Abstract
Tuberculous meningitis (TBM), the most severe form of Mycobacterium tuberculosis infection in humans, is associated with significant morbidity and mortality despite successful treatment with antituberculous drugs. This is due to the irreversible brain damage subsequent to the local inflammatory response of the host to M. tuberculosis. Corticosteroids have been used in conjunction with antituberculous therapy in an attempt to modulate the inflammatory response, but this strategy has been of limited success. Therefore, we examined whether combining antituberculous drugs with the immunomodulatory drug thalidomide or with a new thalidomide analog, immunomodulatory drug 3 (IMiD3), would be effective in reducing morbidity and mortality in an experimental rabbit model of TBM. Intracisternal inoculation of 5 × 104 CFU of Mycobacterium bovis Ravenel in rabbits induced progressive subacute meningitis characterized by high cerebrospinal fluid (CSF) leukocytosis, protein influx, release of tumor necrosis factor (TNF), substantial meningeal inflammation, and mortality by day 28. Treatment with antituberculous drugs or with antituberculous drugs plus thalidomide improved the clinical course of disease somewhat and increased survival to about 50%. In contrast, treatment with antituberculous drugs in combination with IMiD3 limited pathological neurologic changes and resulted in marked improvement (73%) in survival. IMiD3 treatment was also associated with reduced leukocytosis in the CSF and significantly lower levels of TNF in CSF and plasma. Histologically, the meningeal inflammation in animals treated with antituberculous drugs plus IMiD3 was considerably attenuated compared to that of the other treatment groups. These results suggest a potential role for IMiD3 in the management of TBM in patients.
Tuberculous meningitis (TBM), the most severe form of Mycobacterium tuberculosis infection, continues to be associated with significant morbidity and mortality, particularly in children (12, 19, 21, 30, 32, 35). Unfortunately, bacteriologic cure of TBM with antituberculous chemotherapy does not necessarily prevent the severe neurologic sequelae of the infection. This is due to the inflammatory response to the mycobacteria, which causes vasculitis and infarcts, resulting in brain edema and increased intracranial pressure, leading to irreversible tissue damage in the central nervous system (CNS) (8, 12, 13, 23, 24). The inflammatory response is driven by cytokines, including tumor necrosis factor alpha (TNF-α), produced by mononuclear phagocytes in response to infection with M. tuberculosis (8, 13, 23, 24). Immunomodulatory drugs, such as corticosteroids, that reduce cytokine production, thereby dampening the inflammatory response, are often used in addition to the conventional antibiotic treatment (1, 4, 7, 22).
A rabbit model of acute TBM that can be used to study the course of the disease and to design immunomodulatory therapies has been described previously (34). It was observed that a combination of antituberculous drugs plus thalidomide as an adjunct immunomodulator dramatically improved the outcome and survival of rabbits with acute CNS mycobacterial infection. In that study, thalidomide treatment was associated with inhibition of TNF production and downregulation of the mononuclear leukocyte infiltrate into the brain. However, the model used in those studies of mycobacterial meningitis was acute and more rapidly progressive than the naturally occurring M. tuberculosis infection in humans. Also in those studies, the therapeutic interventions used were initiated prior to or simultaneously with experimental infection. Therefore, to more closely mimic the course of human TBM, we have developed a subacute model of mycobacterial CNS infection with a delayed onset of disease. This model enables us to initiate interventions after the expected occurrence of clinical signs and to evaluate the effect of different regimens of antituberculosis and immunomodulatory treatments, such as thalidomide, on the course of the disease.
Thalidomide is a drug with pleiotropic effects. The immunomodulatory effects of thalidomide are at least in part mediated through its ability to downregulate the pathogenic overproduction of TNF-α (5, 6). In vitro studies of TNF-α inhibition in human peripheral blood mononuclear cells have shown that thalidomide has a 50% inhibitory concentration of ∼194 μM (50.2 μg/ml). Preclinical animal studies have shown that 90 days of treatment with thalidomide result in no observed adverse event level (NOAEL) for mice at a dose of 3,000 mg/kg of body weight, whereas for rats, the NOAEL dose is 3,000 mg/kg for females and 30 mg/kg for males (31). New analogs of thalidomide are being produced that are more potent immunomodulators than the parent drug. One group of such analogs was shown to be more potent inhibitors of TNF-α, interleukin 1 beta (IL-1β), IL-6, and IL-12 secretion by monocytes (6, 9). Immunomodulatory drug 3 (IMiD3) is an analog of thalidomide that is under development for potential use as an immunomodulator for the treatment of inflammatory conditions in humans. In in vitro studies of TNF-α inhibition in human peripheral blood mononuclear cells, IMiD3 has a 50% inhibitory concentration of ∼100 nM (25.9 ng/ml) (5, 6). Repeated oral administration of IMiD3 to rats for 28 days shows an NOAEL dose of 300 mg/kg. Both thalidomide and IMiD3 have been tested for teratogenicity in the pregnant rabbit model. When administered to pregnant rabbits, thalidomide causes postimplantation loss and external and visceral fetal defects in the majority of fetuses. In comparison, IMiD3 at either 5, 15, or 25 mg/kg/day shows no evidence of teratogenicity at any dose level (D. Stirling, unpublished observations).
Here we report studies on the effect of combining antituberculous therapy either with thalidomide or with the thalidomide analog IMiD3 on disease manifestation in rabbits infected intrathecally with Mycobacterium bovis Ravenel. We examined the bacillary load in the CNS and other tissues, the inflammatory response in the CNS, the severity of the disease, and the survival of rabbits with subacute mycobacterial meningitis treated with these drug combinations.
MATERIALS AND METHODS
Infecting organism.
M. bovis strain Ravenel (TMC no. 401; Trudeau Mycobacterial Culture Collection), which is highly virulent in rabbits, was selected for this study (17, 33). Mycobacteria were kept frozen in aliquots until used. Before each experiment, a vial was thawed and subjected to brief ultrasonication 3 times for 10 s by using a water bath sonicator (Laboratory Supplies Co., Inc., Hicksville, N.Y.) to break up aggregates. An inoculum of 5 × 104 CFU in 0.1 ml of 0.9% sodium chloride (Abbott Laboratories, North Chicago, Ill.) was used for intrathecal infection.
Induction of meningitis.
