil; Novartis Pharma) were diluted in sterile water and given by gavage.
Treatment was begun 2 h after infection and was continued for 10 days. For R. microsporus and A. corymbifera, five groups of ten mice were used. One group was treated with 4.5 mg/kg of body weight/day of amphotericin B, one group was treated with 100 mg/kg/day of itraconazole, and one group was treated with 150 mg/kg/day of terbinafine. Amphotericin B was given by once-daily injection, and itraconazole and terbinafine were administered in two daily doses. Terbinafine was not tested in mice infected with A. elegans. Control mice were infected but received only 5% glucose, one group by intraperitoneal injection and one group by gavage. Animals were checked twice daily for mortality and for clinical signs. The mice were observed for 5 days after the end of treatment. Each experiment was performed once (i.e., three experiments were conducted). In a subsequent set of three experiments, each conducted with four groups of ten mice, lower doses of amphotericin B of 2, 1, and 0.5 mg/kg/day were tested.
Assessment of organ infection.
Microscopic examination and qualitative culture of organs were performed on mice which died before the end of the experiment and on day 14 postinfection on surviving mice, which were sacrificed by cervical dislocation. In the first set of experiments, five organs were analyzed (brain, kidneys, lungs, spleen, and liver), whereas in the second set of experiments, only brain and kidneys were evaluated. Organs were aseptically removed and crushed in a tube containing 1 ml of saline with 5 glass beads (4-mm diameter). This procedure was used to minimize the homogenization and resulted in a coarse suspension containing small pieces of intact tissues. Half of the suspension (0.5 ml) was plated onto Sabouraud's agar, and plates were incubated at 35°C for 24 to 48 h. Microscopic examination of the suspension was performed with a fluorescence microscope after staining with Blankophor P (Bayer, Leverkusen, Germany) (0.25 mg/ml in 20% KOH). The use of stilbene derivatives is recommended for direct examination to demonstrate the presence of zygomycetes in clinical samples (22). Moreover, it has been shown that Blankophor staining is useful to detect hyphae in patients with zygomycosis (23).
Antifungal assay.
Two groups of five uninfected mice were given itraconazole at 100 mg/kg/day or terbinafine at 150 mg/kg/day by gavage. Mice were sacrificed after 6 days of treatment at 6 hours after the last dose. Mice were bled by cardiac puncture, and serum was obtained by centrifuging the blood for 10 min at 3,000 × g. Organs were removed, weighted, and homogenized in 2 ml of saline, and the material was stored at −20°C until it was assayed. The levels of itraconazole and terbinafine in serum and tissues were measured by bioassay (12). Drug standards were diluted with either pooled mouse serum or pooled mouse kidney or brain homogenate, and the resulting mixtures were used to construct standard curves. Standard curves had a regression coefficient of >0.98. Results were expressed in μg/ml for serum and in μg/g of tissue for organs. All samples were run in duplicate. The minimum drug concentrations that could be detected were 0.12 and 0.25 μg/ml in serum, 1.25 and 2.5 μg/g in kidney, and 0.6 and 1.25 μg/g in brain for itraconazole and terbinafine, respectively. Pooled mouse serum samples and tissue homogenates from normal mice were included as negative controls.
Data analysis.
In each experiment, data for the two control groups were pooled for the analysis. To compare culture results with those of assays for the presence of hyphae and/or spores in tissues in untreated mice, data from all control mice from the two sets of experiments were used. Mortality data were compared by the Kruskal-Wallis test and by using Dunn's post-hoc test for multiple comparisons. Qualitative organ cultures and microscopic examination results were compared by using Fisher's exact test. Statistical analyses were performed using GraphPad Prism version 3.00 for Windows (GraphPad Software, San Diego, Calif.). Statistical significance was defined as P ≤ 0.05.
RESULTS
In vitro susceptibility testing.
The results of susceptibility testing for the three zygomycete isolates used in the study are shown in Table 1. Each of these strains had a different antifungal susceptibility profile. For R. microsporus, amphotericin B and terbinafine were active with MICs ≤ 0.5 μg/ml but itraconazole showed no activity. The three antifungal drugs exhibited low MICs for A. corymbifera, and for A. elegans, amphotericin B exhibited a high MIC of ≥2 μg/ml and itraconazole and terbinafine exhibited MICs of ≤1 μg/ml. Differences of no more than two log 2 dilution steps were noted between the 24- and 48-h incubation time points.
TABLE 1.
