Abstract
We compared the efficacy and the toxicity of zidovudine (AZT) versus stavudine (d4T), in combination with lamivudine (3TC) and indinavir, in AZT-, dideoxyinosine (ddI)-, and/or dideoxycytosine (ddC)-experienced patients in a randomized comparative multicenter trial. One hundred seventy human immunodeficiency virus type 1 (HIV-1)-infected patients, who had received AZT, ddI, and/or ddC for at least 6 months but were naive for d4T, 3TC, and protease inhibitors, were randomized to AZT at 250 to 300 mg twice daily, 3TC at 150 mg twice daily, and indinavir at 800 mg every 8 h or to d4T at 40 mg twice daily, 3TC at 150 mg twice daily, and indinavir at 800 mg every 8 h. The primary endpoint was time to virological failure, defined as plasma HIV-1 RNA levels of >5,000 copies/ml after at least 8 weeks of antiretroviral therapy. Additional endpoints were change from baseline in CD4 cell counts, AIDS-defining events and adverse events, and proportion of patients with HIV-1 RNA levels of <500 copies/ml and HIV-1 RNA levels of <50 copies/ml. At week 80, 15 patients in the AZT arm and 14 patients in the d4T arm had reached the primary endpoint, and time to virological failure did not differ between the two arms (P = 0.98). In the d4T and in the AZT arms, 67 and 73% of patients, respectively, had HIV-1 RNA levels of <500 copies/ml (P = 0.50). The median change from baseline in CD4 cell count was 195 × 106 and 175 × 106/liter for the d4T- and AZT-containing arms, respectively. The proportions of patients with HIV-1 RNA levels of <50 copies/ml at weeks 8, 16, and 24 were similar in the two arms. The occurrence of serious adverse events was not significantly different between arms. In conclusion, in these patients heavily pretreated with AZT, switching from AZT to d4T when initiating indinavir and 3TC did not bring any additional benefit compared to maintaining AZT.
The use of antiretroviral therapy that includes a human immunodeficiency virus (HIV) protease inhibitor (PI) has markedly decreased mortality and morbidity in HIV-infected patients (20). In addition, such combination therapy can suppress viral load for up to 3 years in two-thirds of patients, including subjects previously exposed to nucleosides (nucleoside reverse transcriptase inhibitor [nRTI]) (4). There are several nRTI combinations that can be used with PIs. The zidovudine (AZT)-lamivudine (3TC) combination has been studied extensively, both as double-nucleoside therapy and in combination with either nonnucleoside RT inhibitors (24) or PIs (4, 7, 9, 23). In naive patients, the combination of stavudine (d4T)-3TC-indinavir (IDV) demonstrated antiretroviral activity as potent as that of AZT-3TC-IDV over 48 weeks of treatment (23).
In patients previously exposed to nucleosides, potential cross-resistance between the different compounds of this class may reduce the antiviral activity of second-line nucleoside therapy. Numerous studies have shown the activity of PI in PI-naive, previously nRTI-exposed subjects (4, 7, 9). However, the best second-line nRTI combination has not yet been clearly identified.
In the present trial, we compared the efficacy and the toxicity of AZT-3TC-IDV and d4T-3TC-IDV in patients previously exposed to AZT, dideoxyinosine (ddI), and/or dideoxycytosine (ddC) but naive for d4T, 3TC, and all PIs.
When the trial was initiated, there was a large population of patients treated with dual-nucleoside therapy, PIs started to be recommended as part of a triple drug therapy regimen, and abacavir was not available. At the time of PI initiation, the superiority of switching AZT to d4T over maintaining AZT was questionable. On one hand, the M184V mutation for resistance to 3TC had been reported elsewhere to induce a transient resensitization to AZT when AZT resistance had already emerged (17, 18). On the other hand, there were some concerns regarding the real efficacy of d4T in patients previously treated with AZT but naive for d4T. Finally, AZT-3TC and d4T-3TC were the dual-nucleoside combinations most frequently used as part of a PI-containing regimen.
(This work was presented at the 40th Interscience Conference on Antimicrobial Agents and Chemotherapy, Toronto, Ontario, Canada, 17 to 20 September 2000 [Abstr. 40th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 696, 2000].)
MATERIALS AND METHODS
Study design.
Novavir was a randomized, multicenter open-label trial that compared the safety and the efficacy of d4T versus AZT in combination with 3TC-IDV in HIV type 1 (HIV-1)-infected patients pretreated with AZT, ddI, and/or ddC but naive for d4T, 3TC, and PIs. Randomization was performed centrally in a 1:1 ratio, with stratification according to the number of copies of HIV-1 RNA in the plasma at the time of screening (10,000 or less versus 10,000 or more copies/ml). Patients who completed 80 weeks of study were allowed to continue in an extension phase for an additional 12-month period. Approval was obtained from the Investigational Review Board at the site of the main investigator (V.J.) on 21 February 1997, and patients gave written informed consent.
Treatment regimen.
AZT (Retrovir) and 3TC (Epivir) were provided by Glaxo Wellcome, d4T (Zerit) was provided by Bristol-Myers Squibb, and IDV (Crivixan) was provided by Merck and Co. AZT was given as 250 to 300 mg twice daily, 3TC was given as 150 mg twice daily, d4T was given as 40 mg twice daily (30 mg for patients weighing <60 kg), and IDV was given as 800 mg every 8 h. Patients were advised to take IDV on an empty stomach.
Study population.