New Zealand White rabbits, approximately 2.5 kg, of either sex (Covance Research Products Inc., Denver, Pa.) were used as described previously (34). All rabbits underwent a 1-week period of adaptation after arrival at the animal facility. After this period, a helmet of dental acrylic was attached to the calvaria of the rabbits to facilitate immobilization in a stereotaxic frame. At the start of each experiment, the animals were anesthetized with a combination of ketamine (Fort Dodge, Fort Dodge, Iowa) (35 mg/kg) and xylazine (Lloyd Laboratories, Shenandoah, Iowa) (5 mg/kg) administered intramuscularly (i.m.). The rabbits were then placed in a stereotaxic frame. A spinal needle was introduced into the cisterna magna, and 0.3 ml of cerebrospinal fluid (CSF) was withdrawn. Then, 0.1 ml of M. bovis Ravenel suspension was injected intracisternally. At 2 h after inoculation, a sample of CSF was obtained and plated onto 7H11 agar (Difco, Detroit, Mich.) to determine the number of CFU injected into the CSF of each rabbit. Four to six animals were infected simultaneously. After infection, animals were housed separately in individual cages. Thereafter, samples of CSF (obtained by insertion of a spinal needle) and blood (obtained from the ear artery) were collected weekly. The inflammatory response and the clinical course of infection were monitored for 4 weeks. At the end of each experiment, rabbits were euthanized by an overdose of pentobarbital. One half of the brain and a segment of the lung and spleen were collected aseptically, homogenized, and used for the evaluation of the bacillary load. The other half of the brain and the rest of the lung, liver, and spleen were removed and fixed in 10% buffered formalin acetate (vol/vol) (Fisher Chemical, Fairlawn, N.J.) and prepared for histopathologic examination. A similar protocol for sampling tissue was followed when rabbits were euthanized early because of severe signs of disease (see below) or if the rabbits died before sampling. This protocol was approved by the Rockefeller University Animal Care and Use Committee.
CSF samples.
Immediately after collection, CSF samples were analyzed for numbers of white blood cells (WBCs) by using a cell counter (Beckman Coulter, Inc., Miami, Fla.). Then, 100 μl of CSF was removed for determination of the bacterial load. The remainder of the CSF was centrifuged at 10,000 × g for 5 min, and the supernatant was stored at −70°C until tested for TNF and protein levels. Protein levels were determined by the bicinchoninic acid method (BCA kit; Pierce Chemical, Rockford, Ill.) as described by the manufacturer.
Blood samples.
Blood was collected at different time points from the main auricular artery with a heparinized syringe and then centrifuged at 10,000 × g. Plasma was separated and frozen at −70°C for TNF evaluation.
TNF assay.
TNF biologic activity in CSF and plasma was assayed by using murine L929 fibroblasts as targets in a cytotoxicity assay as described previously (3). This bioassay measures both TNF-α and TNF-β activity and does not distinguish between the two.
CFU assay.
Bacterial loads in the CSF, brains, lungs, and spleens of the infected rabbits were evaluated by plating 10-fold serial dilutions of the CSF and of the organ homogenates onto Middlebrook 7H11 agar plates (Difco). The plates were incubated at 37°C for 2 to 3 weeks. Colonies were counted, and results were expressed in CFU.
Histopathology.
Organ specimens were fixed in 10% formalin (Fisher Chemical). The brains were cut transversely in serial 2- to 3-mm-thick slices from the rostral side to the caudal side. Slices were selected representing the fore-, mid-, and hindbrains, embedded in paraffin (Tissue Prep-2; Fisher Scientific), and then sectioned and stained with hematoxylin and eosin and acid-fast stains (Ziehl-Neelsen).
Clinical scoring system.
To evaluate the clinical course of meningitis and neurological signs, we developed the following scoring system: 0, normal; 1, hyperesthesia, head tilt, and lethargy; 2, monoparesis; 3, hemiparesis and recumbency; 4, quadriplegia; 5, anorexia and CNS depression progressing to a moribund state and death. A score of 4 or 5 was considered an indication for euthanasia. The mean combined clinical score for each group of rabbits was calculated over time. The scoring system was developed with the help of Brian Corning and based on observation of the progression of neurologic and other clinical signs in infected rabbits.
Treatment regimens. (i) Antituberculosis chemotherapy (referred to as antibiotics).
Isoniazid (Nydrazid injection; Apothecon, Bristol-Myers Squibb, Princeton, N.J.) was administered at a dose of 30 mg/day i.m. Rifampin (Rifadin; Merrell Dow Pharmaceuticals, Kansas City, Mo.) was administered orally via a flexible rubber gastric tube at a dose of 30 mg/day in a water suspension. The dose of the antibiotics used in these experiments was selected to be the same as in humans, that is 15 mg/kg of body weight. Since M. bovis is primarily resistant to pyrazinamide, this drug was not included in the treatment. The antituberculosis chemotherapy was started on day 17 after infection and continued to day 28 postinfection.
(ii) Immunomodulatory drugs.
Thalidomide (α-N-phthalimido-glutarimide) and an analog, IMiD3 (α-3-aminophthalimido-glutarimide), were obtained from Celgene Corporation, Warren N.J. In rats, both drugs are absorbed efficiently when administered by gavage, but absorption is somewhat reduced in fed animals. The maximum concentration of drug in serum and terminal elimination half-life have been shown to increase with drug dose (31; D. Stirling, unpublished observations). Clearance and volume distribution values at all dose levels are high, indicating good tissue distribution and rapid clearance. Some changes in red blood cell parameters and increases in blood creatinine and urea, as well as reduced weight gain, have been noted at very high (500 mg/kg/day) doses. Urinary excretion is high, with overall recovery of unchanged drug over 48 h being approximately 67% at all dose levels. Both thalidomide and IMiD3 show no evidence of mutagenic potential as evaluated in assays of reverse mutations in bacteria, induction of chromosome aberrations in human peripheral blood lymphocytes, or induction of mutations in mouse lymphoma L5178Y cells. While thalidomide is a potent teratogen, IMiD3 has shown no evidence of teratogenicity in the pregnant rabbit model (31; D. Stirling, unpublished observations).
(iii) Combination therapy with thalidomide and IMiD3.
