Abstract
Interpretive agreements among the results of fluconazole broth microdilution tests, Etests, and disk diffusion tests were documented by evaluating 495 Candida spp. Microdilution reference test results were in agreement with 96% of the Etest results; most discrepancies were minor differences. Fluconazole resistance of Candida krusei strains often required a full 48 h of incubation in order to be observed by the standard method. For the disk diffusion tests that were performed on Mueller-Hinton agar with glucose and methylene blue, 97% of results were in agreement with those of the reference test, especially when zones of inhibition were measured after the first 24 h of incubation. Some Candida glabrata isolates failed to grow satisfactorily until a full 48 h of incubation was completed. Precision was determined by testing 50 selected isolates in triplicate in each of three laboratories. The reproducibility of results of disk diffusion tests was comparable to that of the reference method. With all procedures, determination of test results was particularly challenging with some strains, and new methods are needed in order to improve endpoint definition.
Compromised patients, such as those infected with human immunodeficiency virus, are often colonized by and/or infected with fungi, especially Candida spp. Consequently, they frequently receive antifungal agents such as fluconazole for relatively long periods of time during hospitalization. Prolonged exposure to an azole such as fluconazole can select strains with diminished susceptibilities (6, 7), and consequently, increasing doses may be necessary. For this reason, the susceptibilities of isolates recovered from compromised patients receiving prolonged fluconazole treatment should be monitored in order to determine when strains with decreased susceptibilities have been selected (3). In order to do this in a cost-effective way, simple, inexpensive testing procedures are needed, but such tests must be accurate and precise. The purpose of this report is to document the relative levels of accuracy and precision of three procedures for testing fluconazole: broth microdilution, Etest, and disk diffusion. The accuracy of each procedure was assessed by comparing the results to those obtained by the 48-h microdilution reference method of the National Committee for Clinical Laboratory Standards (NCCLS) (8). Precision was measured by performing replicate tests in three separate laboratories.
MATERIALS AND METHODS
Microorganisms.
For this study, M. A. Pfaller selected 495 clinical isolates of Candida spp. for which there was a broad range of fluconazole MICs. The five species that were represented are shown in Table 1. For testing reproducibility, 50 of the 495 isolates were selected in order to maximize the number of strains for which MICs were near the interpretive breakpoints of ≤8.0 μg/ml (susceptible) and ≥64 μg/ml (resistant). This subset of strains included 26 strains of Candida albicans and 6 strains each of Candida glabrata, Candida krusei, Candida parapsilosis, and Candida tropicalis. Quality control strains of C. parapsilosis (ATCC 22019) and C. krusei (ATCC 6258) were included in every test run.
TABLE 1.
Susceptibility of 495 Candida spp. isolates to fluconazole as determined by two different methods and two incubation times
| Species (no. of isolates tested) | Incubation time (h) | Methoda | MIC (μg/ml)b
|
||
|---|---|---|---|---|---|
| Range | 50% | 90% | |||
| C. albicans (303) | 24 | MD | 0.016->256 | 0.25 | 0.5 |
| ET | 0.12->256 | 0.5 | 1.0 | ||
| 48 | MD | 0.016->256 | 0.25 | 1.0 | |
| ET | 0.12->256 | 0.5 | 1.0 | ||
| C. parapsilosis (84) | 24 | MD | 0.06-16 | 1.0 | 2.0 |
| ET | 0.12-32 | 1.0 | 2.0 | ||
| 48 | MD | 0.12-32 | 1.0 | 4.0 | |
| ET | 0.12-64 | 1.0 | 4.0 | ||
| C. glabrata (37) | 24 | MD | 0.25-8.0 | 1.0 | 4.0 |
| ET | 0.5-2.0 | 0.5 | 2.0 | ||
| 48 | MD | 0.25-32 | 4.0 | 8.0 | |
| ET | 0.5-64 | 2.0 | 4.0 | ||
| C. tropicalis (38) | 24 | MD | 0.12-4.0 | 0.25 | 1.0 |
| ET | 0.5-8.0 | 1.0 | 2.0 | ||
| 48 | MD | 0.12-16 | 0.5 | 1.0 | |
| ET | 0.5-32 | 1.0 | 4.0 | ||
| C. krusei (33) | 24 | MD | 2.0-32 | 16 | 16 |
| ET | 2.0->256 | 32 | 64 | ||
| 48 | MD | 32-128 | 64 | 128 | |
| ET | 8.0->256 | 32 | 64 | ||
MD, broth microdilution tests; ET, Etests on RPMI 1640 agar with 2% glucose.