Enrolled patients had documented HIV-1 infection as determined by positive enzyme-linked immunosorbent assay and confirmed by Western blotting, were aged 18 years or older, and had >6 months of prior AZT, ddI, and/or ddC cumulative treatment, either as monotherapy or in combination. Patients had HIV-1 plasma RNA levels of between 5,000 and 200,000 copies/ml. Exclusion criteria included previous treatment with d4T, 3TC, or PIs; previous intolerance to AZT; investigational drugs within 60 days of study entry; opportunistic infection within 30 days of study entry; chronic diarrhea; peripheral neuropathy of ≥grade 2 from the AIDS Clinical Trials Group toxicity scale, and pregnancy or breastfeeding. Patients with a hemoglobin level of <8.0 g/dl, an absolute neutrophil count of <750 cells/mm3, a platelet count of <75,000/mm3, an aspartate aminotransferase, alanine aminotransferase, or alkaline phosphatase level >5 times the upper limit of normal (ULN), an amylase level >2 times the ULN, a creatinine level >1.5 times the ULN, or a uric acid level >2 times the ULN were also excluded.
Evaluation of patients.
The patients were evaluated at screening (day −28), at randomization, at weeks 4 and 8, and every 8 weeks thereafter with a clinical assessment and routine laboratory monitoring. CD4 and CD8 cell counts and real-time plasma HIV RNA levels were determined at screening, baseline, weeks 4 and 8, and then every 16 weeks thereafter. In addition, plasma samples were stored at time of clinical assessment, i.e., every 8 weeks, allowing for retrospective batched determination of viral load. HIV RNA assays were performed at each site with the method routinely used at the time of the trial: bDNA (Chiron; lower limit of detection, 500 copies/ml) in 8 laboratories, Amplicor HIV-1 Monitor assay (Roche Molecular Systems; lower limit of detection, 200 copies/ml) in 28 laboratories, and both assays in 2 laboratories. All participating laboratories were members of the quality control Agence Nationale de Recherche sur le SIDA virology network. An ultrasensitive HIV-1 RNA PCR assay (Roche; lower limit of detection, 50 copies/ml) was performed centrally on plasma samples obtained at weeks 8, 16, and 24. Mutation genotypic resistance studies were performed on viral plasma RNA collected at baseline (day 0), by the consensus technique of the Agence Nationale de Recherche sur le SIDA (21). Primary and secondary RT mutations were identified according to the consensus statement from the International AIDS Society Resistance Testing-USA panel (10).
The study was reviewed twice by a data and safety monitoring board. At the second review, the data and safety monitoring board recommended continuing the follow-up of patients with HIV-1 RNA levels of <5,000 copies/ml every 3 months for 12 months.
Endpoints.
The primary measure of antiretroviral activity was the time to virological failure, defined as the first HIV RNA level of >5,000 copies/ml after at least 8 weeks of therapy, confirmed in a second specimen. The cutoff value of 5,000 copies/ml was retained since, when the trial was initiated, there were few options for salvage therapy in patients failing a PI-containing regimen. Sample size was calculated assuming that virological failure would be observed in 30% of patients in the less effective arm. Assuming that the more effective treatment would reduce the percentage of failing patients from 30 to 15%, a total of 157 patients per arm was required to establish a significant difference, in order to achieve 90% power at the 5% level of significance. Secondary outcome measures were grade 3 or grade 4 adverse events, AIDS-defining events or death, plasma HIV RNA and CD4 cell count changes, proportion of patients with less than 500 copies/ml at week 80, and proportion of patients with less than 50 copies/ml at weeks 8, 16, and 24.
Statistical analysis.
Statistical analyses were performed with the Statistical Analysis System (SAS Institute, Inc., Cary, N.C.). Comparison of qualitative variables was determined by Fisher's exact test. The significance of the differences in continuous variables was evaluated by the Kruskal-Wallis nonparametric test. In the primary analysis, times to virological failure were estimated by the method of Kaplan and Meier and compared by the log-rank test Analyses were based on the Cox proportional hazards model for predicting the time to virological failure. All variables for which the univariate P value was lower than 0.20 were used in a stepwise regression to determine independent prognostic factors of time to virological failure.
Secondary analyses included the proportion of patients who had plasma HIV-1 RNA levels of <50 copies/ml at weeks 8, 16, and 24 and the proportion of patients who had plasma HIV-1 RNA levels of <500 copies/ml at week 80. For the latter analysis, plasma HIV-1 RNA measurements were handled in the following way: a missing HIV-1 RNA value at any point was considered a failure (>500 copies/ml), with the exception of missing values for which the preceding and subsequent measurements indicated treatment success (<500 copies/ml), in which case the data were considered a success. In the latter analysis, the number of available HIV-1 RNA data and imputed data are given. All statistical tests were two sided, and all analyses were conducted with an intent-to-treat approach.
RESULTS
Baseline characteristics.
The trial was run in 43 centers in France. A total of 170 patients were enrolled from March 1997 to March 1998; 85 subjects were assigned to AZT-3TC-IDV, and 85 subjects were assigned to d4T-3TC-IDV. Although the expected number of patients was not reached, the trial was closed to accrual in March 1998 given the low number of patients who were included in the trial since September 1997. This decrease in accrual was related to the extensive use of PIs in first-line antiretroviral therapy since the end of summer 1997. Table 1 shows the baseline characteristics for patients by treatment arms. Seventy-nine percent of subjects were male, the median age was 37 years, and the median duration of previous nRTI therapy was 19.5 months. Ninety-six percent of the patients had received dual-nucleoside therapy. The median CD4 cell count was 291 cells/mm3, and the median HIV-1 RNA level was 4.36 log10 copies/ml. There was no significant difference in baseline plasma HIV-1 RNA level or in CD4 cell count between the randomized arms. Other baseline characteristics were likewise balanced across treatment arms.
TABLE 1.