In addition to isoniazid and rifampin (antituberculosis treatment) some of the rabbits received thalidomide. Thalidomide was prepared as a suspension in sterile 0.9% sodium chloride and administered by a gastric tube. The dose was 200 mg/day/rabbit. Treatment was initiated on day 16 after infection and continued through day 28 postinfection. A third group of rabbits received a combination of isoniazid and rifampin plus IMiD3 (also known as analog CI-B) (6, 9). The dose was 50 mg/day/rabbit, administered as a suspension in sterile 0.9% sodium chloride via a gastric tube. Treatment was started on day 16 after infection and continued through day 28 postinfection.
Quantitation of thalidomide and IMiD3 in CSF.
CSF samples were collected at different time points after a single dose of 200 mg of thalidomide or 50 mg of IMiD3. Solid-phase extraction was performed to purify the samples. After centrifugation at 8,500 rpm (Eppendorf centrifuge 5415; Brinkman Instruments, Inc., Westbury, N.Y.) for 5 min to precipitate any residue, the CSF supernatants were loaded onto OASIS HLB extraction cartridges (Waters Corporation, Milford, Mass.) and run through. The cartridges retaining the analytes were eluted twice with 0.5 ml of methanol-acetonitrile-acetic acid (50:50:0.1%) and collected. The elutes were evaporated to dryness under a stream of 100% nitrogen. The residues were dissolved in 0.2 ml of reconstitution solution (water:acetonitrile:acetic acid [90:10:0.1%]) and analyzed by liquid chromatography coupled with tandem mass spectrometry by using a Waters 2790 liquid chromatograph with an XTerra MS column (C18, 5 μm, 2.1 by 50 mm; Waters Corporation) for the separation. The mobile-phase (acetonitrile:water:acetic acid [100:25:0.2]) flow rate was 0.2 ml/min, and the run time of each sample was 4.5 min. The Quattro liquid chromatography tandem mass spectrometer (Micromass, Beverly, Mass.) was operated in electrospray (positive ion) mode with multiple-reaction monitoring. The ions monitored were 260.0 → 149.0 for IMiD3 and 259 → 84 for thalidomide. Quantitation was based on a standard calibration curve by comparison of the chromatographic peak areas for thalidomide, IMiD3, and internal standards. The major operating parameters were nebulizer gas flow (110 liters/h), desolvation gas flow (640 liters/h), cone voltage (30.0 V), collision energy (15.0 V), dwell time (0.5 s), and injection volume (50 μl). Reconstitution solution and untreated rabbit CSF were used as controls.
Statistical analysis.
The independent Student t test or the Mann-Whitney test for nonparametric independent data was used for analysis (SPSS software). A Kruskal-Wallis test was used to determine the statistical significance of the differences in severity of clinical signs of disease of rabbits (36). Kaplan-Meier analysis was used to determine the statistical significance of the differences in survival time of rabbits. A P value of <0.05 was considered significant.
RESULTS
Response of rabbits to intrathecal infection with M. bovis Ravenel.
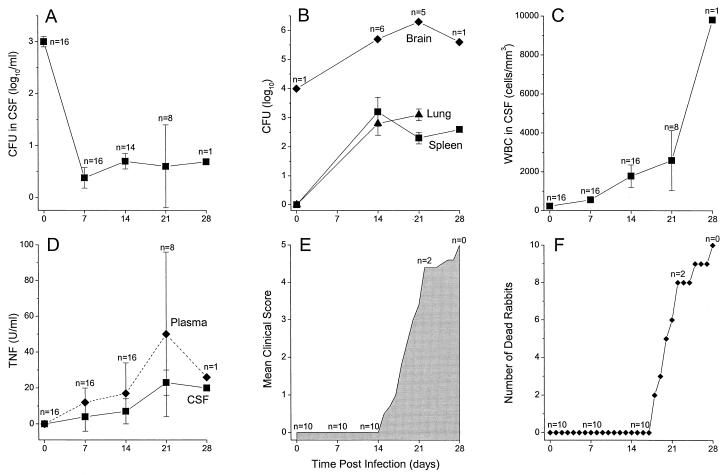
To achieve a subacute CNS infection, rabbits were inoculated intracisternally with one dose of 5 × 104 CFU of M. bovis Ravenel, a virulent mycobacterium in rabbits (16, 17). The animals were monitored for clinical, immunologic, and pathological parameters for 4 weeks after the initial infection. CSF and blood samples were obtained weekly. At 2 h after injection of the bacilli into the cisterna magna (time zero), there was a bacillary load of 3 log10 CFU/ml in the CSF (Fig. 1A). Microscopic examination of cytospin preparations of CSF at time zero revealed that the bacilli were within monocytes. This was confirmed by the CFU assay showing that the bacilli were in the cell pellet and not free in the CSF. By 7 days postinfection the number of bacilli had dropped to fewer than 0.5 log10/ml of CSF and persisted at about this level for the duration of the experiment (28 days). Protein levels in the CSF were monitored as an indicator of onset of infection-induced inflammation and also of breach of the blood brain barrier (BBB). These were within the normal range (<1 mg/ml) during the first week postinfection. By day 14, the levels had increased to 2.5 mg/ml, indicating breach of the BBB (Table 1). Thereafter, protein levels remained significantly elevated (>1 mg/ml) for the duration of the study.
FIG. 1.
Response of rabbits infected intracisternally with 5 × 104 CFU of M. bovis Ravenel and monitored for 28 days. (A) Numbers (CFU per milliliter) of M. bovis Ravenel in the CSF. (B) Numbers (CFU per organ) of M. bovis Ravenel in the tissues. (C) WBC density (cells per cubic millimeter) in CSF. (D) TNF concentration (units per milliliter) in CSF and plasma. (E) Severity of signs of disease (clinical score) of infected rabbits. (F) Mortality of infected rabbits. All values are means ± standards errors of the means for the number of animals available for evaluation at each time point.
TABLE 1.
Protein levels in CSF of rabbits infected with M. bovis Ravenel
| Time postinfection (days) | Level of protein in CSF (mg/ml)a | P valueb |
|---|---|---|
| 0 | 0.72 ± 0.07 | |
| 7 | 0.82 ± 0.20 | |
| 14 | 2.61 ± 0.9 | 0.03 |
| 21 | 1.59 ± 0.15 | 0.05 |
Results are means ± standard errors of the means for 8 rabbits at 0 days postinfection and 6 rabbits at 7, 14, and 21 days postinfection.
P value compared to time zero.
