Abstract
The combination of epigallocatechin gallate (EGCg, a main constituent of tea catechins) with penicillin showed synergism against 21 clinical isolates of penicillinase-producing Staphylococcus aureus. Besides binding directly to peptidoglycan, the inhibition of penicillinase activity by EGCg is responsible for the synergism. EGCg inhibited the penicillinase activity in a dose-dependent fashion, with a 50% inhibitory concentration of 10 μg/ml.
The emergence and spread of bacterial strains producing β-lactamase have greatly diminished the usefulness of β-lactams (12). Even β-lactamase-stable β-lactams can be slowly hydrolyzed by staphylococcal β-lactamases (8), and overproduction of β-lactamases could result in borderline resistance (11, 16). The combinations of β-lactams and β-lactamase inhibitors (such as sulbactam, clavulanic acid, and tazobactam) are a successful strategy to overcome infections caused by bacteria producing β-lactamases (9). But the recent emergence of bacterial strains producing inhibitor-resistant enzymes could be related to the frequent use of clavulanate (1, 2, 17). Furthermore, the appearance of extended-spectrum β-lactamases and IMP-1 (a new β-lactamase) is now threatening the value of broad-spectrum cephalosporins and carbapenems against various bacterial infections (4, 7, 18). It seems that the introduction of any new β-lactam will be followed by the appearance of a new β-lactamase. Therefore, any strategy to prevent inactivation of β-lactams by β-lactamases is of particular significance.
We recently confirmed that epigallocatechin gallate (EGCg, a main constituent of tea catechins) enhances the activity of β-lactams against methicillin-resistant Staphylococcus aureus owing to interference with the integrity and biosynthesis of the bacterial cell wall through direct binding to peptidoglycan (19). In this report, we present in vitro evidence that EGCg inhibits penicillinase activity, thus restoring the antibacterial activity of penicillin against penicillinase-producing S. aureus.
EGCg with a purity of 98% was extracted from green tea. Penicillin, ampicillin, and purified penicillinase from Bacillus cereus (Sigma, St. Louis, Mo.), nitrocefin (Oxoid, Basingstoke, United Kingdom), and potassium clavulanate (Wako Pure Chemical Industries, Osaka, Japan) were used.
Twenty-one clinical isolates of penicillinase-producing S. aureus were from the Clinical Microbiology Laboratories of Showa University Hospital. All the strains were identified by PCR analysis for the presence of blaZ gene as reported previously (10). The production of β-lactamase was tested with a nitrocefin assay (15). Two standard strains, S. aureus ATCC 25923 and Escherichia coli ATCC 25922, were used as controls. Mueller-Hinton broth (MHB) supplemented with 25 mg of Ca2+/liter and 12.5 mg of Mg2+/liter was used. The MICs and fractional inhibitory concentration (FIC) indices were determined by the broth microdilution and checkerboard methods (5, 6). Synergy between penicillin and EGCg was indicated by an FIC index of ≤0.5.
To prepare the cell-free supernatant of penicillinase, S. aureus 226, a strain producing high levels of penicillinase even without any inducer, was cultured in MHB at 35°C to an optical density at 600 nm of 0.4. After centrifugation and filtration, the cell-free supernatant was collected as the crude extract of penicillinase. The supernatant contained about 0.015 U of penicillinase per ml, as confirmed by a nitrocefin assay.
To confirm the protection of penicillin or ampicillin from penicillinase by EGCg, the penicillin-susceptible S. aureus strain ATCC 25923 (5 × 104 cells) was inoculated in 100 μl of the cell-free supernatant of penicillinase in the presence of various concentrations of penicillin and EGCg. The ampicillin-susceptible strain E. coli ATCC 25922 (5 × 104 cells) was inoculated in 100 μl of MHB containing various concentrations of ampicillin and EGCg as well as the purified penicillinase. After culture at 35°C for 24 h (S. aureus) or 18 h (E. coli), the MICs were determined. Changes in penicillin and ampicillin MICs were considered evidence of EGCg-induced protection of penicillin and ampicillin from penicillinase.