Baseline characteristics of patients
| Characteristic | Value for treatment arm
|
Value for total (n = 170) | |
|---|---|---|---|
| d4T-3TC-IDV (n = 85) | AZT-3TC-IDV (n = 85) | ||
| Sex, no. (%) male | 68 (80) | 67 (79) | 135 (79) |
| Age, yr | |||
| Median | 36 | 37 | 37 |
| 25%-75% | 32-42 | 33-47 | 33-44 |
| Prior nRTI | |||
| Median duration (mo) | 19.6 | 18.9 | 19.5 |
| 25%-75% | 12-37.3 | 13-38.3 | 12-37.9 |
| No. (%) of patients exposed to: | |||
| AZT-ddI | 49 (58) | 40 (47) | 89 (52) |
| AZT-ddC | 29 (34) | 35 (41) | 64 (38) |
| AZT-ddC and AZT-ddI | 5 (6) | 8 (9) | 13 (8) |
| CDCa status, no. (%) | |||
| CDC-A | 41 (48) | 39 (46) | 80 (47) |
| CDC-B | 33 (39) | 39 (46) | 72 (42) |
| CDC-C | 11 (13) | 7 (8) | 18 (11) |
| CD4 cells/mm3 | |||
| Median | 291 | 292 | 291 |
| 25%-75% | 216-370 | 225-368 | 221-368 |
| HIV RNA level, log10 copies/ml | |||
| Median | 4.42 | 4.33 | 4.36 |
| 25%-75% | 4.07-4.68 | 4.1-4.64 | 4.08-4.66 |
CDC, Centers for Disease Control and Prevention.
Genotypic data for the HIV-1 RT were available at study entry on isolates from 153 patients. Mutations in RT were common, reflecting the prior use of nRTIs. One hundred nineteen patients (78%) had the T215Y/F resistance mutation, and 135 (88%) patients had one or more thymidine-associated resistance mutations at RT codon 41, 67, 70, 210, 215, or 219. There was no statistical difference in the incidence of thymidine-associated resistance mutations at baseline between the two treatment arms. No M184V resistance mutation to 3TC was found at baseline.
Subject disposition and follow-up.
All 170 patients began the treatment and were included in the analysis. The median duration of follow-up was 18.4 months in both groups. Six patients were lost to follow-up, and 39 patients discontinued the randomized treatment before week 80 (Table 2), 15 subjects in the d4T arm and 24 subjects in the AZT arm, with no statistical difference between the two arms (log-rank test, P = 0.13). Among these patients who discontinued the randomized treatment, the nucleoside assigned by randomization, AZT or d4T, was maintained in 11 out of the 15 patients in the d4T arm and 9 out of the 24 patients in the AZT arm. Three patients died during the trial, but no death was related to HIV disease or to drug toxicity.
TABLE 2.
Subject disposition
| Reason subjects discontinued study drugs | No. of subjects (%)
|
|
|---|---|---|
| d4T-3TC-IDV | AZT-3TC-IDV | |
| Before wk 80 | 15 (18) | 24 (28) |
| Lost to follow-up | 1 (1) | 1 (1) |
| Death | 2 (2) | 0 |
| Adverse events | 7 (8) | 11 (13) |
| Subject request | 2 (2) | 3 (4) |
| Virological failure | 1 (1) | 6 (7) |
| Physician decision | 0 | 1 (1) |
| Other reason or unknown | 2 (2) | 2 (2) |
| During the extended follow-up | 9 (16) | 13 (22) |
| Lost to follow-up | 0 | 1 (2) |
| Death | 1 (2) | 0 |
| Adverse events | 4 (7) | 0 |
| Subject request | 0 | 4 (7) |
| Virological failure | 0 | 0 |
| Physician decision | 3 (5) | 2 (3) |
| Other reason or unknown | 1 (2) | 6 (10) |
The 12-month extended follow-up was run in 115 out of the 141 patients (82%) who did not reach an endpoint, 55 patients and 60 patients in the d4T and AZT arms, respectively. One patient was lost to follow-up after 24 months, and another subject died (cerebral stroke) during the extended follow-up. Among patients participating in the extended follow-up, a further 22 patients discontinued the randomized treatment (9 patients in the d4T arm and 13 patients in the AZT arm). There was no statistical difference for the entire period between the two treatment arms (log-rank test, P = 0.08) although a larger proportion of patients discontinued the randomized treatment in the AZT arm than in the d4T arm.
Virological and immunologic response to treatment.
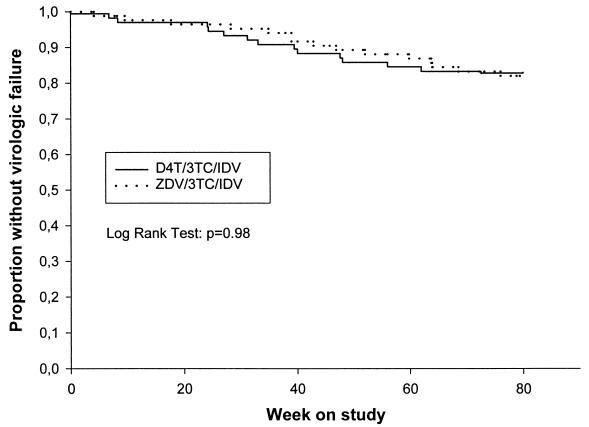
Virological failure occurred in 14 (16%) patients receiving d4T-3TC-IDV and 15 (18%) patients receiving AZT-3TC-IDV before week 80. Six additional patients reached primary endpoint during the extended follow-up with three failures in each arm. Time to failure was not different between the two groups (Fig. 1; log-rank test, P = 0.98). A high proportion of patients in both treatment arms achieved and maintained a plasma HIV-1 RNA level of less than 500 copies/ml after 8 weeks on treatment (Fig. 2). At week 80, 67 and 73% of patients had HIV-1 RNA levels of less than 500 copies/ml in the d4T and the AZT arms, respectively, with no statistical difference between the two groups (P = 0.50). At the end of the extended follow-up, the proportion of patients with less than 500 copies/ml was 76 and 78% in the d4T and AZT arms, respectively. The proportions of patients in each treatment arm who had HIV RNA levels of <50 copies/ml at weeks 8, 16, and 24 are shown in Table 3.
FIG. 1.
Kaplan-Meier estimates of the proportion of patients who did not reach the primary study endpoint (viral load of >5,000 copies/ml).