One of the main hallmarks of TBM is leukocytosis in the CSF. The number of leukocytes seen in the CSF during the first week of infection was within the normal range (<103/mm3) (Fig. 1C). Significantly elevated leukocytosis in the CSF was apparent from day 7 on (P = 0.001), and leukocytosis remained significantly elevated throughout the experiment. The differential WBC count in the CSF revealed that >90% of the leukocytes were mononuclear. In addition, TNF levels increased significantly both in the CSF (P = 0.01 at day 14) and systemically (in plasma) (P < 0.001 at day 14) (Fig. 1D). By day 21 the level of TNF in CSF and plasma peaked and remained elevated until the death of the animals. These inflammatory parameters (CSF protein, leukocytosis, and TNF) correlated with the clinical course of mycobacterial meningitis. For the first 2 weeks postinfection, the rabbits gained weight normally. However, by day 15 there was a significant (P = 0.01) increase in clinical score. The third week of infection was critical: most of the infected rabbits lost appetite, started losing weight, and developed severe neurologic signs such as somnolence or irritability, loss of coordination, pareses, and paralyses (P < 0.001) (Fig. 1E). Rabbits started dying after day 17 of infection (Fig. 1F). By day 28 all the rabbits were either moribund or dead.
At the 2-h (time zero) time point, about 20% of the bacillary inoculum was within brain tissue as evaluated by the CFU assay of brain homogenates (Fig. 1B). At days 21 and 28 postinfection, high numbers of bacilli (about 106) were recovered from the brains of those infected rabbits that had survived to these time points. Mycobacteria were also recovered in significant numbers from the lungs and spleens of infected animals at 3 weeks postinfection, suggesting that breach of the BBB had allowed the infection to spread from the CNS to the peripheral organs (Fig. 1B).
Pharmacokinetics of thalidomide and IMiD3.
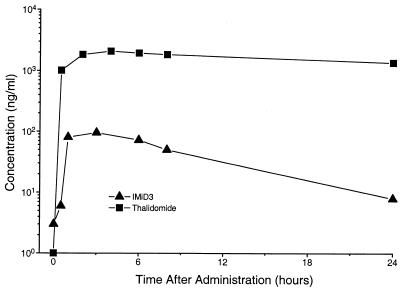
To establish whether thalidomide or IMiD3 administered by gavage penetrated into the CNS, we tested CSF for the presence of drugs at various time points. Oral administration of a single dose of thalidomide (200 mg/rabbit) to naive rabbits showed very fast penetration of the drug into the CNS. The concentration in the CSF ranged from 500 to 1,500 ng/ml at 30 min and increased to 1,000 to 3,400 ng/ml by 4 h (Fig. 2). By 24 h (the time of the next dose delivery), CSF levels of thalidomide were still about 1,400 ng/ml. Administration of a single dose of IMiD3 (50 mg/rabbit) resulted in a 10-fold-lower level of drug in CSF. By 24 h the concentration of IMiD3 had dropped to about 10 ng/ml (Fig. 2).
FIG. 2.
Pharmacokinetics in rabbit CSF after a single oral dose of 200 mg of thalidomide or 50 mg of IMiD3. Levels of the drugs in the CSF are shown in nanograms per milliliter. Results are means of observations obtained from 2 rabbits per treatment.
Effect of treatment on bacillary load in tissues of infected rabbits.
Previous studies showed a potential role for thalidomide as an adjunctive immune modulator in acute TBM (34). We therefore examined the effect of thalidomide in the present subacute model of meningitis. The onset of clinical signs of disease and death in infected rabbits was usually noted at about day 14 or 15 postinfection (Fig. 1E). In order to mimic the pattern of therapeutic intervention, which in human TBM is initiated after the onset of symptoms, we started immunomodulatory and antibiotic therapy on days 16 and 17 postinfection, respectively. Infected rabbits were divided into four groups. The first group received 30 mg of isoniazid/day i.m. and 30 mg of rifampin/day orally. A parallel group of rabbits received 200 mg of thalidomide/day orally in addition to the antituberculosis drugs. A third group was treated with 50 mg of IMiD3/day and antituberculosis drugs. Control animals (fourth group) were infected but not treated with any drugs.
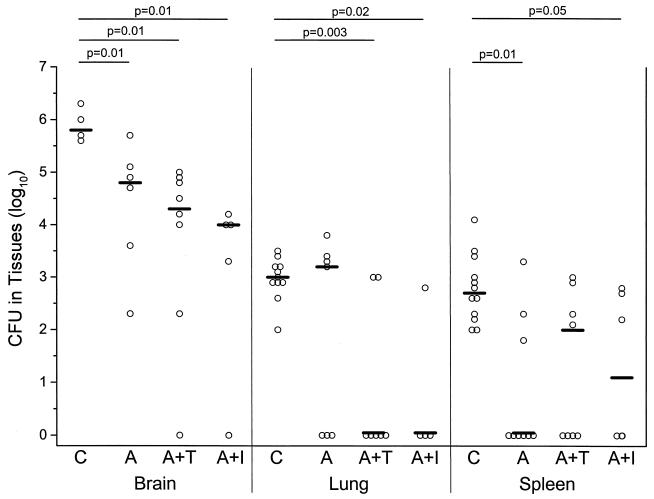
To measure the efficacy of antituberculous treatment in the presence or absence of immunomodulatory adjunctive therapy, mycobacteria were cultured from the brain, lungs, and spleens of rabbits at necropsy. Significantly reduced numbers of CFU (1 to 2 log10 lower) were noted in the brains of rabbits in the three treatment groups compared to the control untreated rabbits (Fig. 3). Although somewhat lower CFU were seen in the presence of immunomodulatory drugs, there was no significant difference between the group that received antibiotics only or the groups that received antibiotics together with either thalidomide or IMiD3. In the lungs, a significant reduction in the bacterial load was noted only in the groups that received antibiotics together with either thalidomide or IMiD3 (Fig. 3). In the spleen, a significant reduction in CFU was noted in response to antibiotic treatment as well as to antibiotic treatment combined with IMiD3 treatment (Fig. 3).
FIG. 3.
Effect of treatment of infected rabbits on bacterial load (CFU in tissues) in brains, lungs, and spleens at necropsy. Control rabbits were infected and not treated (C). Another group of rabbits was infected and treated with antibiotics (isoniazid plus rifampin) (A). A third group of rabbits was infected and treated with the same antibiotics combined with thalidomide (A+T). The fourth group was infected and treated with the same antibiotics combined with IMiD3 (A+I). Treatment was initiated on days 16 and 17 as described in Materials and Methods and continued to day 28 postinfection. Results are for individual animals. The bar denotes the median for the animals tested. Statistically significant differences are denoted by the P values shown at the tops of the graphs.