To prove the direct inhibition of penicillinase by EGCg, the purified penicillinase (10 U/ml) was preincubated in 100 μl of MHB with various concentrations of EGCg for 30 min and 18 h at 35°C in a 96-well culture plate. Nitrocefin (250 μg/ml) was then added as the substrate. β-Lactamase broke down the ring of nitrocefin, resulting in a color change from yellow to dark red. The color changes were recorded by detecting the optical density at 492 of each well at 10 min with a spectrophotometer. The concentration of EGCg required for 50% inhibition (IC50) of the enzyme activity was determined graphically according to the standard curve of penicillinase under the same assay conditions.
The MICs and FIC indices of penicillin in combination with EGCg against the 21 clinical isolates are presented in Table 1. The MIC range of penicillin was 2 to 128 μg/ml (the susceptibility breakpoint was ≤0.125 μg/ml). The MIC of EGCg was 100 μg/ml, and EGCg alone did not enhance the production of penicillinase. Synergism occurred against 48, 81, and 100% of the tested strains in combinations of penicillin with EGCg at 3.125, 6.25, and 12.5 μg/ml, respectively. The MIC at which 50% of strains were inhibited (MIC50) of penicillin decreased from 32 μg/ml to 8, 8, 2, and 0.5 μg/ml and the MIC90 decreased from 128 μg/ml to 64, 32, 16, and 8 μg/ml in the presence of EGCg at 3.125, 6.25, 12.5, and 25 μg/ml, respectively. The synergistic bactericidal activity of the penicillin-EGCg combination was also observed in a time-kill assay (data not shown).
TABLE 1.
MICs and FIC indices of penicillin in combination with EGCg against 21 isolates of penicillinase-producing S. aureusa
| Strain | MIC (μg/ml)
|
FIC index
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| A | B | C | D | E | B | C | D | E | |
| 97 | 32 | 16 | 8 | 2 | 0.25 | 0.53 | 0.31 | 0.19 | 0.26 |
| 226 | 128 | 64 | 64 | 16 | 2 | 0.53 | 0.56 | 0.25 | 0.27 |
| 419 | 64 | 32 | 16 | 8 | 8 | 0.53 | 0.31 | 0.25 | 0.38 |
| 1-152 | 128 | 64 | 32 | 16 | 2 | 0.53 | 0.31 | 0.25 | 0.27 |
| 1-150 | 32 | 8 | 8 | 4 | 1 | 0.28 | 0.31 | 0.25 | 0.28 |
| 1-813 | 128 | 32 | 32 | 16 | 8 | 0.28 | 0.31 | 0.25 | 0.31 |
| 1-814 | 64 | 16 | 8 | 2 | 1 | 0.28 | 0.19 | 0.16 | 0.27 |
| 1-820 | 64 | 16 | 8 | 2 | 1 | 0.28 | 0.19 | 0.16 | 0.27 |
| 1-836 | 16 | 2 | 1 | 0.25 | 0.063 | 0.16 | 0.13 | 0.14 | 0.25 |
| 1-909 | 16 | 2 | 1 | 0.5 | 0.063 | 0.16 | 0.13 | 0.16 | 0.25 |
| 3-651 | 2 | 2 | 1 | 0.25 | 0.125 | 1.03 | 0.56 | 0.25 | 0.31 |
| 5-28 | 128 | 32 | 8 | 8 | 1 | 0.28 | 0.13 | 0.19 | 0.26 |