In vitro susceptibility to amphotericin B, itraconazole, and terbinafine of three zygomycete isolates used for in vivo studies
| Isolate and incubation time (h) | MIC (μg/ml)a
|
||
|---|---|---|---|
| Amphotericin B | Itraconazole | Terbinafine | |
| R. microsporus AZN 1185 | |||
| 24 | 0.25 | >16 | 0.25 |
| 48 | 0.5 | >16 | 0.5 |
| A. corymbifera AZN 4095 | |||
| 24 | 0.06 | 0.03 | 0.12 |
| 48 | 0.12 | 0.12 | 0.25 |
| A. elegans AZN 1829 | |||
| 24 | 2 | 0.5 | 0.5 |
| 48 | 4 | 1 | 1 |
MICs were determined after 24 and 48 h of incubation.
Amphotericin B, itraconazole, and terbinafine were unable to kill the A. elegans and R. microsporus strains with MFCs of >16 μg of amphotericin B/ml and of terbinafine/ml and >32 μg of itraconazole/ml. The MFCs of amphotericin B, itraconazole, and terbinafine for A. corymbifera were 4, 8, and 4 μg/ml, respectively.
In vivo experiments.
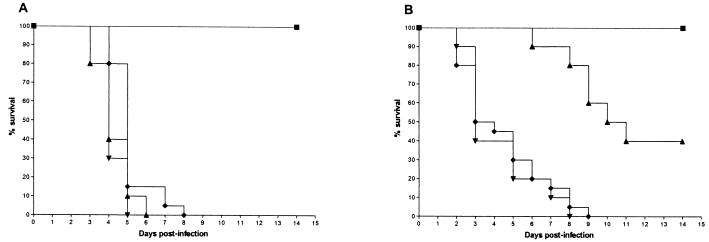
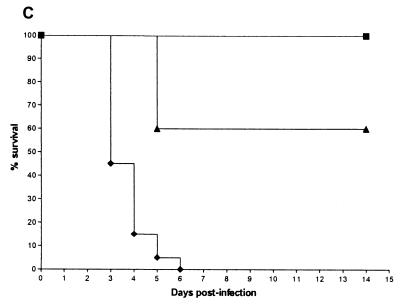
Survival curves (Fig. 1) demonstrate that all three isolates caused acute and lethal infections in control mice. The median survival times of mice infected with each of the three isolates are shown in Table 2.
FIG. 1.
Cumulative mortality for mice infected with strain R. microsporus AZN 1185 (A), A. corymbifera AZN 4095 (B), and A. elegans AZN 1829 (C) in treated and control groups. Each experiment was done once with 10 mice in each treated group and 20 mice in the control group. Mice were treated for 10 days starting 2 h after inoculation. ▴, itraconazole at 100 mg/kg of body weight/day given by gavage; ▪, amphotericin B at 4.5 mg/kg/day given intraperitoneally; ▾, terbinafine at 150 mg/kg/day given by gavage; ⧫, 5% glucose control.
TABLE 2.
Survival times for mice infected with three different zygomycete isolates and treated with amphotericin B at 4.5 mg/kg of body weight/day, itraconazole at 100 mg/kg/day, or terbinafine at 150 mg/kg/daya
| Group | Median survival time (days) and range for isolateb
|
||
|---|---|---|---|
| R. microsporus AZN 1185 | A. corymbifera AZN 4095 | A. elegans AZN 1829 | |
| Control | 5 (4-8) | 3.5 (2-9) | 3 (3-6) |
| Amphotericin B | 15 (15)* | 15 (15)** | 15 (15)** |
| Itraconazole | 4 (3-6) | 10.5 (6-15)* | 15 (5-15)** |
| Terbinafine | 4 (4-5) | 3 (2-8) | NDc |
Treatment was started 2 h after inoculation and given for 10 days.
*, P < 0.01 (compared with controls); **, P < 0.001 (compared with controls).
ND, not done.
With R. microsporus (Fig. 1A), all control mice died within 8 days. Treatment with amphotericin B at 4.5 mg/kg of body weight/day resulted in 100% survival, and the median survival time was significantly improved compared to that of untreated mice (P < 0.01). All mice treated with itraconazole or terbinafine died by day 6, which was not significantly different from the results for the control mice. Figure 1B shows the survival results for mice infected with A. corymbifera. All control mice died within 9 days. No mice treated with amphotericin B died, which was a significantly better result than that seen for the controls (P < 0.001). Forty percent of the mice treated with itraconazole survived, which was better than the result seen for the controls (P < 0.01). Terbinafine did not significantly prolong survival compared to that of the controls. The survival curves for mice infected with A. elegans are shown in Fig. 1C. All mice given glucose either by gavage or intraperitoneally died within 6 days. No mice treated with amphotericin B died, which was significantly better than the results seen for the mice with no therapy (P < 0.001). Itraconazole significantly prolonged survival compared to that of the controls (P < 0.01), with 60% survival by the end of the experiment. Terbinafine was not tested in mice infected with A. elegans.