Effect of treatment on inflammatory response in CSF of rabbits with subacute mycobacterial meningitis.
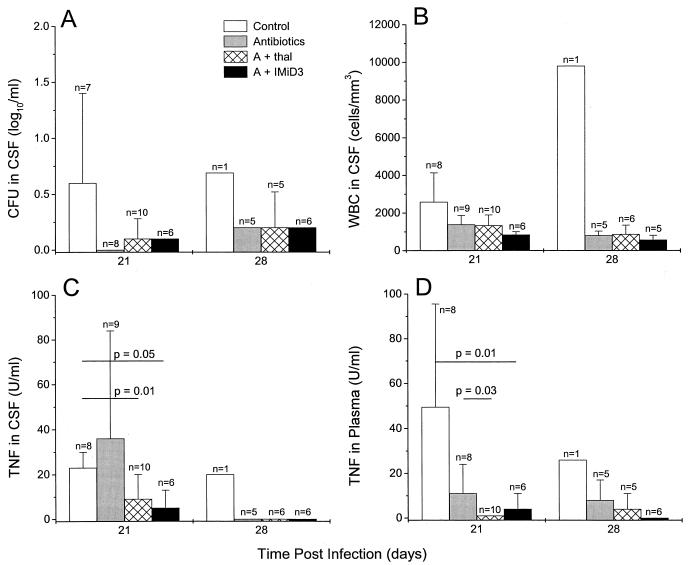
To measure the effect of treatment on the number of bacilli in the CSF, mycobacteria were cultured from the CSF during therapy and quantitated by the CFU assay. Reduced numbers of CFU in CSF were observed in all treated rabbits on day 21 as well as on day 28, compared to the control group in which mycobacteria persisted (Fig. 4A). Leukocytosis in the CSF was also reduced following treatment; the decrease was somewhat more pronounced in the animals treated with the antibiotics plus IMiD3 (Fig. 4B). Treatment with antibiotics plus thalidomide or IMiD3 was also associated with a significant inhibition of TNF production: on day 21 little, if any, TNF was detected in the CSF or plasma of rabbits receiving a combination of antibiotics and an immunomodulatory drug (Fig. 4C and D). In contrast, in animals treated with antibiotics alone, increased levels of TNF in the CSF were noted on day 21. These high levels usually preceded the death of the infected rabbits.
FIG. 4.
Effect of treatment of infected rabbits on inflammatory response in CSF. All rabbits were infected intracisternally with M. bovis Ravenel. Control animals received no treatment (white bars), one group of rabbits was treated with antibiotics only (gray bars), another group received 200 mg of thalidomide/day plus antibiotics (hatched bars), and the fourth group of rabbits was treated with antibiotics combined with IMiD3 (black bars). Treatment was initiated on days 16 and 17 postinfection and continued to day 28 postinfection. (A) Numbers (CFU per milliliter) of M. bovis Ravenel in CSF. (B) WBC density (cells per cubic millimeter) in CSF. (C) TNF concentration (units per milliliter) in CSF. (D) TNF concentration (units per milliliter) in plasma. All values are means ± standard errors of the means for the number of animals indicated in each group.
Effect of treatment on course of disease and survival of infected rabbits.
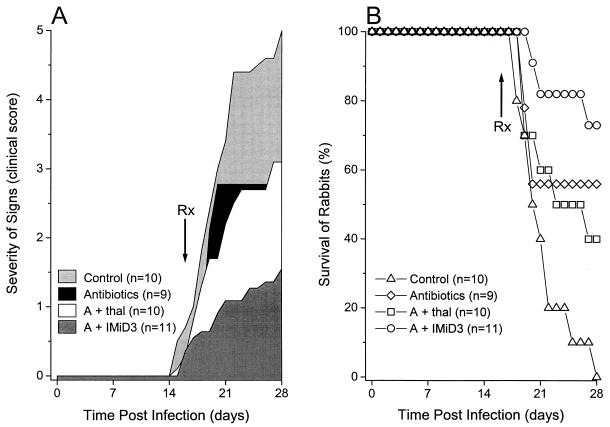
Using a clinical scoring system we recently developed (see Material and Methods), we evaluated the effect of treatment on the progression of disease. Infected rabbits treated with antituberculous drugs or a combination of antituberculous drugs plus thalidomide developed less severe signs of disease than rabbits from the infected untreated control group. However, these differences were not statistically significant (P > 0.05) (Fig. 5A). The majority of animals treated with antibiotics plus IMiD3 showed much reduced signs of disease with only mild and transitory neurological signs. Only this group of animals had statistically significant improvement in the clinical score compared to the untreated control rabbits (P = 0.01).
FIG. 5.
Clinical course and survival of infected rabbits. (A) Severity of signs (see “Clinical scoring system” in Material and Methods). Control rabbits were infected but not treated, one group of rabbits was infected and treated with antibiotics (isoniazid plus rifampin) (antibiotics), another group of rabbits was infected and treated with the same antibiotics combined with thalidomide (A + thal), and a fourth group of rabbits was infected and treated with the same antibiotics combined with IMiD3 (A + IMiD3). Results are expressed as the area under the curve for the numbers of animals indicated. (B) Survival of infected rabbits. Control rabbits were infected but not treated, one group of rabbits was infected and treated with antibiotics only, another group of rabbits was infected and treated with antibiotics combined with thalidomide, and a fourth group of rabbits was infected and treated with antibiotics combined with IMiD3. The number of animals in each group is indicated. Significant effects on the survival of rabbits were observed with the antibiotics plus IMiD3 in comparison to the control group (Kaplan-Meier analysis, P < 0.05).
A striking difference was observed in the 28-day survival of infected rabbits from the different treatment groups. Improved survival was observed in the groups treated with antibiotics alone or with antibiotics plus thalidomide (60 or 40%, respectively) compared to that of the control rabbits (Fig. 5B). However, this was not statistically significant (P = 0.2 and P = 0.3, respectively). The highest rate of survival (73%) was noted in the rabbits that received combined antibiotic therapy with IMiD3 (P = 0.008). Among those animals that survived, rabbits treated with antibiotics plus thalidomide or IMiD3 either had no signs of disease (clinical score = 0) or only mild neurologic signs (clinical score = 1 or 2) (Table 2). In contrast, one of the five surviving animals treated with antibiotics alone had a clinical score of 3, indicating severe signs of brain damage (Table 2).