FIG. 2.
Proportion of patients with serum HIV RNA levels of less than 500 copies/ml. Bars are 95% confidence intervals.
TABLE 3.
Number (percent) of patients with HIV-1 RNA levels of <50 copies/ml
| Wk | No. (%) of patients
|
||
|---|---|---|---|
| d4T arm | AZT arm | Total | |
| 8 | 24 (32) | 19 (23) | 43 (28) |
| 16 | 58 (75) | 57 (71) | 115 (73) |
| 24 | 58 (77) | 59 (75) | 117 (76) |
Baseline characteristics and early response to treatment, measured as decrease in plasma HIV RNA levels (limit of detection, 50 copies/ml) at weeks 8, 16, and 24 or increase in CD4 cell counts at weeks 4 and 8, were included in proportional hazard models. The final model indicates that HIV RNA levels below 50 copies/ml at weeks 16 and 24, decreased HIV RNA levels from baseline to week 24 (per 1 log decrease), and the presence of the T215Y/F mutation were independently associated with a reduction in the risk of virological failure (Table 4). For example, a decrease of 1.0 log in the concentration of HIV RNA from baseline to week 24 was associated with a significant lowering to 0.47 in the hazard ratio of virological failure (i.e., a 53% reduction in the risk of failure). The presence of the T215Y/F mutation at baseline was associated with a 61% reduction in the risk of virological failure, with a P value of 0.015.
TABLE 4.
Multivariate relative risk for predicting virological failure
| Variable | Relative risk (95% CIa) | P value |
|---|---|---|
| Plasma HIV RNA level | ||
| <50 versus >50 copies/ml at wk 16 | 0.32 (0.13-0.77) | 0.011 |
| <50 versus >50 copies/ml at wk 24 | 0.25 (0.09-0.70) | 0.008 |
| Decrease from baseline to wk 24 in log copies/ml | 0.47 (0.32-0.70) | <0.001 |
| Presence of the T215Y/F mutation | 0.39 (0.18-0.83) | 0.015 |
CI, confidence interval.
The CD4 cell count increased in both treatment arms (Fig. 3). At week 80, the median increase from baseline was 195 × 106 cells/liter in the d4T-containing regimen versus 175 × 106 cells/liter in the AZT-containing regimen (P = 0.29). From week 80 to the end of the extended follow-up, there was still a substantial increase in the CD4 cell count, with a global median increase from baseline of 260 × 106 and 237 × 106 cells/liter in the d4T arm and in the AZT arms, respectively.
FIG. 3.
Changes from baseline in the CD4 cell count during the 80 weeks of the study. Median values are shown. Bars are 25th and 75th percentiles. IQR, interquartile range.
Adverse events.
Serious adverse events, defined as those requiring hospitalization, those considered life threatening, or neoplasia, occurred in 45 of 170 patients (26.4%), regardless of etiology: 22 in the AZT arm and 23 in the d4T arm. In 14 patients, these serious adverse events were clinical nephrolithiasis, 6 in the AZT arm and 8 in the d4T arm. Overall, grade 3 or 4 clinical adverse events were reported for 16 of 170 patients (9.4%), 7 in the AZT arm and 9 in the d4T arm. Paresthesia of any grade was observed for 13 patients, 2 in the AZT arm and 11 in the d4T arm (P = 0.018). A total of 47 patients had clinical nephrolithiasis, 28 patients in the AZT arm and 19 patients in the d4T arm. Lipodystrophy was assessed clinically at the end of the extended follow-up, and results will be reported elsewhere. Grade 3 or 4 laboratory adverse events were reported for 30 patients, 14 in the AZT arm and 16 in the d4T arm (Table 5).
TABLE 5.
Grade 3 or 4 laboratory toxicities according to treatment group
| Type of toxicity | No. of events (no. of patients)
|
|
|---|---|---|
| d4T arm | AZT arm | |
| Total bilirubin | 6 (1) | 1 (1) |
| ASATa | 1 (1) | 4 (2) |
| ALATb | 3 (3) | 3 (3) |
| Serum glucosec | 2 (1) | 4 (4) |
| Triglyceridesc | 14 (9) | 3 (3) |
| Serum amylase | 1 (1) | 2 (1) |
| Total no. | 27 (16) | 17 (14) |
ASAT, aspartate aminotransferase.
ALAT, alanine aminotransferase.
Tests were obtained when patients were in a nonfasted state.
DISCUSSION
The response of previously treated HIV-infected patients to new agents is of great concern due to the existence of cross-resistance between compounds of the same class, which may limit further available options. At first reported for PIs and nonnucleoside RT inhibitors, intraclass cross-resistance has also been reported for nucleosides. Different studies have shown that patients previously treated with AZT had suboptimal responses to subsequent therapy with d4T-containing regimens (8, 13; D. V. Havlir, G. Friedland, R. Pollard, C. Tierney, L. Smeaton, L. Fox, D. D. Richman, and the ACTG 290/298 Teams, 5th Conf. Retrovir. Opportunistic Infect., abstr. 2, 1998). By contrast, Gallant et al. did not find any significant difference in either HIV RNA or CD4 cell count response to a d4T-containing regimen between AZT-naive and AZT-experienced patients (3). However, in contrast to previous studies, 63% of patients received concomitant PI therapy. We studied a population of AZT-pretreated patients and could assess in a randomized trial whether switching from AZT to d4T conferred a benefit over maintaining AZT as part of the triple drug therapy.
Although the median duration of previous NRTI therapy was 19.5 months and 88% of subjects had AZT resistance mutations at baseline, 70% of the whole population of patients achieved viral load levels of less than 500 copies/ml after 18 months of follow-up. This may be explained by the potent antiviral effect of 3TC and IDV initiated simultaneously. The lack of the M184V mutation at baseline confirms that our patients were truly naive for 3TC. These data are in agreement with the results of earlier trials evaluating the antiviral effect of AZT-3TC-IDV (5, 6) or ritonavir (16) in patients previously exposed to AZT but naive for 3TC and PIs.