| 6-5 | 128 | 128 | 64 | 32 | 8 | 1.03 | 0.56 | 0.38 | 0.31 |
| 6-42 | 8 | 2 | 1 | 1 | 0.25 | 0.28 | 0.19 | 0.25 | 0.28 |
| 6-43 | 4 | 2 | 2 | 0.5 | 0.125 | 0.53 | 0.56 | 0.25 | 0.28 |
| 6-78 | 64 | 8 | 8 | 2 | 0.5 | 0.16 | 0.19 | 0.16 | 0.26 |
| 6-326 | 16 | 8 | 4 | 1 | 0.25 | 0.53 | 0.31 | 0.19 | 0.27 |
| 6-339 | 8 | 8 | 2 | 0.25 | 0.063 | 1.03 | 0.31 | 0.16 | 0.26 |
| 6-346 | 32 | 16 | 4 | 1 | 0.5 | 0.53 | 0.19 | 0.16 | 0.27 |
| 6-350 | 16 | 8 | 4 | 1 | 0.5 | 0.53 | 0.31 | 0.19 | 0.28 |
| 6-404 | 16 | 2 | 1 | 0.5 | 0.125 | 0.16 | 0.13 | 0.16 | 0.26 |
| ATCC 25923 | 0.125 | 0.125 | 0.063 | 0.063 | 0.016 | 1.03 | 0.56 | 0.63 | 0.38 |
A, penicillin alone; B to E, penicillin plus EGCg at 3.125, 6.25, 12.5, and 25 μg/ml, respectively. The MIC of EGCg alone for the strains was 100 μg/ml. All strains except ATCC 25923 were positive for the blaZ gene and were positive for β-lactamase production in the ntirocefin assay.
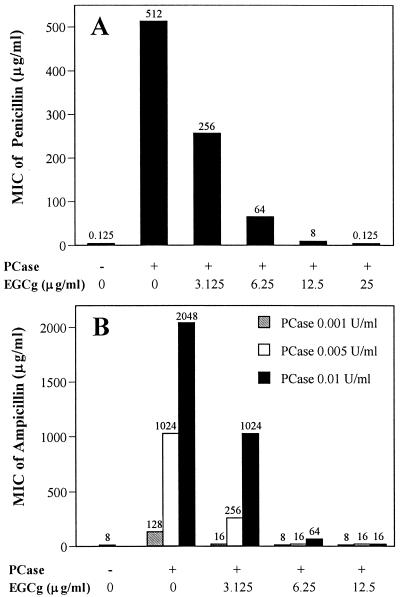
The MIC of penicillin for S. aureus ATCC 25923 rose from 0.125 to 512 μg/ml in the cell-free supernatant containing penicillinase. EGCg blocked the penicillinase activity in a dose-dependent manner and thus restored the MICs of penicillin from 512 μg/ml to 256, 64, 8, and 0.125 μg/ml at concentrations of 3.125, 6.25, 12.5, and 25 μg/ml, respectively (Fig. 1A).
FIG. 1.
Protection of penicillin (A) and ampicillin (B) from penicillinase by EGCg. (A) Penicillin-susceptible S. aureus ATCC 25923 cells were inoculated in the cell-free supernatant containing about 0.015 U of penicillinase (PCase) per ml in the presence of twofold serial dilutions of penicillin and EGCg. (B) Ampicillin-susceptible E. coli ATCC 25922 cells were inoculated in MHB containing purified penicillinase at 0.001, 0.005, and 0.01 U/ml in the presence of twofold serial dilutions of ampicillin and EGCg. After incubation at 35°C for 24 h (S. aureus) and 18 h (E. coli), the MICs were determined.
Similarly, the MICs of ampicillin for E. coli ATCC 25922 rose from 8 μg/ml to 128, 1,024, and 2,048 μg/ml in the presence of the purified penicillinase at 0.001, 0.005, and 0.01 U/ml, respectively. EGCg protected the antibacterial activity of ampicillin. In the presence of 0.01 U of penicillinase per ml, for example, the MICs of ampicillin were restored from 2,048 μg/ml to 1,024, 64, and 16 μg/ml by EGCg at 3.125, 6.25, and 12.5 μg/ml, respectively (Fig. 1B). The direct effect of EGCg against E. coli can be omitted, because the MIC of EGCg was more than 800 μg/ml and there was no synergism between EGCg and ampicillin against E. coli (19).