Table 3 summarizes the results of qualitative culture and microscopic examination of five organs in untreated mice. An active infection, as defined by the presence of hyphae in tissues, was present in the kidneys and brain in all of the mice infected with R. microsporus. For mice infected with A. corymbifera, hyphae were noted in the kidneys and brain in almost all of the animals but spleen and liver were infected in only 5% of the cases and lungs were not infected. Mice infected with A. elegans had a disseminated infection involving kidneys, liver, spleen, and to a minor extent, brain and lungs. Interestingly, culture results did not correlate with the presence of hyphae in organs. Particularly, in mice infected with A. corymbifera and A. elegans only a small number of infected brain and kidneys were culture positive. On the other hand, positive cultures were obtained for organs in which hyphae were not seen by direct examination. It is notable that for some of these organs (e.g., spleen and liver of mice infected with A. corymbifera), positive cultures correlated with the presence of ungerminated spores. Therefore, detection of the presence of hyphae by direct examination was used to determine the levels of organ clearance in treatment groups. The percentages of positive organs, as defined by the presence of hyphae assessed microscopically in tissues, in control and treated animals infected with each of the three strains are shown in Table 4. Amphotericin B significantly reduced the number of infected organs compared to those of the controls for all three strains (P < 0.05 to P < 0.0001). In contrast, in mice infected with R. microsporus or A. corymbifera, terbinafine and itraconazole did not significantly reduce the number of positive organs in comparison with those of the control groups. In mice infected with A. elegans, itraconazole significantly reduced the number of infected organs (P < 0.0001) except for the brain.
TABLE 3.
Microscopic examination of and qualitative culture results for five organs for control mice
| Strain and organ | No. of micea | Percentage of positive results for organ by:
|
||
|---|---|---|---|---|
| Microscopic examination for:
|
Culture | |||
| Hyphae | Spores | |||
| R. microsporus AZN 1185 | ||||
| Kidney | 30 | 100 | 13 | 100 |
| Spleen | 20 | 90 | 95 | 100 |
| Lung | 20 | 35 | 60 | 100 |
| Liver | 20 | 40 | 100 | 100 |
| Brain | 30 | 100 | 30 | 100 |
| A. corymbifera AZN 4095 | ||||
| Kidney | 30 | 100 | 10 | 90 |
| Spleen | 20 | 5 | 90 | 100 |
| Lung | 20 | 0 | 5 | 85 |
| Liver | 20 | 5 | 95 | 95 |
| Brain | 30 | 97 | 0 | 23 |
| A. elegans AZN 1829 | ||||
| Kidney | 30 | 100 | 0 | 30 |
| Spleen | 20 | 80 | 0 | 85 |
| Lung | 20 | 35 | 0 | 10 |
| Liver | 20 | 95 | 0 | 95 |
| Brain | 30 | 53 | 0 | 0 |
Control groups from two experiments were pooled for analysis.
TABLE 4.
Microscopic examination results (presence of hyphae) for control and treated mice infected with R. microsporus A. corymbifera, and A. elegansa
| Strain and group (no. of mice in group) | No. of survivors | Percentage of positive results forb,d:
|
||||
|---|---|---|---|---|---|---|
| Kidney | Spleen | Lung | Liver | Brain | ||
| R. microsporus AZN 1185 | ||||||
| Control (20) | 0 | 100 | 90 | 35 | 40 | 100 |
| Amphotericin B (10) | 10 | 0*** | 0*** | 0 | 0* | 0*** |
| Itraconazole (10) | 0 | 100 | 80 | 0 | 20 | 100 |
| Terbinafine (10) | 0 | 100 | 90 | 30 | 0* | 100 |
| A. corymbifera AZN 4095 | ||||||
| Control (20) | 0 | 100 | 5 | 0 | 5 | 95 |
| Amphotericin B (10) | 10 | 0*** | 0 | 0 | 0 | 10*** |
| Itraconazole (10) | 4 | 80 | 0 | 10 | 0 | 78c |
| Terbinafine (10) | 0 | 100 | 0 | 0 | 10 | 80 |
| A. elegans AZN 1829 | ||||||
| Control (20) | 0 | 100 | 80 | 35 | 95 | 55 |
| Amphotericin B (10) | 10 | 0*** | 0*** | 0 | 0*** | 0** |
| Itraconazole (10) | 6 | 0*** | 0*** | 0 | 0*** | 67c |
Amphotericin B was given at 4.5 mg/kg of body weight/day, itraconazole was given at 100 mg/kg/day, and terbinafine was given at 150 mg/kg/day. Treatment was started 2 h after inoculation and given for 10 days.