In the present study, AZT-3TC-IDV and d4T-3TC-IDV exhibited the same levels of antiviral activity. However, one main limitation of our study was its limited statistical power. The study was powered in assuming a 30% virological failure rate in the less effective arm. Given that the observed overall failure rate was only 17% and that only 170 patients were enrolled, we could not meet the original study objectives as stated in Materials and Methods. Considering an observed failure rate of 17% in the less effective arm with a sample size of 85 patients in each treatment arm, we had a 62% power to detect a rate difference of 15% between the two groups. However, the small observed rate difference of 2% between the two treatment arms likely implies similar antiviral efficacies and could not be statistically significant even with a large sample size.
The lack of benefit of d4T compared to continuing AZT in AZT-pretreated patients was in agreement with the growing body of data on cross-resistance between these two drugs. Cross-resistance between AZT and d4T has been reported more extensively, in vitro and during clinical trials. In the ALTIS study, the presence of the T215Y/F mutation and a resistant d4T phenotype, defined as an increase in 50% inhibitory concentration of at least 1.8 compared to the mean 50% inhibitory concentration of susceptible viruses, was associated with a poor virological response (decrease lower than 0.3 log copies/ml), arguing for a relative phenotypic and genotypic cross-resistance between d4T and AZT (V. Calvez, D. Costagliola, D. Descamps, S. Matheron, A. Simon, M. A. Valantin, C. Katlama, and F. Brun-Vézinet, 4th Int. Workshop HIV Drug Resist. Treatment Strategies, abstr. 107, 2000). In the AIDS Clinical Trials Group 302 trial, the virological substudy, performed with patients receiving d4T monotherapy after more than 3 years of exposure to AZT, showed that responders had fewer AZT resistance mutations than did nonresponders (N. Shulman, R. Shafer, R. Winters, R. Machekano, S. Liou, M. Hughes, A. Zolopa, and D. Katzenstein for ACTG 302, 8th Conf. Retrovir. Opportunistic Infect., abstr. 437, 2001). Izopet et al. reported that patients with AZT resistance mutations had a significantly lower RNA suppression after 3 and 6 months of treatment with d4T-ddI than did patients with wild-type virus (12). Montaner et al. reported that AZT resistance mutations, especially at codon 215, were associated with a reduced virological response to d4T-3TC (19). Reduced inhibition of purified HIV-1 RT derived from an AZT-resistant isolate by AZT triphosphate and d4T triphosphate compared to RT from a paired wild-type isolate has been recently described and may provide a biochemical explanation for the lower efficacy of d4T in patients with prior AZT treatment (C. Y. Duan, D. Poticha, T. C. Stoeckli, J. Lu, C. Petropoulos, J. Whitcomb, C. S. McHenry, and D. R. Kuritzkes, 8th Conf. Retrovir. Opportunistic Infect., abstr. 442, 2001). These data showing the existence of cross-resistance between AZT and d4T suggest strongly that, in the present trial, the activity of d4T was reduced to the activity of AZT against AZT-resistant strains, explaining the similar antiviral effects of the two regimens. Another hypothesis to explain the reduction in d4T efficacy in AZT-pretreated patients would be pharmacological antagonism between d4T and AZT, resulting from the alteration of d4T phosphorylation by AZT. At first reported in 1998 (J. P. Somadossi, X. J. Zhou, J. Moore, D. V. Havlir, G. Friedland, C. Tierney, L. Smeaton, L. Fox, D. Richman, R. Pollard, and the ACTG 290 Team, 5th Conf. Retrovir. Opportunistic Infect., abstr. 3, 1998), before the description of cross-resistance, this mechanism could explain the antagonism of the AZT-d4T combination but does not seem to be involved in the case of patients receiving d4T after prior exposure to AZT (11). Finally, as mentioned above, 3TC-IDV is a potent combination. We cannot exclude the possibility that, when d4T was used as part of a PI-containing regimen, the benefit in efficacy associated with d4T administration was not important enough to translate into differences in HIV RNA and CD4 cell responses.
In multivariate analysis, we found that initial responses to highly active antiretroviral therapy (decrease in HIV RNA from baseline to week 24 and HIV RNA below 50 copies/ml at weeks 16 and 24), but not baseline HIV RNA levels, were associated with a reduction in the risk of subsequent virological failure. This result is in agreement with findings of some previous studies (2, 14, 22). The lack of significant relationship between baseline viral load and time to virological failure in multivariate analysis suggests that baseline HIV RNA levels interfere with response to treatment through its effect on the speed with which undetectable plasma HIV RNA levels can be achieved. Baseline RNA values may be useful for designing initial therapy, but nadir RNA values appear to be a more reliable predictor of virological outcome. In patients with persistent detectable viral load, incomplete viral suppression under therapy favors the development of drug-resistant virus and eventually virological failure (1).
Unexpectedly, we found that the presence of the T215Y/F mutation was associated with a reduction in the risk of virological failure. Previous studies have shown, for AZT-pretreated patients, that genotypic resistance to AZT did not impact the antiviral activity of AZT-3TC combined with ritonavir (16) or IDV (L. Demeter, V. Degruttola, S. Eshleman, J. B. Jackson, M. Hughes, and S. Hammer for the ACTG 320 Study Team, 6th Conf. Retrovir. Opportunistic Infect., abstr. 131, 1999). Our data agree with results of the ACTG 370 trial (15), in which the presence of at least one AZT resistance mutation in AZT-3TC-pretreated patients conferred a reduced risk of virological failure on subsequent treatment with IDV-AZT combined with 3TC or delavirdine (V. A. Johnson, R. L. Bassett, J. L. Koel, R. A. Rhodes, R. K. Young, H. Barrett, and D. R. Kuritzkes, Abstr. 40th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 2111, 2000). We were able to show in a substudy that poor adherence to therapy likely explained the poor virological outcome in patients with sensitive virus at baseline (D. Descamps, P. Flandre, J. Izopet, C. Tamalet, C. Ruffault, F. Zeng, V. Meiffredy, G. Peytavin, J. P. Aboulker, V. Joly, P. Yeni, and F. Brun-Vézinet, 8th Conf. Retrovir. Opportunistic Infect., abstr. 438, 2001).