50% and 90%, MICs at which 50 and 90% of the isolates tested are inhibited, respectively.
Microdilution susceptibility tests.
The NCCLS procedure (8) was followed as carefully as possible. Serial dilutions of fluconazole (from 256 to 0.03 μg/ml) were prepared in RPMI 1640 broth (HyClone lot no. AHC 7888A) and then dispensed into microdilution test panels. The trays were then stored at −70°C until needed. The inoculum was adjusted to provide 0.5 × 103 to 2.5 × 103 CFU/ml (4, 8, 9) as confirmed by periodic colony counts throughout the study. The trays were incubated at 35°C in ambient air. After 24 h and again after 48 h, MICs were recorded as the lowest concentrations that were visually determined to result in substantial decreases in the amount of growth (about 50 to 80% inhibition compared to that in a growth control well). Quality control strains of Candida spp. were evaluated throughout the study, and the fluconazole MICs fell within the expected ranges (2).
Agar-based susceptibility tests.
Etest strips (AB Biodisk, Solna, Sweden) were tested on RPMI 1640 broth with 2% glucose and 1.5% Bacto Agar (Difco Laboratories). For disk diffusion tests, 25-μg fluconazole disks (BBL lot no. 801553) were also applied to the same test plate. In addition, disk tests were also performed on Mueller-Hinton agar with 2% glucose and methylene blue (0.5 μg/ml). The latter reagent was added to help clarify the zone of inhibition. Because this medium gave encouraging results with disk tests in the initial phase of this study, it was added to the later evaluations of reproducibility. For either test, agar plates were swab inoculated with a suspension of yeast cells in a manner that is currently being used for testing antibacterial agents. When adjusted to the turbidity of a 0.5 McFarland standard, a suspension of yeasts contains about 106 CFU/ml (9). The diameter of each zone of inhibition was taken as the area that showed a sharp decline in the density of growth. Etest MICs were determined according to the manufacturer's instructions and were rounded up to the nearest even log2 concentration in order to simplify analysis.
Precision of test procedures.
A challenge set of 50 strains was selected, and each strain was subcultured to nine slants. These slants were then labeled with random code numbers, and three slants of each strain were distributed to the authors' laboratories. Broth microdilution trays, agar plates, 25-μg fluconazole disks, and Etest strips were provided by a common source. Each participant was asked to perform fluconazole susceptibility tests with subcultures of each of the 150 coded slants. In this way, triplicate tests were performed in a truly blind fashion that generated nine values for each strain. To evaluate the overall precision of each method, the median MIC or zone diameter was calculated and the reported results were evaluated in terms of the number of twofold dilution intervals away from the median MIC or the number of millimeters away from the median zone diameter for the strain.
RESULTS AND DISCUSSION
MICs.
Table 1 shows the MICs for each species as determined by microdilution and Etest procedures and incubation for 24 or 48 h. The two procedures gave MICs that were in general agreement (± one or two doubling concentrations). For C. albicans, C. tropicalis, and C. krusei, most Etest MICs were the same or 1 dilution higher than those provided by microdilution tests. The reverse was true when we tested C. glabrata. With both methods, fluconazole resistance among C. krusei isolates was more apparent when the isolates were incubated a full 48 h. For the purposes of this study, the 48-h microdilution susceptibility test results (8) were used as the standard reference for evaluating other procedures.
Accuracy of tests.
All strains tested for susceptibility were interpreted as belonging to one of three categories (1, 8, 10): susceptible (strains for which MICs were ≤8 μg/ml or zone diameters were ≥19 mm), susceptible-dose dependent (strains for which MICs were 16 to 32 μg/ml or zone diameters were 15 to 18 mm), or resistant (strains for which MICs were ≥64 μg/ml or zone diameters were ≤14 mm). Percentages of results that were interpreted as being in agreement or discrepant were then calculated. Table 2 summarizes these estimates of accuracy for the different methods. When microdilution panels were observed after only 24 h of incubation, 98% of the strains had grown sufficiently to permit estimations of MICs and 94% of the MICs agreed with the results of the 48-h reference test. Very major discrepancies involved tests of C. krusei strains which were susceptible at 24 h but resistant at 48 h. This species also provided 20 of the 22 minor discrepancies.
TABLE 2.