Presence of hyphae.
Brain from one mouse was not tested.
*, P < 0.05 (compared with controls); **, P < 0.005 (compared with controls); ***, P < 0.0001 (compared with controls).
In an additional set of experiments, the activity of amphotericin B at 2, 1, and 0.5 mg/kg/day was evaluated. The survival rates, median survival times, and results of microscopic examination of brain and kidneys in control and amphotericin B-treated mice for the three isolates are presented in Table 5. A uniform response was obtained in the treated groups for all three strains, with survival rates of 90 to 100% and significantly prolonged median survival times compared with those of the controls (P < 0.01 to P < 0.001). All three doses of amphotericin B dramatically reduced or eliminated infection in kidneys (P < 0.001 to P < 0.0001). However, spores were observed in kidneys of all animals infected with R. microsporus and cultures were positive, indicating that the treatment failed to eradicate the fungus even in the surviving mice. Brain infection was significantly reduced in all treatment groups for mice infected with R. microsporus and A. corymbifera (P < 0.005 to P < 0.0001), but a dose-response effect was not observed. For mice infected with R. microsporus, all brains contained spores and were culture positive.
TABLE 5.
Survival rates and times for mice infected with three different zygomycete isolates and treated with different doses of amphotericin Ba
| Strain and groupb | Survival (%) | Median survival time (days) and ranged | Percentage of positive results for:
|
|||
|---|---|---|---|---|---|---|
| Kidney for:
|
Brain for:
|
|||||
| Hyphaec,d | Culture | Hyphaec,d | Culture | |||
| R. microsporus AZN 1185 | ||||||
| Control | 0 | 4 (4-6) | 100 | 100 | 100 | 100 |
| AMB 2 | 100 | 15 (15)§ | 0¶ | 100 | 20§ | 100 |
| AMB 1 | 90 | 15 (6-15)** | 0¶ | 100 | 20§ | 100 |
| AMB 0.5 | 100 | 15 (15)§ | 20§ | 100 | 30*** | 100 |
| A. corymbifera AZN 4095 | ||||||
| Control | 0 | 3 (2-6) | 100 | 100 | 100 | 30 |
| AMB 2 | 100 | 15 (15)§ | 0¶ | 0 | 10¶ | 0 |
| AMB 1 | 90 | 15 (4-15)** | 0¶ | 30 | 20§ | 0 |
| AMB 0.5 | 90 | 15 (4-15)** | 0¶ | 20 | 10¶ | 0 |
| A. elegans AZN 1829 | ||||||
| Control | 0 | 3.5 (3-5) | 100 | 40 | 50 | 0 |
| AMB 2 | 100 | 15 (15)§ | 0¶ | 0 | 10 | 0 |
| AMB 1 | 100 | 15 (15)§ | 0¶ | 0 | 0* | 0 |
| AMB 0.5 | 90 | 15 (4-15)** | 10¶ | 0 | 10 | 0 |
Ten mice in each group. Treatment was started 2 h after inoculation and given for 10 days.
AMB 2, AMB 1, and AMB 0.5, amphotericin B given at 2, 1, and 0.5 mg/kg of body weight/day, respectively.
Presence of hyphae detemined by microscopic examination.
*, P < 0.05 (compared with controls); **, P < 0.01 (compared with controls); ***, P < 0.005 (compared with controls); §, P < 0.001 (compared with controls); ¶, P < 0.0001 (compared with controls).
The levels of itraconazole and terbinafine in serum following 6 days of administration were 22.0 ± 5.0 and < 0.25 μg/ml, respectively. The concentration of itraconazole was 14.0 ± 5.3 μg/g in kidneys and lower than the limit of quantitation of the test (< 0.6 μg/g) in brain. Although terbinafine was undetectable in serum, an adequate absorption of the drug was obtained (with wide interindividual variability), as demonstrated by the high concentrations of 25.4 ± 18.9 and 10.1 ± 11.9 μg/g observed in kidneys and brain, respectively.