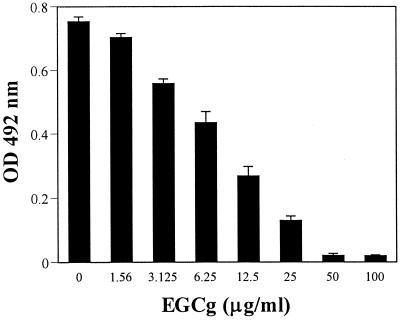
Figure 2 shows that EGCg directly inhibited the activity of penicillinase in a dose-dependent manner. The IC50s of EGCg were 10 μg/ml (21 μM) and 44 μg/ml (96 μM) for the 18-h and 30-min preincubations, respectively. The IC50s of clavulanic acid, a control inhibitor run under the same assay conditions, were 2.5 μg/ml (10.5 μM) and 13.5 μg/ml (56 μM), respectively.
FIG. 2.
Direct inhibition of penicillinase activity by EGCg. Purified penicillinase (10 U/ml) was incubated with EGCg in 100 μl of MHB at 35°C for 18 h prior to the addition of nitrocefin as its substrate. The optical density at 492 nm was then determined with a spectrophotometer.
The above results demonstrated that besides the effect of EGCg on the cell wall, the direct inhibition of penicillinase activity by EGCg is responsible for synergism. EGCg destroys the penicillinase activity, protecting penicillin or ampicillin from inactivation.
The safe consumption of tea for thousands of years indicates the low toxicity of tea and EGCg. EGCg is absorbed through the digestive tract and distributed to many organs in animals and humans (3, 13, 14). EGCg at 5.6 μg/ml in rat blood plasma was detected after administration of 500 mg/kg of body weight (13). EGCg at 2 μg/ml in human blood plasma was detected 90 min after 525-mg EGCg capsules were taken (14). In this experiment, potent synergy against 48, 81, and 100% of the tested isolates was observed with the combination of penicillin and EGCg at 3.125, 6.25, and 12.5 μg/ml, respectively, indicating that a bioavailable concentration with in vivo activity may be achievable.
Acknowledgments
We thank Rika Wakuta and Kunihide Gomi, Central Clinical Laboratories, Showa University Hospital, for providing S. aureus isolates.
This work was supported in part by a grant from the Ministry of Education, Culture, Sports, Science and Technology, Japan.
REFERENCES
- 1.Blasquez, J., M.-R. Baquero, I. Canton, I. Alos, and F. Baquero. 1993. Characterization of a new TEM-type β-lactamase resistant to clavulanate, sulbactam, and tazobactam. Antimicrob. Agents Chemother. 37:2059-2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chaibi, E. B., D. Sirot, G. Paul, and R. Labia. 1999. Inhibitor-resistant TEM β-lactamase: phenotypic, genetic and biochemical characteristics. J. Antimicrob. Chemother. 43:447-458. [DOI] [PubMed] [Google Scholar]
- 3.Chen, L., M. J. Lee, H. Li, and C. S. Yang. 1997. Absorption, distribution, elimination of tea polyphenols in rats. Drug Metab. Dispos. 25:1045-1050. [PubMed] [Google Scholar]
- 4.Heritage, J., F. H. M'Zali, D. Gascoyne-Binzi, and P. M. Hawkey. 1999. Evolution and spread of SHV extended-spectrum β-lactamases in Gram-negative bacteria. J. Antimicrob. Chemother. 44:309-318. [DOI] [PubMed] [Google Scholar]
- 5.Hu, Z.-Q., W.-H. Zhao, N. Asano, Y. Yoda, Y. Hara, and T. Shimamura. 2002. Epigallocatechin gallate synergistically enhances the activity of carbapenems against methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 46:558-560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hu, Z.-Q., W.-H. Zhao, Y. Hara, and T. Shimamura. 2001. Epigallocatechin gallate synergy with ampicillin-sulbactam against 28 clinical isolates of methicillin-resistant Staphylococcus aureus. J. Antimicrob. Chemother. 48:361-364. [DOI] [PubMed] [Google Scholar]