No unexpected toxicity of either regimen was observed, and serious or grade ≥3 adverse events did not occur with a significantly greater frequency in one arm over the other, although there was a trend toward grade ≥3 hypertriglyceridemia in the d4T arm. However, this result should be considered with caution since patients were sampled in a nonfasted state. Preliminary results of a lipodystrophy substudy performed during extended follow-up show that triglyceride levels measured in a fasted state were similar between the two arms (V. Joly, P. Flandre, V. Meiffredy, S. Hazebrouck, M. Harel, J. P. Aboulker, and P. Yeni, 8th Conf. Retrovir. Opportunistic Infect., abstr. 539, 2001). Twenty-eight percent of the patients had at least one episode of nephrolithiasis according to a broad clinical definition. This high rate of nephrolithiasis may be explained by the long duration of follow-up. Gulick et al. reported that 36% of patients had at least one episode of nephrolithiasis after 3 years of treatment with AZT-3TC-IDV (6). The good tolerance to this nucleoside-containing regimen must be tempered by the fact that patients entered the trial with many months of nucleoside therapy and so were preselected for good tolerance to these compounds, particularly AZT.
In conclusion, we were unable to detect a difference in virological efficacy between continuing AZT and switching to d4T, when adding 3TC and IDV in this population of AZT-pretreated patients. Combined with other data, this suggests that, in the face of extensive resistance to AZT, the benefits of switching to d4T are limited.
Acknowledgments
This work was supported by the French Agency for AIDS Research (ANRS).
APPENDIX
The Novavir Study Group comprised the following persons and institutions.
The Scientific Committee consisted of J.-P. Aboulker, B. Bazin, F. Brun-Vézinet, I. Carrière, A. Certain, J. Dormont, Y. Esnault, P. Flandre, P.-M. Girard, V. Joly, C. Katlama, V. Meiffredy, C. Michelet, J.-M. Molina, A. Prieur, F. Raffi, D. Séréni, and P. Yeni.
The participating clinical centers (all in France or its overseas departments) were as follows: Centre Hospitalier de Belfort, Belfort (J.-P. Faller and P. Elinger); Centre Hospitalier La Milétrie, Poitiers (J.-P. Breux and M. Duballet); CHRU Pointe à Pitre-Abymes, Guadeloupe (B. Contamin and M. Strobel); Hôpital Robert Debré, Reims (I. Beguinot and G. Remy); Hôpital Corvisart, Charleville Mézières (M. Bouvier-Alias, P. Lanoux, and C. Penalba); Hôpital Avicenne, Bobigny (B. Jarrousse and P. Honoré); Hôpital Saint-Jacques, Besançon (G. Achard, C. Drobacheff, H. Gil, B. Hoen, and C. Roche); Hôpital André Mignot, Le Chesnay (J. Doll); Hôpital Cochin, Paris (J. Krulik, D. Sicard, D. Séréni, and B. Silbermann); Hôpital Bicêtre, Le Kremlin Bicêtre (J.-F. Delfraissy, C. Goujard, M. Mole, and M.-T. Rannou); Hôpital Lariboisière, Paris (J.-M. Salord, V. Vincent, and M. Parrinello); Hôpital Henri Duffaut, Avignon (G. Brun and G. Lepeu); Hôpital Jean Verdier, Bondy (V. Jeantils and C. Thaleb); Hôpital Rothschild, Paris (N. Adda, T. Nguyen, and W. Rozenbaum); Hôpital Antoine Béclère, Clamart (F. Boue, V. Chambrin, and G. Lubart); Hôpital Bichat Claude-Bernard, Paris (Y. Bennai, L. Belardi, E. Bouvet, I. Fournier, C. Gaudebout, V. Joly, C. Mandet, S. Masson, and P. Yeni); Centre Médico-Chirurgical Foch, Suresnes (I. Vergne and D. Zucmann); Hôpital Henri Mondor, Créteil (M. Lechevallier and A. Sobel); Hôpital Pitié-Salpétrière, Paris (C. Katlama, S. Maury, and H. Schoen); Hôpital Saint-Louis, Paris (J. Modaï and M.-N. Sombardier); Hôpital Pierre Zobda-Quitman, Fort de France (A. Cabie, V. Beaujolais, and G. Sobesky); Hôpital d'Angers, Angers (J.-M. Chennebault, P. Fialaire, and E. Vivien); CHR Pellegrin, Bordeaux (T. Galperine and J.-M. Ragnaud); Hôpital Saint-André, Bordeaux (M. Bonarek and P. Morlat); Hôpital Hôtel Dieu, Lyon (C. Carré, L. Cotte, and C. Trépo); Hôpital Sainte Marguerite, Marseille (T. Dinh, G. Fabre, and J.-A. Gastaut); CHU Gui de Chauliac, Montpellier (C. Merle, J. Reynes, and M. Vidal); Hôpital Hôtel Dieu, Nantes (M.-F. Charonnat, A. Huart, F. Raffi, and M. Sicot); Hôpital De L'Archet, Nice (P. Clevenbergh and P. Dellamonica); Hôpital Pontchaillou, Rennes (C. Arvieux, F. Cartier, and F. Souala); Centre Hospitalier La Beauchée, Saint Brieuc (C. Devaurs and C. Hascoet); CHRU de Strasbourg, Strasbourg (V. Krantz and J.-M. Lang); Hôpital de Purpan, Toulouse (M.-F. Garbay and P. Massip); CHU Côte de Nacre, Caen (C. Bazin, P. Goubin, and M. Six); Hôpital de Tourcoing, Tourcoing (Y. Mouton); Hôpital de Brabois, Vandoeuvre les Nancy (C. Burty, P. Canton, and T. May); Hôpital Charles Nicolle, Rouen (F. Borsa-Lebas and Y. Debab); and Centre Hospitalier de Compiègne, Compiègne (L. Geffray and D. Merrien).