DISCUSSION
Zygomycosis remains a difficult-to-treat infection and is associated with high mortality (22). Amphotericin B is the treatment of choice for these infections, and the other antifungal drugs, including the azoles, are considered ineffective against zygomycetes (13, 28). Nevertheless, in vitro susceptibility data for zygomycetes obtained with standardized techniques have been limited to a small number of isolates and there have been few studies that examine the in vivo efficacy of antifungals in experimentally infected animals (7, 8, 19, 30, 31, 33).
For this study, we tested the in vivo efficacy of three presently available antifungal drugs against three zygomycete strains in animal models of disseminated infection. Indices used to assess the outcome of infection were the mean survival time, the percentage of surviving animals by the end of the experiment, and the percentage of infected organs at autopsy (3-5). Nevertheless, it is generally assumed that zygomycetes are difficult to isolate from clinical specimens in infected patients (13, 22, 29). Moreover, in preliminary experiments conducted in our laboratory it was surprising that brains from mice with clinical evidence of neurological disorders were culture negative. It is probable that the coenocytic hyphae were damaged by the homogenization procedure applied to the sample and that the fungus became nonviable as a result. For this reason, we used a gentle homogenization procedure, and organ culture was performed along with a direct examination of the tissues to detect the presence of hyphae and/or ungerminated spores.
Infection by intravenous administration of A. corymbifera spores resulted in an active infection (hyphae) in brain and kidneys, but most of the infected brains yielded negative cultures. Many spores were retained by the spleen and the liver but did not germinate. This result is in accordance with previous reports of experimental Absidia infection in mice. Corbel et al. showed that intravenous inoculation of mice with A. corymbifera resulted in generalized distribution of spores, particularly in the liver and spleen, whereas the development of active lesions containing hyphae was confined to the brain and the kidneys (1, 2). Moreover, in the same experimental model it was shown that the fungus was not always cultured from the brain of animals in which hyphae could be demonstrated microscopically (1). In mice challenged with A. elegans, a disseminated infection was observed but 70% of the infected kidneys and all of the infected brains were culture negative. Spores were never observed in tissues, a result which could be related to the low LD90 of this strain (3.103 CFU/mouse, 250- and 2,300-fold lower than the LD90s for A. corymbifera and R. microsporus, respectively). To our knowledge, this is the first report of an animal model of zygomycosis caused by A. elegans. This fungus is an uncommon human pathogen that is being reported with increasing frequency as a cause of aggressive infection in nonimmunocompromised patients (22), and effective treatment of A. elegans infections is not standardized. In mice infected with R. microsporus, a disseminated infection was also observed but organ cultures were always positive. It is difficult to know whether positive results for cultures were due to the presence of a large number of spores retained in the organs or due to hyphae that were less sensitive to the homogenization procedure. Similar results were obtained in previous experiments in which mice infected intravenously by R. microsporus or Rhizopus oryzae presented a disseminated infection involving lungs, kidneys, liver, and spleen and for which all cultures were positive (32). These data obtained for direct examination and qualitative cultures illustrate the problems associated with the estimation of fungal burden due to coenocytic filamentous fungi. Even when a gentle homogenization procedure was used, infected organs were often culture negative. Therefore, it is useful to perform both direct examinations to demonstrate the presence of an active infection and culture to provide information about the presence of viable spores retained in tissues.
In these models, amphotericin B showed very good therapeutic efficacy. In the first set of experiments, a high dose of 4.5 mg/kg of body weight/day was used based on the high MIC for amphotericin B against A. elegans. A previous report, using a very similar model, indicated that a strain of Aspergillus terreus against which amphotericin B had a MIC of 2 μg/ml was resistant in vivo to 4.5 mg/kg/day of the drug (5). For the three strains tested, all mice treated with this high dose of amphotericin B survived and an active infection was almost never observed in tissues. Nevertheless, for R. microsporus, organ cultures showed that spores remained viable in the tissues and that even such a high dose of amphotericin B was not fungicidal in vivo. In the second set of experiments, lower doses of amphotericin B ranging from 0.5 to 2 mg/kg/day showed good efficacy but no concentration-effect relationships were observed. The lack of a concentration-effect relationship may be related to tissue accumulation of amphotericin B and the start of treatment 2 h after inoculation. Consistent with these results, previous studies using a similar model of disseminated zygomycosis in nonneutropenic mice have demonstrated that amphotericin B prolonged survival in infected animals but did not clear the fungus from any of the organs tested (19). Using nonimmunocompromised guinea pigs, similar results were obtained in animals infected with R. oryzae (19, 33). Only one study showed amphotericin B clearing the fungal infection from most of the organs in guinea pigs infected with R. microsporus (33). It has been shown that inhibition of spore germination and killing of spores depend on different mechanisms and that bronchoalveolar macrophages from normal mice are unable to kill R. oryzae spores in vivo (35).