- 7.Ito, H., Y. Arakawa, S. Ohsuka, R. Wacharotayankun, N. Kato, and M. Ohta. 1995. Plasmid-mediated dissemination of the metallo-β-lactamase gene blaIMP among clinically isolated strains of Serratia marcescens. Antimicrob. Agents Chemother. 39:824-829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kernodle, D. S., C. S. Stratton, L. W. McMurray, J. R. Chipley, and P. A. McGraw. 1989. Differentiation of beta-lactamase variants in Staphylococcus aureus by substrate hydrolysis profiles. J. Infect. Dis. 159:103-108. [DOI] [PubMed] [Google Scholar]
- 9.Maddux, M. 1991. Effects of β-lactamase-mediated antimicrobial resistance: the role of β-lactamase inhibitors. Pharmacotherapy 11(Suppl.):40-50. [PubMed] [Google Scholar]
- 10.Martineau, F., F. J. Picard, L. Grenier, P. H. Roy, M. Ouellette, the ESPRIT Trial, and M. G. Bergeron. 2000. Multiplex PCR assays for the detection of clinically relevant antibiotic resistance genes in staphylococci isolated from patients infected after cardiac surgery. J. Antimicrob. Chemother. 46:527-533. [DOI] [PubMed] [Google Scholar]
- 11.McDougal, L. K., and C. Thornsberry. 1986. The role of β-lactamase in staphylococcal resistance to penicillinase-resistant penicillins and cephalosporins. J. Clin. Microbiol. 23:832-839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Medeiros, A. A. 1984. Beta-lactamases. Br. Med. Bull. 40:18-27. [DOI] [PubMed] [Google Scholar]
- 13.Nakagawa, K., and T. Miyazawa. 1997. Absorption and distribution of tea catechin, (−)-epigallocatechin-3-gallate, in rats. J. Nutr. Sci. Vitaminol. (Tokyo) 43:679-684. [DOI] [PubMed] [Google Scholar]
- 14.Nakagawa, K., S. Okuda, and T. Miyazawa. 1997. Dose-dependent incorporation of tea catechins, (−)-epigallocatechin-3-gallate and (−)-epigallocatechin, into human plasma. Biosci. Biotechnol. Biochem. 61:1981-1985. [DOI] [PubMed] [Google Scholar]
- 15.O'Callaghan, C. H., A. Morris, S. M. Kirby, and A. H. Shingler. 1972. Novel method for detection of β-lactamases by using a chromogenic cephalosporin substrate. Antimicrob. Agents Chemother. 1:283-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tomasz, A., H. B. Drugeon, H. M. de Lencastre, D. Jabes, L. McDougal, and J. Bille. 1989. New mechanism for methicillin resistance in Staphylococcus aureus: clinical isolates that lack the PBP 2a gene and contain normal penicillin-binding proteins with modified penicillin-binding capacity. Antimicrob. Agents Chemother. 33:1869-1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vedel, G., A. Belaaouaj, G. Lilly, R. Labia, A. Philippon, P. Nevot, and G. Paul. 1992. Clinical isolates of Escherichia coli producing TRI β-lactamases: novel TEM-enzymes conferring resistance to β-lactamase inhibitors. J. Antimicrob. Chemother. 30:449-462. [DOI] [PubMed] [Google Scholar]
- 18.Watanabe, M., S. Iyobe, M. Inoue, and S. Mitsuhashi. 1991. Transferable imipenem resistance in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 35:147-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao, W-H., Z.-Q. Hu, S. Okubo, Y. Hara, and T. Shimamura. 2001. Mechanism of synergy between epigallocatechin gallate and β-lactams against methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 45:1737-1742. [DOI] [PMC free article] [PubMed] [Google Scholar]