Overall interpretive agreement between results of fluconazole susceptibility tests and of standard 48-h broth microdilution reference tests
| Method and medium | Incubation time (h) | No. of testsa | No. (%) of discrepant resultsb
|
% Total agreement | ||
|---|---|---|---|---|---|---|
| Minor | Major | Very major | ||||
| Microdilution in RPMI 1640 broth | 24 | 486 | 22 (4.5) | 0 | 6 (1.2) | 94.2 |
| Etest on RPMI 1640-glucose-agarc | 24 | 467 | 18 (3.9) | 0 | 2 (0.4) | 95.7 |
| 48 | 492 | 15 (3.0) | 2 (0.4) | 1 (0.2) | 96.3 | |
| Disk diffusion on RPMI 1640-glucose-agarc | 24 | 467 | 22 (4.7) | 0 | 8 (1.7) | 93.6 |
| 48 | 492 | 21 (4.3) | 2 (0.4) | 9 (1.8) | 93.5 | |
| Disk diffusion on Mueller-Hinton agar-glucose-methylene blued | 24 | 490 | 14 (2.9) | 0 | 1 (0.2) | 96.9 |
| 48 | 495 | 28 (5.7) | 5 (1.0) | 0 | 93.3 | |
A total of 495 Candida spp. were tested by all methods. Differences represent the number of strains that failed to grow satisfactorily on the designated medium for the given incubation times.
For susceptible strains, MICs were ≤8 μg/ml or zone diameters were ≥19 mm; for dose-dependent-susceptibility strains, MICs were 16 to 32 μg/ml or zone diameters were 15 to 18 mm; and for resistant strains, MICs were ≥64 μg/ml or zone diameters were ≤14 mm. Minor discrepancies are results indicating dose-dependent susceptibility by one method but susceptibility or resistance by the other method. Major discrepancies are results indicating resistance by the test method but susceptibility by the reference test. Very major discrepancies are results indicating susceptibility by the test method but resistance by the reference test.
RPMI 1640 broth with 2% glucose and 1.5% agar.
Mueller-Hinton agar with 2% glucose and 0.5 μg of methylene blue/ml.
On RPMI 1640-glucose-agar, 94% of the strains grew sufficiently to permit the recording of test results after 24 h. The majority of early-growth failures involved tests of the C. glabrata strains that required a full 48 h for testing, and even then, the lawns of growth were rather light. Etest MICs with this medium showed 96% agreement with the reference microdilution test results. The majority of discrepancies were minor differences, but results for two C. krusei isolates showed very major discrepancies at 24 h. Results of disk diffusion tests on the same agar plates displayed 93.5% agreement; all very major discrepancies involved tests of C. krusei, and results for two C. albicans isolates provided major discrepancies but only when they were read at 48 h.
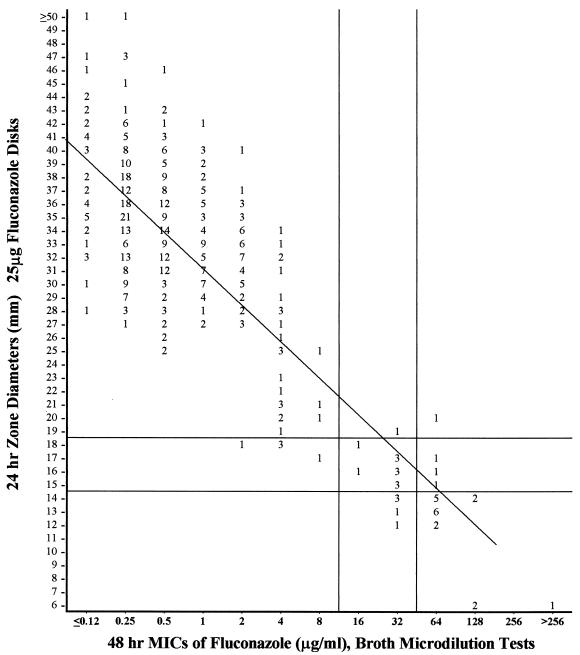
Disk tests were improved by using Mueller-Hinton agar supplemented with glucose for better growth and a low concentration of methylene blue to help clarify the zones of inhibition. At the first 24 h, only five strains (three C. parapsilosis and two C. albicans strains) grew too poorly to permit measurement of zone diameters but they all grew sufficiently after 48 h. On this supplemented medium, results of the 24-h disk tests showed the best (97%) agreement with results of the 48-h broth microdilution test: only one very major discrepancy was recorded (C. krusei). The 14 minor discrepancies involved tests of eight C. krusei, three C. parapsilosis, two C. glabrata, and one C. albicans isolate . When disks were held for 48 h before inhibitory zones were measured, five isolates (three C. glabrata, one C. albicans, and one C. parapsilosis isolate) were judged to be “false resistant” (major errors). The few strains that grew poorly on the first day were accurately tested after an additional day of incubation. Figure 1 presents a scattergram plotted to compare the 24-h zones on this medium to the reference microdilution MICs for each of the 490 strains that grew sufficiently well.