The Data and Safety Monitoring Board consisted of E. Hirsch, J. Puel, M. Seligmann, and M. Vray.
The coordinating trial center was INSERM SC10 (J.-P. Aboulker, B. Bazin, P. Flandre, M. Harel, S. Hazebrouck, N. Leturque, V. Meiffredy, A. Prieur, and Y. Saïdi).
ARCAM consisted of R. Léonard, A. Dauphin, and R. Laillier.switching to d4T are limited.
REFERENCES
- 1.Condra, J. H., W. A. Schleif, O. M. Blahy, L. J. Gabryelski, D. J. Graham, J. C. Quintero, A. Rhodes, H. I. Robbins, E. Roth, M. Shivaprakash, D. Titus, T. Yang, H. Teppler, K. E. Squires, P. J. Deutsch, and E. A. Emini. 1995. In vivo emergence of HIV-1 variants resistant to multiple protease inhibitors. Nature 374:569-571. [DOI] [PubMed] [Google Scholar]
- 2.Cozzi-Lepri, A., V. Miller, A. N. Phillips, H. Rabenau, C. A. Sabin, and S. Staszewski. 2001. The virological response to highly active antiretroviral therapy over the first 24 weeks of therapy according to the pre-therapy viral load and the weeks 4-8 viral load. AIDS 15:47-54. [DOI] [PubMed] [Google Scholar]
- 3.Gallant, J. E., R. E. Chaisson, J. C. Keruly, and R. D. Moore. 1999. Stavudine in zidovudine (ZDV)-experienced compared with ZDV-naive patients. AIDS 13:225-229. [DOI] [PubMed] [Google Scholar]
- 4.Gulick, R. M., J. W. Mellors, D. Havlir, J. J. Eron, C. Gonzalez, D. McMahon, D. D. Richman, F. T. Valentine, L. Jonas, A. Meibohm, E. A. Emini, and J. A. Chodakewitz. 1997. Treatment with indinavir, zidovudine and lamivudine in adults with human immunodeficiency virus infection and prior antiretroviral therapy. N. Engl. J. Med. 337:734-739. [DOI] [PubMed] [Google Scholar]
- 5.Gulick, R. M., J. W. Mellors, D. Havlir, J. J. Eron, C. Gonzalez, D. McMahon, L. Jonas, A. Meibohm, D. Holder, W. A. Schleif, J. H. Condra, E. A. Emini, R. Isaacs, J. A. Chodakewitz, and D. D. Richman. 1998. Simultaneous vs sequential initiation of therapy with indinavir, zidovudine and lamivudine for HIV-1 infection. JAMA 280:35-41. [DOI] [PubMed] [Google Scholar]
- 6.Gulick, R. M., J. W. Mellors, D. Havlir, J. J. Eron, A. Meibohm, J. H. Condra, F. T. Valentine, D. McMahon, C. Gonzalez, L. Jonas, E. A. Emini, J. A. Chodakewitz, R. Isaacs, and D. D. Richman. 2000. 3-year suppression of HIV viremia with indinavir, zidovudine, and lamivudine. Ann. Intern. Med. 133:35-39. [DOI] [PubMed] [Google Scholar]
- 7.Hammer, S., K. E. Squires, M. D. Hughes, J. M. Grimes, L. M. Demeter, J. S. Currier, J. J. Eron, J. E. Feinberg, H. H. Balfour, M. D. Lawrence, L. R. Deyton, J. A. Chodakewitz, and M. A. Fischl. 1997. A controlled trial of two nucleoside analogues plus indinavir in persons with human immunodeficiency virus infection and CD4 cell counts of 200 per cubic millimeter or less. N. Engl. J. Med. 337:725-733. [DOI] [PubMed] [Google Scholar]
- 8.Havlir, D., C. Tierney, G. H. Friedland, R. B. Pollard, L. Smeaton, J. P. Somadossi, L. Fox, H. Kessler, K. H. Fife, and D. D. Richman. 2000. In vivo antagonism with zidovudine plus stavudine combination therapy. J. Infect. Dis. 182:321-325. [DOI] [PubMed] [Google Scholar]
- 9.Hirsch, M., R. Steigbigel, R., S. Stazewski, J. Mellos, E. Scerpella, B. Hirschel, J. Lange, K. Squires, S. Rawlins, A. Meibohm, and R. Leavitt for the Protocol 039 Study Group. 1999. A randomized controlled trial of indinavir, zidovudine and lamivudine in adults with advanced HIV-1 infection and prior antiretroviral therapy. J. Infect. Dis. 180:659-665. [DOI] [PubMed] [Google Scholar]
- 10.Hirsch, M. S., F. Brun-Vézinet, R. T. D'Aquila, S. M. Hammer, V. A. Johnson, D. R. Kuritzkes, C. Loveday, J. W. Mellors, B. Clotet, B. Conway, L. M. Demeter, S. Vella, D. M. Jacobsen, and D. D. Richman. 2000. Antiretroviral drug resistance testing in adult HIV-1 infection. Recommendations of an International AIDS Society-USA panel. JAMA 283:2417-2426. [DOI] [PubMed] [Google Scholar]
- 11.Hoggard, P. G., S. D. Sales, D. Phiboonbanakit, J. Lloyd, B. A. Maher, S. H. Khoo, E. Wilkins, P. Carey, C. A. Hart, and D. J. Back. 2001. Influence of prior exposure to zidovudine on stavudine phosphorylation in vivo and ex vivo. Antimicrob. Agents Chemother. 45:577-582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Izopet, J., A. Bicart-See, C. Pasquier, K. Sandres, E. Bonnet, B. Marchou, J. Puel, and P. Massip. 1999. Mutations conferring resistance to zidovudine diminish the antiviral effect of stavudine plus didanosine. J. Med. Virol. 59:507-511. [PubMed] [Google Scholar]