TABLE 2.
Severity of disease in mycobacterium-infected rabbits surviving to day 28
| Treatment | No. of survivors/total no. of rabbits | No. of rabbits with clinical score of:
|
||||
|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | ||
| None (control) | 0/10 | 0 | 0 | 0 | 0 | 0 |
| Antibotics | 5/9 | 3 | 0 | 1 | 1 | 0 |
| Antibiotics + thalidomide | 4/10 | 3 | 1 | 0 | 0 | 0 |
| Antibiotics + IMiD3 | 8/11 | 7 | 0 | 1 | 0 | 0 |
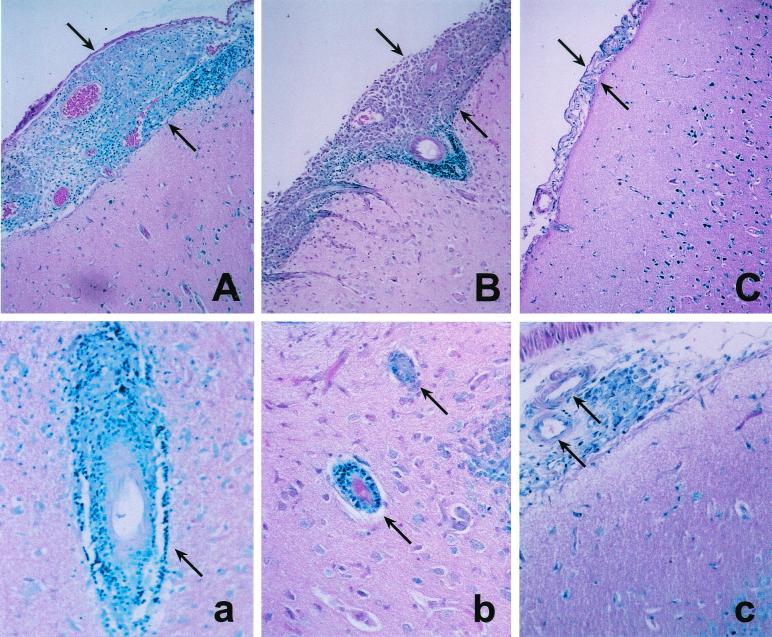
To investigate the underlying pathological mechanism of the brain damage seen in infected rabbits, we carried out histologic examinations of the brain at the time of euthanasia. Infected rabbits that were untreated did not survive beyond the first 4 weeks of infection. Rabbits treated with antituberculous drugs alone demonstrated severe inflammation of the leptomeninges, with distention of the subarachnoid space by large numbers of macrophages and lymphocytes (Fig. 6A). In addition, severe vasculitis was noted, characterized by mononuclear cells accumulating around blood vessels within the brain parenchyma (Fig. 6a). Antituberculous treatment combined with thalidomide resulted in some attenuation of the inflammation in the brain (Fig. 6B). However, the subarachnoid space was still distended and infiltrated, and vasculitis was prominent (Fig. 6b). In contrast, when the infected rabbits were treated with antituberculous drugs plus IMiD3, the inflammatory process was dramatically reduced (Fig. 6C). Only minimal leukocyte infiltration was seen in the subarachnoid space. Although some residual mononuclear cells were apparent adjacent to the vasculature in the brain, the vessels themselves appeared normal (Fig. 6c).
FIG. 6.
Histology of the brain and meninges of surviving rabbits 4 weeks after intrathecal infection with M. bovis Ravenel. Infected rabbits were treated with antituberculous drugs (A and a), antibiotics plus 200 mg of thalidomide/day (B and b), or antibiotics plus 50 mg of IMiD3/day (C and c). Distension of the subarachnoid space is indicated by the arrows. Panels A, B, and C show representative areas of the meninges. Panels a, b, and c show representative vasculature within the parenchyma of the brain. Few, if any, acid-fast organisms were seen in any of the sections. The sections were stained with Ziehl-Neelsen stain. Magnification, ×10 (for panels A, B, and C) and ×20 (for panels a, b, and c).
DISCUSSION
In this study we describe a rabbit model of subacute mycobacterial meningitis that closely mirrors TBM in human patients. To fully mimic the management of human TBM, where onset of symptoms is followed by therapy, we initiated treatment after the appearance of neurologic signs in the experimental animals. We used this model to evaluate a new immunomodulatory drug, IMiD3, which, because of its ability to limit inflammation in the CNS, protected the infected animals from severe neurologic damage and death.
Interestingly, antituberculous therapy alone did not improve outcome significantly despite reductions in bacillary load. Rather, even after initiation of therapy, about 50% of the treated animals progressed to severe neurologic signs and death (Fig. 5). This may be due to antibiotic killing of mycobacteria and release of cell wall products which further increased inflammation in the CNS, resulting in damage of the vasculature accompanied by infarcts, brain edema, and necrosis. In our experimental system, we noted that TNF levels in the CSF were increased after the initiation of therapy. TNF levels were higher at day 21 in antibiotic-treated animals than in untreated control rabbits and were associated with severe clinical signs and mortality. There are several reports of a paradoxical response to antituberculous drugs in humans, whereby initiation of antibiotic therapy was observed to lead to an initial worsening of the clinical state of patients with TBM (2, 10, 15, 20).
In order to reduce the inflammation accompanying human TBM, standard antituberculosis therapy usually includes anti-inflammatory treatment, such as corticosteroids. However, there are conflicting reports on the effectiveness of corticosteroids in the treatment of tuberculosis and TBM in patients (11, 18, 28, 29). Previous studies of the rabbit model of acute TBM had suggested a possible role for the TNF-α inhibitor thalidomide as an adjunct anti-inflammatory drug (34). However, treatment with thalidomide did not achieve efficient enough protection against the inflammatory sequelae of TBM in experimental animals. The results reported in the present study indicate that the thalidomide analog IMiD3 may be more effective than thalidomide. Pharmacokinetic analyses show that although delivered in a fourfold-lower dose than the parent drug thalidomide, IMiD3 crosses the BBB and persists in the CSF in effective concentrations. The analog does not appear to interfere with the penetration into the CSF of antituberculous drugs, since the number of bacilli in the CSF was not increased in this group of rabbits compared to animals treated with antibiotics alone. Treatment with the analog did, however, result in improved clinical outcome and survival.