FIG. 1.
Zones of inhibition around 25-μg-fluconazole disks containing Mueller-Hinton agar supplemented with 2% glucose and methylene blue (0.5 μg/ml). Values are numbers of isolates from the 490 isolates of Candida spp. that grew on this medium after 24 h. The least-squares method was used to calculate a regression line (y = 55.4 − 2.66x, with r = 80).
Precision of MICs.
Table 3 summarizes data concerning the reproducibility of fluconazole MICs. By the reference microdilution test, 93 to 94% of all MICs varied over a broad range of ± two doubling concentrations on either side of the median. This range of variation is in agreement with observations made during the initial evaluation of the microdilution procedure (4). With the Etest, >90% of all MICs were no more than one dilution interval from the all-laboratory median. Compared to RPMI 1640-glucose-agar, Mueller-Hinton agar with glucose and methylene blue did not improve the precision of Etests. The reference method used to evaluate the other procedures suffers from inconsistencies due largely to difficulties in defining the endpoint when there is a trailing growth pattern. The agar-based Etest (5, 11) seems to have a slight advantage over broth dilution tests in this regard. By both methods, MICs recorded after 24 h were more consistent than those determined after 48 h.
TABLE 3.
Precision of fluconazole MICs when 50 Candida isolates were tested in triplicate in each of three laboratories
| MIC or range of MICs | % of MICs at median or within rangea
|
|||||
|---|---|---|---|---|---|---|
| Microdilution in RPMI 1640 broth
|
Etest on RPMI 1640-glucose-agarb
|
Etest on Mueller-Hinton agar-glucose- methylene bluec
|
||||
| 24 h | 48 h | 24 h | 48 h | 24 h | 48 h | |
| Median MIC | 55.6 | 50.4 | 64.0 | 60.7 | 55.6 | 63.1 |
| Median MIC ± 1 dilution | 87.3 | 86.2 | 96.4 | 94.0 | 91.1 | 90.2 |
| Median MIC ± 2 dilutions | 93.8 | 93.1 | 98.7 | 97.4 | 96.7 | 96.7 |
| Median MIC ± 3 dilutions | 95.6 | 95.3 | 99.5 | 98.7 | 99.3 | 99.8 |
For each test strain, 9 MICs were recorded and the precision of each MIC was expressed as the number of twofold dilutions away from the overall median MIC for that strain.
RPMI 1640 broth with 2% glucose and 1.5% agar.
Mueller-Hinton agar with 2% glucose and 0.5 μg of methylene blue/ml.
Precision of disk tests.
Table 4 describes the same type of data for disk diffusion tests. From the regression statistics calculated from the data in Fig. 1, a twofold concentration change in MICs was accompanied by a 3- to 4-mm change in the diameters of the zones of inhibition. Consequently, the precision of disk tests can be compared to that of MIC determinations. A range of MICs that represent the median ± one doubling concentration is equal to a median zone of ±3 or 4 mm, and a range of MICs that represent the median ± two doubling concentrations is equal to a median zone of ±6 to 8 mm. As can be seen in Table 4, the precision of disk diffusion tests is similar to that observed for the standard reference procedure. When Mueller-Hinton agar with glucose and methylene blue was used, the zone diameters were more reproducible than those on RPMI 1640-glucose-agar. Furthermore, zones of inhibition measured after 24 h of incubation were more reproducible than those measured after 48 h.
TABLE 4.
Precision of fluconazole disk diffusion susceptibility tests on each of two agar media
| Range of zone diameters | % of zone diameters within rangea
|
|||
|---|---|---|---|---|
| RPMI 1640-glucose-agarb
|
Mueller-Hinton agar-glucose- methylene bluec
|
|||
| 24 h | 48 h | 24 h | 48 h | |
| Median ± 3 mm | 67.5 | 64.5 | 72.8 | 70.9 |
| Median ± 4 mm | 79.2 | 73.9 | 82.0 | 79.1 |
| Median ± 6 mm | 91.1 | 87.1 | 94.0 | 90.4 |
| Median ± 8 mm | 97.0 | 94.4 | 97.8 | 96.2 |
A total of 50 Candida isolates were tested on two agar media in triplicate in each of three laboratories, and the median of nine zone diameters was calculated. Precision was expressed as the extent to which each measurement deviated from that median.