- 13.Katlama, C., M. A. Valantin, S. Matheron, A. Coutellier, V. Calvez, D. Descamps, C. Longuet, M. Bonmarchand, R. Tubiana, M. De Sa, R. Lancar, H. Agut, F. Brun-Vezinet, and D. Costagliola. 1998. Efficacy and tolerance of stavudine and lamivudine in treatment-naive and treatment-experienced patients with HIV-1 infection. Ann. Intern. Med. 129:525-531. [DOI] [PubMed] [Google Scholar]
- 14.Kempf, D. J., R. A. Rode, Y. Xu, E. Sun, M. E. Heath-Chiozzi, J. Valdes, A. J. Japour, S. Danner, C. Boucher, A. Molla, and J. M. Leonard. 2001. The duration of viral suppression during protease inhibitor therapy for HIV-1 infection is predicted by plasma HIV-1 RNA at the nadir. AIDS 12:F9-F14. [DOI] [PubMed] [Google Scholar]
- 15.Kuritzkes, D., R. L. Bassett, V. A. Johnson, I. C. Marschner, J. J. Eron, J. P. Somadossi, E. P. Acosta, R. L. Murphy, K. Fife, K. Wood, D. Bell, A. Martinez, and C. B. Pettinelli for the Adult AIDS Clinical Trials Group 370 Protocol Team. 2000. Continued lamivudine versus delavirdine in combination with indinavir and zidovudine or stavudine in lamivudine-experienced patients: results of Adult AIDS Clinical Trials Group protocol 370. AIDS 14:1553-1561. [DOI] [PubMed] [Google Scholar]
- 16.Kuritzkes, D. R., A. Sevin, B. Young, M. Bakhtiari, H. Wu, M. St. Clair, E. Connick, A. Landay, J. Spritzler, H. Kessler, and M. M. Lederman for the ACTG Protocol 315 Team. 2000. Effect of zidovudine resistance mutations on virologic response to treatment with zidovudine-lamivudine-ritonavir: genotypic analysis of human immunodeficiency virus type 1 isolates from AIDS Clinical Trials Group Protocol 315. J. Infect. Dis. 181:491-497. [DOI] [PubMed] [Google Scholar]
- 17.Larder, B. A., S. A. Kemp, and R. Harrigan. 1995. Potential mechanism for sustained antiretroviral efficacy of AZT-3TC combination therapy. Science 269:696-699. [DOI] [PubMed] [Google Scholar]
- 18.Masquelier, B., D. Descamps, I. Carrière, F. Ferchal, G. Collin, M. Denayrolles, A. Ruffault, B. Chanzy, J. Izopet, C. Buffet-Janvresse, M. P. Schmitt, E. Race, H. J. Fleury, J. P. Aboulker, P. Yeni, and F. Brun-Vézinet. 1999. Zidovudine resensitization and dual HIV-1 resistance to zidovudine and lamivudine in the Delta lamivudine rollover study. Antivir. Ther. 4:69-77. [PubMed] [Google Scholar]
- 19.Montaner, J. S., T. Mo, J. M. Raboud, S. Rae, C. S. Alexander, C. Zala, D. Rouleau, and P. R. Harrigan. 2000. Human immunodeficiency virus-infected persons with mutations conferring resistance to zidovudine show reduced virologic responses to hydroxyurea and stavudine-lamivudine. J. Infect. Dis. 181:729-732. [DOI] [PubMed] [Google Scholar]
- 20.Palella, F. J., Jr., K. M. Delaney, A. C. Moorman, M. O. Loveless, J. Fuhrer, G. A. Satten, D. J. Aschman, S. D. Holmberg, and the HIV Outpatient Study Investigators. 1998. Declining morbidity and mortality among patients with advanced human immunodeficiency infection. N. Engl. J. Med. 338:853-860. [DOI] [PubMed] [Google Scholar]
- 21.Pasquier, C., N. Millot, R. Njouom, K. Sandres, M. Cazabat, J. Puel, and J. Izopet. 2001. HIV-1 subtyping using phylogenetic analysis of pol gene sequences. J. Virol. Methods 94:45-54. [DOI] [PubMed] [Google Scholar]
- 22.Powderly, W. G., M. S. Saag, S. Chapman, G. Yu, B. Quart, and N. J. Clendeninn. 1999. Predictors of optimal virological response to potent antiretroviral therapy. AIDS 13:1873-1880. [DOI] [PubMed] [Google Scholar]
- 23.Squires, K. E., R. Gulick, P. Tebas, J. Santana, V. Mulanovich, R. Clark, B. Yangco, S. I. Marlowe, D. Wright, C. Cohen, T. Cooley, J. Mauney, K. Uffelman, N. Schoellkopf, R. Grosso, and M. Stevens. 2000. A comparison of stavudine plus lamivudine versus zidovudine plus lamivudine in combination with indinavir in antiretroviral naive individuals with HIV infection: selection of thymidine analog regimen therapy (START I). AIDS 14:1591-1600. [DOI] [PubMed] [Google Scholar]
- 24.Staszewski, S., J. Morales-Ramirez, K. T. Tashima, A. Rachlis, D. Skiest, J. Stanford, R. Stryker, P. Johnson, D. F. Labriola, D. Farina, D. J. Manion, N. M. Ruiz, et al. 1999. Efavirenz plus zidovudine and lamivudine, efavirenz plus indinavir, and indinavir plus zidovudine and lamivudine in the treatment of HIV-1 infection in adults. N. Engl. J. Med. 341:1865-1873. [DOI] [PubMed] [Google Scholar]