Furthermore, we found that amphotericin B was fungistatic in vitro against R. microsporus and A. elegans and fungicidal against A. corymbifera but only at relatively high concentrations. This observation corresponds with that of the inability of amphotericin B to sterilize the tissues in our model. Nevertheless, the in vivo efficacy of the drug correlated with the low MICs detected in vitro against R. microsporus and A. corymbifera. However, it is to be noticed that despite a relatively high MIC of 2 μg/ml against A. elegans, amphotericin B was active in vivo even at the lowest dose tested. The limited value of the in vitro data to predict clinical outcome and the low incidence of zygomycosis in humans emphasize the usefulness of experimental animal models to test the therapeutic efficacy of present and new antifungal drugs. In the model used in the study reported here, treatment was started early after infection, which is not a common clinical situation. Nevertheless, similar models of mold infections have been previously used to evaluate the efficacy of antifungal drugs (19).
Although azole drugs are considered ineffective against zygomycetes (13, 28), some studies have shown that azole compounds alone or in combination may have an activity in animal models of zygomycosis (7, 30). In this study, itraconazole exhibited a high MIC against the R. microsporus isolate and was not effective in vivo. Treatment of experimental zygomycosis with itraconazole has been reported for few studies. In one study, itraconazole, saperconazole, and ketoconazole were inactive in treating nonimmunocompromised guinea pigs infected with either R. oryzae or R. microsporus (33). In another report, two strains of R. oryzae for which the mode MICs of itraconazole were 2 and >16 μg/ml were used to infect guinea pigs and mice and in both animal models, the drug was ineffective (19).
In contrast, the A. corymbifera strain used in this study was inhibited in vitro by low concentrations of itraconazole; this result is in accordance with previous reports (10, 20). A. elegans was less susceptible to itraconazole, whose MICs ranged from 0.5 to 1 μg/ml. Itraconazole was partially active in both strains, tending to be more active against A. elegans. This could be explained by the lower frequency of brain infection in mice challenged with A. elegans (55 and 95% for A. elegans and A. corymbifera, respectively). The low tissue concentration of itraconazole in brain and its consequent inability to clear infection could account for the better activity of the drug in mice infected with A. elegans. Recently, in a murine model of A. corymbifera infection, itraconazole, although less active than amphotericin B, was shown to increase the rate of survival of infected animals (16). As the new azoles have in vitro activity when used alone (E. Dannaoui, J. Meletiadis, J. F. G. Meis, J. W. Mouton, and P. E. Verweij, Abstr. 40th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 939, 2000) as well as in combination (E. Dannaoui, J. F. G. Meis, J. W. Mouton, and P. E. Verweij, Abstr. 40th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 934, 2000), further studies of the potential of itraconazole as well as of the new azoles in the treatment of zygomycosis are warranted.
Primarily designed for superficial mycoses, terbinafine is a sterol biosynthesis inhibitor which may be effective for the treatment of systemic fungal infections such as aspergillosis (26) or scedosporiosis (34). Moreover, a combination of oral terbinafine with amphotericin B has been successfully used to treat a case of invasive zygomycosis (18). In the study presented here, relatively low MICs were obtained for terbinafine against R. microsporus and A. corymbifera. Nevertheless, terbinafine was inactive in vivo despite high tissue levels of the drug in both kidneys and brain. Similar poor in vivo efficacy of terbinafine, despite good activity in vitro, has been reported in animal models of aspergillosis (27), sporotrichosis (11), and phaeohyphomycosis (6) and could be related to nonsaturated protein binding of the drug (25).
In conclusion, our results demonstrated very limited in vitro-in vivo correlations for the three tested microorganisms. These observations highlight the potential of animal models to test new approaches for the treatment of zygomycosis. Particularly, the in vivo efficacy of itraconazole warrants further studies with this drug and with the new azole compounds that are presently under development.