RPMI 1640 broth with 2% glucose and 1.5% agar.
Mueller-Hinton agar with 2% glucose and 0.5 μg of methylene blue/ml.
We concluded that the results of fluconazole susceptibility tests by the disk diffusion method were just as reproducible as the results of the standard reference microdilution procedure. Furthermore, of the tests that we evaluated, the Etest produced the most reproducible results. The precision of disk diffusion tests was maximized by using Mueller-Hinton agar with glucose and methylene blue and by incubating the disks for only 24 h. The 1% of strains that failed to grow adequately at the 24-h examination gave satisfactory results when they were incubated for another day. The results of disk diffusion tests done in this way showed excellent (97%) agreement with the results of the reference broth microdilution method.
Acknowledgments
This study was made possible by a grant from Pfizer Pharmaceuticals, New York, N.Y.
REFERENCES
- 1.Barry, A. L., and S. D. Brown. 1996. Fluconazole disk diffusion procedure for determining susceptibility of Candida species. J. Clin. Microbiol. 34:2154-2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barry, A. L., M. A. Pfaller, S. D. Brown, A. Espinel-Ingroff, M. A. Ghannoum, C. Knapp, R. P. Rennie, J. H. Rex, and M. G. Rinaldi. 2000. Quality control limits for broth microdilution susceptibility tests of ten antifungal agents. J. Clin. Microbiol. 38:3457-3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bille, J. 1997. When should Candida isolates be tested for susceptibility to azole antifungal agents? Eur. J. Clin. Microbiol. Infect. Dis. 16:281-282. [DOI] [PubMed] [Google Scholar]
- 4.Espinel-Ingroff, A., G. W. Kish, T. M. Kerkering, R. A. Fromtling, M. Bartizal, J. N. Galgiani, K. Villareal, M. A. Pfaller, T. Gerarden, M. G. Rinaldi, and A. Fothergill. 1992. Collaborative comparison of broth macrodilution and microdilution antifungal susceptibility tests. J. Clin. Microbiol. 30:3138-3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Espinel-Ingroff, A., M. Pfaller, M. E. Erwin, and R. N. Jones. 1996. Interlaboratory evaluation of Etest method for testing antifungal susceptibilities of pathogenic yeasts to five antifungal agents by using Casitone agar and solidified RPMI 1640 medium with 2% glucose. J. Clin. Microbiol. 34:848-852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fan-Harvard, P., D. Capano, S. M. Smith, A. Mangia, and R. H. K. Eng. 1991. Development of resistance in Candida isolates from patients receiving prolonged antifungal therapy. Antimicrob. Agents Chemother. 35:2302-2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Korting, H. C., M. Ollert, A. Georgii, and M. Froschl. 1988. In vitro susceptibility and biotypes of Candida albicans isolates from the oral cavities of patients infected with human immunodeficiency virus. J. Clin. Microbiol. 26:2626-2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.National Committee for Clinical Laboratory Standards. 1997. Reference method for broth dilution antifungal susceptibility tests of yeasts. Approved standard M-27A. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 9.Pfaller, M. A., L. Burmeister, M. A. Bartlett, and M. G. Rinaldi. 1988. Multicenter evaluation of four methods of yeast inoculum preparation. J. Clin. Microbiol. 26:1437-1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rex, J., M. Pfaller, J. Galgiani, M. Bartlett, A. Espinel-Ingroff, M. Ghannoum, M. Lancaster, F. Odds, M. Rinaldi, T. Walsh, and A. Barry. 1997. Development of interpretive breakpoints for antifungal susceptibility testing: conceptual framework and analysis of in vitro and in vivo correlation data for fluconazole and itraconazole and Candida infections. Clin. Infect. Dis. 24: 235-247. [DOI] [PubMed] [Google Scholar]
- 11.Sewell, D. L., M. A. Pfaller, and A. L. Barry. 1994. Comparison of broth macrodilution, broth microdilution, and E test antifungal susceptibility tests for fluconazole. J. Clin. Microbiol. 32:2099-2102. [DOI] [PMC free article] [PubMed] [Google Scholar]