The mechanism of action of thalidomide and its analogs is currently receiving much attention. Thalidomide appears to downregulate production of TNF-α and possibly other proinflammatory cytokines produced by stimulated monocytes. Thalidomide also acts as a costimulatory signal to T cells, inducing increased T-cell proliferation and enhanced gamma interferon and IL-2 production (5, 14). The new thalidomide analogs, and IMiD3 in particular, have been shown to be even more-potent inhibitors of monocyte TNF-α, IL-1β, and IL-6 production and to be more-effective inducers of Th1-type cytokines (6). In addition, a recent report has shown that thalidomide and IMiD3 augment the number of NK cells in vivo and NK cell cytotoxic activity in vitro (9). In the present study, it appears that IMiD3 inhibits TNF, and this is associated with reduced pathology in the brain. Whether IMiD3 also induces increased Th1 T-cell responses in this experimental system is yet to be established.
In humans, adjunctive thalidomide therapy of a tuberculous abscess and of stage II childhood TBM was initially reported to be safe and well tolerated (26, 27). However, a subsequent randomized, placebo-controlled trial of thalidomide therapy, administered for 1 month in addition to standard antituberculosis therapy and steroids in children with severe TBM (stages II and III), was terminated because thalidomide therapy was associated with a worse neurological outcome after 1 month than placebo therapy (25). This was noted particularly in children with the most severe disease (stage III). No difference in clinical outcome between thalidomide- and placebo-treated patients was noted at 6 months (25). This patient-based study underscored the importance of developing better drugs, which may be safer for clinical use. Indeed, our experiments indicate that a combination of antituberculous drugs plus the thalidomide analog IMiD3 reduces the inflammatory response and markedly improves the clinical outcome and survival of rabbits with TBM, despite the lower delivery dose and lower levels of drug in CSF. In addition, IMiD3 appears to not be teratogenic in the pregnant rabbit model (D. Stirling, unpublished observations). Together, these results suggest a potential role for IMiD3 in the treatment of human TBM.
Acknowledgments
This work was supported by Public Health Service grants AI 22616 (to G.K.) and CA09673 (to L.T.) from the National Institutes of Health and by the Stony Wold-Herbert Fund.
We thank Willem A. Hanekom for valuable suggestions, Amy Bergtold for technical assistance, Judy Adams for preparation of the figures, and Marguerite Nulty for secretarial work. We thank Knut M. Wittkowski for help with statistical analyses.
REFERENCES
- 1.Alzeer, A. H., and J. M. FitzGerald. 1993. Corticosteroids and tuberculosis: risks and use as adjunct therapy. Tuber. Lung Dis. 74:6-11. [DOI] [PubMed] [Google Scholar]
- 2.Andrade Filho, A., A. G. Gomes, A. C. De Lemos, M. C. Neves, Y. M. Souza, S. L. Pereira, A. P. De Souza, and P. L. Dos Santos. 1999. Paradoxical expansive lesions of cerebral tuberculosis during antitubercular drug therapy. Arq. Neuro-psiquiatr. 57:471-475. [DOI] [PubMed] [Google Scholar]
- 3.Burroughs, M. H., L. Tsenova-Berkova, K. Sokol, J. Ossig, E. Tuomanen, and G. Kaplan. 1995. Effect of thalidomide on the inflammatory response in cerebrospinal fluid in experimental meningitis. Microb. Pathog. 19:245-255. [DOI] [PubMed] [Google Scholar]
- 4.Cisneros, J. R., and K. M. Murray. 1996. Corticosteroids in tuberculosis. Ann. Pharmacother. 30:1298-1303. [DOI] [PubMed] [Google Scholar]
- 5.Corral, L. G., P. A. J. Haslett, G. W. Muller, R. Chen, L. M. Wong, C. J. Ocampo, R. T. Patterson, D. I. Stirling, and G. Kaplan. 1999. Differential cytokine modulation and T cell activation by two distinct classes of thalidomide analogs that are potent inhibitors of TNF-alpha. J. Immunol. 163:380-386. [PubMed] [Google Scholar]
- 6.Corral, L. G., and G. Kaplan. 1999. Immunomodulation by thalidomide and thalidomide analogs. Ann. Rheum. Dis. 58(Suppl. 1):I107-I113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coyle, P. K. 1999. Glucocorticoids in central nervous system bacterial infection. Arch. Neurol. 56:796-801. [DOI] [PubMed] [Google Scholar]
- 8.Dastur, D. K., D. K. Manghani, and P. M. Udani. 1995. Pathology and pathogenetic mechanisms in neurotuberculosis. Radiol. Clin. N. Am. 33:733-752. [PubMed] [Google Scholar]
- 9.Davies, F. E., N. Raje, T. Hideshima, S. Lentzsch, G. Young, Y. T. Tai, B. Lin, K. Podar, D. Gupta, D. Chauhan, S. P. Treon, P. G. Richardson, R. L. Schlossman, G. J. Morgan, G. W. Muller, D. I. Stirling, and K. C. Anderson. 2001. Thalidomide and immunomodulatory derivatives augment natural killer cell cytotoxicity in multiple myeloma. Blood 98:210-216. [DOI] [PubMed] [Google Scholar]
- 10.Donald, P. R., J. F. Schoeman, N. Beyers, E. D. Nel, S. M. Carlini, K. D. Olsen, and G. H. McCracken. 1995. Concentrations of interferon gamma, tumor necrosis factor alpha, and interleukin-1 beta in the cerebrospinal fluid of children treated for tuberculous meningitis. Clin. Infect. Dis. 21:924-929. [DOI] [PubMed] [Google Scholar]
- 11.Dooley, D. P., J. L. Carpenter, and S. Rademacher. 1997. Adjunctive corticosteroid therapy for tuberculosis: a critical reappraisal of the literature. Clin. Infect. Dis. 25:872-887. [DOI] [PubMed] [Google Scholar]
- 12.Farinha, N. J., K. A. Razali, H. Holzel, G. Morgan, and V. M. Novelli. 2000. Tuberculosis of the central nervous system in children: a 20-year survey. J. Infect. 41:61-68. [DOI] [PubMed] [Google Scholar]
- 13.Garg, R. K. 1999. Tuberculosis of the central nervous system. Postgrad. Med. J. 75:133-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haslett, P. A. J., L. G. Corral, M. Albert, and G. Kaplan. 1998. Thalidomide costimulates primary human T lymphocytes, preferentially inducing proliferation, cytokine production, and cytotoxic responses in the CD8+ subset. J. Exp. Med. 187:1885-1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kumar, S., V. Puri, M. M. Mehndiratta, S. Gupta, A. Bhutani, and C. Sharma. 1995. Paradoxical response to antitubercular drugs. Indian J. Pediatr. 62:695-701. [DOI] [PubMed] [Google Scholar]
- 16.Lurie, M. B. 1964. Native organ and species resistance to tuberculosis: preliminary studies, p. 3-29. In M. B. Lurie (ed.), Resistance to tuberculosis: experimental studies in native and acquired defensive mechanisms. Harvard University Press, Cambridge, Mass.