Acknowledgments
This work was supported by a European Community grant (TMR-Eurofung network, contract ERBFMRXCT97-0145).
REFERENCES
- 1.Corbel, M. J., and S. M. Eades. 1975. Factors determining the susceptibility of mice to experimental phycomycosis. J. Med. Microbiol. 8:551-564. [DOI] [PubMed] [Google Scholar]
- 2.Corbel, M. J., and S. M. Eades. 1978. Observations on the localization of Absidia corymbifera in vivo. Sabouraudia 16:125-132. [PubMed] [Google Scholar]
- 3.Dannaoui, E., E. Borel, M. F. Monier, M. A. Piens, S. Picot, and F. Persat. 2001. Acquired itraconazole resistance in Aspergillus fumigatus. J. Antimicrob. Chemother. 47:333-340. [DOI] [PubMed] [Google Scholar]
- 4.Dannaoui, E., E. Borel, F. Persat, M. F. Monier, and M. A. Piens. 1999. In-vivo itraconazole resistance of Aspergillus fumigatus in systemic murine aspergillosis. J. Med. Microbiol. 48:1087-1093. [DOI] [PubMed] [Google Scholar]
- 5.Dannaoui, E., E. Borel, F. Persat, M. A. Piens, and S. Picot. 2000. Amphotericin B resistance of Aspergillus terreus in a murine model of disseminated aspergillosis. J. Med. Microbiol. 49:601-606. [DOI] [PubMed] [Google Scholar]
- 6.Dixon, D. M., and A. Polak. 1987. In vitro and in vivo drug studies with three agents of central nervous system phaeohyphomycosis. Chemotherapy 33:129-140. [DOI] [PubMed] [Google Scholar]
- 7.Goldani, L. Z., and A. M. Sugar. 1994. Treatment of murine pulmonary mucormycosis with SCH 42427, a broad-spectrum triazole antifungal drug. J. Antimicrob. Chemother. 33:369-372. [DOI] [PubMed] [Google Scholar]
- 8.Honda, A., K. Kamei, H. Unno, K. Hiroshima, T. Kuriyama, and M. Miyaji. 1998. A murine model of zygomycosis by Cunninghamella bertholletiae. Mycopathologia 144:141-146. [DOI] [PubMed] [Google Scholar]
- 9.Jessup, C. J., N. S. Ryder, and M. A. Ghannoum. 2000. An evaluation of the in vitro activity of terbinafine. Med. Mycol. 38:155-159. [DOI] [PubMed] [Google Scholar]
- 10.Johnson, E. M., A. Szekely, and D. W. Warnock. 1998. In-vitro activity of voriconazole, itraconazole and amphotericin B against filamentous fungi. J. Antimicrob. Chemother. 42:741-745. [DOI] [PubMed] [Google Scholar]
- 11.Kan, V. L., and J. E. Bennett. 1988. Efficacies of four antifungal agents in experimental murine sporotrichosis. Antimicrob. Agents Chemother. 32:1619-1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kan, V. L., D. K. Henderson, and J. E. Bennett. 1986. Bioassay for SF 86-327, a new antifungal agent. Antimicrob. Agents Chemother. 30:628-629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kwon-Chung, K. J., and J. E. Bennett. 1992. Medical mycology. Lea & Febiger, Philadelphia, Pa.