- 17.Lurie, M. B., S. Abramson, J. B. Swartz, and A. G. Heppleston. 1950. An evaluation of the method of quantitative airborne infection and its use in the study of the pathogenesis of tuberculosis. Am. Rev. Tuberc. 61:765-797. [PubMed] [Google Scholar]
- 18.Miyoshi, Y., S. Noda, H. Murai, and H. Itoh. 2000. Repeated deterioration of tuberculous meningitis due to a reduction in the corticosteroid dosage during chemotherapy. Rinsho Shinkeigaku 40:1018-1022. [PubMed] [Google Scholar]
- 19.Paganini, H., F. Gonzalez, C. Santander, L. Casimir, G. Berberian, and M. T. Rosanova. 2000. Tuberculous meningitis in children: clinical features and outcome in 40 cases. Scand. J. Infect. Dis. 32:41-45. [DOI] [PubMed] [Google Scholar]
- 20.Parons, M. 1988. Treatment, p. 32-60. In M. Parsons (ed.), Tuberculous meningitis. Oxford University Press, New York, N.Y.
- 21.Porkert, M. T., M. Sotir, P. Parrott-Moore, and H. M. Blumberg. 1997. Tuberculous meningitis at a large inner-city medical center. Am. J. Med. Sci. 313:325-331. [DOI] [PubMed] [Google Scholar]
- 22.Prasad, K., J. Volmink, and G. R. Menon. 2000. Steroids for treating tuberculous meningitis. Cochrane Database Syst. Rev. 3:CD002244. [DOI] [PubMed] [Google Scholar]
- 23.Ramilo, O., X. Saez-Llorens, J. Mertsola, H. Jafari, K. D. Olsen, E. J. Hansen, M. Yoshinaga, S. Ohkawara, H. Nariuchi, and G. H. McCracken, Jr. 1990. Tumor necrosis factor alpha/cachectin and interleukin 1 beta initiate meningeal inflammation. J. Exp. Med. 172:497-507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saez-Llorens, X., O. Ramilo, M. M. Mustafa, J. Mertsola, and G. H. McCracken, Jr. 1990. Molecular pathophysiology of bacterial meningitis: current concepts and therapeutic implications. J. Pediatr. 116:671-684. [DOI] [PubMed] [Google Scholar]
- 25.Schoeman, J. F. 2000. Thalidomide therapy in childhood tuberculous meningitis. J. Child Neurol. 15:838.. [DOI] [PubMed] [Google Scholar]
- 26.Schoeman, J. F., A. Ravenscroft, and H. B. Hartzenberg. 2001. Possible role of adjunctive thalidomide therapy in the resolution of a massive intracranial tuberculous abscess. Child's Nerv. Syst. 17:370-372. [DOI] [PubMed] [Google Scholar]
- 27.Schoeman, J. F., P. Springer, A. Ravenscroft, P. R. Donald, L. G. Bekker, A. J. van Rensburg, W. A. Hanekom, P. A. Haslett, and G. Kaplan. 2000. Adjunctive thalidomide therapy of childhood tuberculous meningitis: possible anti-inflammatory role. J. Child Neurol. 15:497-503. [DOI] [PubMed] [Google Scholar]
- 28.Schoeman, J. F., L. E. Van Zyl, J. A. Laubscher, and P. R. Donald. 1997. Effect of corticosteroids on intracranial pressure, computed tomographic findings, and clinical outcome in young children with tuberculous meningitis. Pediatrics 99:226-231. [DOI] [PubMed] [Google Scholar]
- 29.Senderovitz, T., and K. Viskum. 1994. Corticosteroids as adjuvants in the treatment of tuberculosis. Ugeskr. Laeg. 156:5268-5272. [PubMed] [Google Scholar]
- 30.Starke, J. R. 1999. Tuberculosis of the central nervous system in children. Semin. Pediatr. Neurol. 6:318-331. [DOI] [PubMed] [Google Scholar]
- 31.Teo, S. K., N. J. Trigg, M. E. Shaw, J. M. Morgan, and S. D. Thomas. 1999. Subchronic toxicity of thalidomide in rodents after 13 weeks of oral administration. Int. J. Toxicol. 18:337-352. [Google Scholar]
- 32.Topley, J. M., S. Bamber, H. M. Coovadia, and P. D. Corr. 1998. Tuberculous meningitis and co-infection with HIV. Ann. Trop. Paediatr. 18:261-266. [DOI] [PubMed] [Google Scholar]
- 33.Tsenova, L., V. H. Freedman, and G. Kaplan. 2000. Experimental tuberculous meningitis, p. 107-118. In P. K. Peterson and J. S. Remington (ed.), New concepts in the immunopathogenesis of CNS infections. Blackwell Science, Oxford, United Kingdom.
- 34.Tsenova-Berkova, L., K. Sokol, V. H. Freedman, and G. Kaplan. 1998. A combination of thalidomide plus antibiotics protects rabbits from mycobacterial meningitis associated death. J. Infect. Dis. 177:1563-1572. [DOI] [PubMed] [Google Scholar]
- 35.Walia, R., and W. Hoskyns. 2000. Tuberculous meningitis in children: problem to be addressed effectively with thorough contact tracing. Eur. J. Pediatr. 159:535-538. [DOI] [PubMed] [Google Scholar]
- 36.Wittkowski, K. M. 1988. Friedman-type statistics and consistent multiple comparisons for unbalanced designs. J. Am. Stat. Assoc. 83:1163-1170. [Google Scholar]