- 14.Meis, J. F., B. J. Kullberg, M. Pruszczynski, and R. P. Veth. 1994. Severe osteomyelitis due to the zygomycete Apophysomyces elegans. J. Clin. Microbiol. 32:3078-3081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Misra, P. C., K. J. Srivastava, and K. Lata. 1979. Apophysomyces, a new genus of the Mucorales. Mycotaxon 8:377-382. [Google Scholar]
- 16.Mosquera, J., P. A. Warn, J. L. Rodriguez-Tudela, and D. W. Denning. 2001. Treatment of Absidia corymbifera infection in mice with amphotericin B and itraconazole. J. Antimicrob. Chemother. 48:583-586. [DOI] [PubMed] [Google Scholar]
- 17.National Committee for Clinical Laboratory Standards. 1998. Reference method for broth dilution antifungal susceptibility testing of conidium-forming filamentous fungi; proposed standard. Document M-38P. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 18.Norden, G., S. Bjorck, H. Persson, C. Svalander, X. G. Li, and L. Edebo. 1991. Cure of zygomycosis caused by a lipase-producing Rhizopus rhizopodiformis strain in a renal transplant patient. Scand. J. Infect. Dis. 23:377-382. [DOI] [PubMed] [Google Scholar]
- 19.Odds, F. C., F. Van Gerven, A. Espinel-Ingroff, M. S. Bartlett, M. A. Ghannoum, M. V. Lancaster, M. A. Pfaller, J. H. Rex, M. G. Rinaldi, and T. J. Walsh. 1998. Evaluation of possible correlations between antifungal susceptibilities of filamentous fungi in vitro and antifungal treatment outcomes in animal infection models. Antimicrob. Agents Chemother. 42:282-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Otcenasek, M., and V. Buchta. 1994. In vitro susceptibility to 9 antifungal agents of 14 strains of Zygomycetes isolated from clinical specimens. Mycopathologia 128:135-137. [DOI] [PubMed] [Google Scholar]
- 21.Padhye, A. A., and L. Ajello. 1988. Simple method of inducing sporulation by Apophysomyces elegans and Saksenaea vasiformis. J. Clin. Microbiol. 26:1861-1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ribes, J. A., C. L. Vanover-Sams, and D. J. Baker. 2000. Zygomycetes in human disease. Clin. Microbiol. Rev. 13:236-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ruchel, R., and M. Schaffrinski. 1999. Versatile fluorescent staining of fungi in clinical specimens by using the optical brightener Blankophor. J. Clin. Microbiol. 37:2694-2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ryder, N. S., and B. Favre. 1997. Antifungal activity and mechanism of action of terbinafine. Rev. Contemp. Pharmacother. 8:275-288. [Google Scholar]
- 25.Ryder, N. S., and I. Frank. 1992. Interaction of terbinafine with human serum and serum proteins. J. Med. Vet. Mycol. 30:451-460. [DOI] [PubMed] [Google Scholar]
- 26.Schiraldi, G. F., and M. D. Colombo. 1997. Potential use of terbinafine in the treatment of aspergillosis. Rev. Contemp. Pharmacother. 8:349-356. [Google Scholar]
- 27.Schmitt, H. J., J. Andrade, F. Edwards, Y. Niki, E. Bernard, and D. Armstrong. 1990. Inactivity of terbinafine in a rat model of pulmonary aspergillosis. Eur. J. Clin. Microbiol. Infect. Dis. 9:832-835. [DOI] [PubMed] [Google Scholar]
- 28.Sheehan, D. J., C. A. Hitchcock, and C. M. Sibley. 1999. Current and emerging azole antifungal agents. Clin. Microbiol. Rev. 12:40-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sugar, A. M. 2000. Agents of mucormycosis and related species, p. 2685-2695. In G. Mandell, J. Bennett, and R. Dolin (ed.), Principles and practice of infectious diseases, 5th ed. Churchill Livingstone, Philadelphia, Pa.
- 30.Sugar, A. M., and X. P. Liu. 2000. Combination antifungal therapy in treatment of murine pulmonary mucormycosis: roles of quinolones and azoles. Antimicrob. Agents Chemother. 44:2004-2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Van Cutsem, J., and J. R. Boelaert. 1989. Effects of deferoxamine, feroxamine and iron on experimental mucormycosis (zygomycosis). Kidney Int. 36:1061-1068. [DOI] [PubMed] [Google Scholar]
- 32.Van Cutsem, J., J. Fransen, and P. A. Janssen. 1988. Experimental zygomycosis due to Rhizopus spp. infection by various routes in guinea-pigs, rats and mice. Mycoses 31:563-578. [DOI] [PubMed] [Google Scholar]
- 33.Van Cutsem, J., F. Van Gerven, J. Fransen, and P. A. Janssen. 1989. Treatment of experimental zygomycosis in guinea pigs with azoles and with amphotericin B. Chemotherapy 35:267-272. [DOI] [PubMed] [Google Scholar]
- 34.Verweij, P. E., N. J. Cox, and J. F. Meis. 1997. Oral terbinafine for treatment of pulmonary Pseudallescheria boydii infection refractory to itraconazole therapy. Eur. J. Clin. Microbiol. Infect. Dis. 16:26-28. [DOI] [PubMed] [Google Scholar]
- 35.Waldorf, A. R., S. M. Levitz, and R. D. Diamond. 1984. In vivo bronchoalveolar macrophage defense against Rhizopus oryzae and Aspergillus fumigatus. J. Infect. Dis. 150: 752-760. [DOI] [PubMed] [Google Scholar]