Abstract
We have compared the activities of liposomal amphotericin B (LAMB) at 3, 5, 10, and 20 mg/kg/day and amphotericin B deoxycholate (AMB) at 1.5 and 2.5 mg/kg/day in a murine systemic infection by Fusarium verticillioides. Survival was improved by all treatments except AMB at 1.5 mg/kg/day. The tissue burden in liver was reduced by LAMB at all dosages and by AMB at 2.5 mg/kg/day. The two highest dosages of LAMB showed significant reductions in the spleen.
Fusarium species are common soil saprophytes and plant pathogens which have also frequently been reported as etiologic agents of opportunistic infections in humans (3, 9). The increasing number of severe cases reported and the resistance to available antifungal drugs explain the clinical interest in these fungi. Fusarium verticillioides is, after Fusarium solani, the most common species causing fusariosis, which involves multiple organs, such as the liver, lung, kidney, heart, spleen, and pancreas, with a high mortality rate (8, 9, 21, 22, 25, 27). To date, 15 cases of disseminated human infection by F. verticillioides have been reported, and more than 60% of them have shown a fatal outcome despite antifungal treatment (7, 9, 10, 17). However, it is possible that the real incidence of this species is underestimated because, unfortunately, in an important number of the published cases the etiological agent has not been identified to the species level. Amphotericin B (AMB), the standard antifungal therapy, is frequently ineffective and can cause important secondary effects. Other drugs, such as flucytosine, ketoconazole, and miconazole, have also been used, all of them showing a very low efficacy (22, 24). Combinations of AMB with flucytosine (5, 21), ketoconazole (19), and rifampin (8, 13) have also been used, again with poor results. Unfortunately, the novel antifungals such as posaconazole, LY303366, nikkomycin Z, FK-463, and caspofungin do not seem to offer better chances of success (2, 6, 14, 23). Interestingly, one case of disseminated infection by F. verticillioides in a patient with acute lymphoblastic leukemia was resolved by using liposomal amphotericin B (LAMB) at 3 mg/kg/day after the failure of AMB (4). LAMB has demonstrated efficacy against infections produced by filamentous fungi (12, 15, 16). Due to the poor activity of AMB in vitro (20) and in vivo against F. verticillioides and the scarce data on the clinical use of LAMB in fusariosis, we have evaluated the efficacy of this drug in a murine model of disseminated infection by such fungus.
We used the clinical isolate FMR 4382. The in vitro AMB MIC against this strain was 4 μg/ml. The inoculum was prepared as described previously (18). AMB (Fungizona; Squibb Industria Farmacéutica S.A., Barcelona, Spain) and LAMB (AmBisome; Gilead Sciences S.A., Madrid, Spain) were reconstituted with sterile distilled water and further diluted in 5% sterile dextrose solution to reach the desired concentrations. OF-1 male mice (Charles River, Criffa S.A., Barcelona, Spain) weighing 30 g were used. Conditions were approved by the Animal Welfare Committee of the Faculty of Medicine of our university. Groups of 10 animals were infected by the intravenous (i.v.) inoculation of 0.2 ml of the conidial suspension via the lateral tail vein. In a preliminary study, five different inocula (2.5 × 106, 2.5 × 107, 1 × 108, 5 × 108, and 1 × 109 conidia/ml) were tested. Treatments started 24 h postchallenge and continued for 10 days. Seven groups were established. AMB at 1.5 and 2.5 mg/kg/day was administered intraperitoneally, because doses higher than 1 mg/kg/day have shown to be toxic when administered i.v. The control group received 0.2 ml of 5% dextrose solution i.v. Surviving mice were sacrificed by an overdose of halotane on day 11. Liver, spleen, and kidneys were aseptically removed and homogenized in 1 ml of sterile saline solution. Five 10-fold serial dilutions of this homogenate were made with sterile saline. Volumes of 0.1 and 0.5 ml of the homogenate and 0.1 ml of each dilution were inoculated onto potato dextrose agar plates and incubated at 30°C for 3 days. The mean survival time (MST) was estimated by the Kaplan-Meier method and compared among groups by using the log rank test. P values were corrected for multiple comparisons. Colony counts were analyzed by analysis of variance. Statistical significance was declared when the P value was <0.05. Calculations were performed with SPSS for Windows version 10.0.
In the preliminary study, all of the mice infected with 2.5 × 106, 2.5 × 107, 1 × 108, or 5 × 108 conidia/ml survived (data not shown). Mice in the groups inoculated with the three lowest inocula did not show the typical external signs associated with disseminated fusarial infections. Approximately 50% of the mice in the group infected with 5 × 108 conidia/ml showed these symptoms, and a high number of F. verticillioides CFU were recovered from their kidneys. However, this inoculum was not useful for evaluating the mortality rate of the animals. By contrast, approximately 50% of the mice died within a few minutes after challenge with the highest inoculum (1 × 109 conidia/ml). This was probably due to embolisms caused by the high density of the conidial suspension. However, when this inoculum was halved and administered at an interval of 15 min, mortality was prevented in the first 24 h, and it was 50% after 11 days. All of the mice infected with 1 × 109 conidia/ml developed the typical external signs of the infection. A high number of fungal colonies were recovered from the dead animals. Similar results were obtained in repeated experiments (data not shown). Fusarium spp., like many other opportunistic fungi, have a weak virulence for immunocompetent animals, including human beings. In our study we have confirmed this by observing that inocula as high as 1 × 108 and 5 × 108 conidia/ml were unable to kill the animals tested. Only the suspension of 1 × 109 conidia/ml caused an infection with an important degree of mortality. Otherwise, clinical data show that once these fungi infect immunocompromised patients, their effects can be devastating (3, 9).
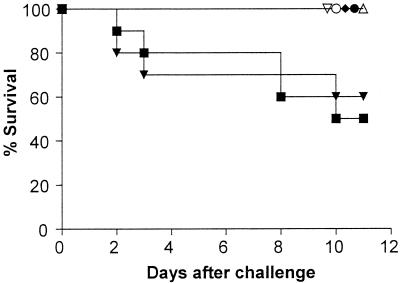
The results of the different treatments on the survival of mice infected with F. verticillioides are shown in Fig. 1. The results for treatment with AMB at 1.5 mg/kg/day (MST = 8.30 days, with 60% survival) were not statistically different from those for the control (MST = 8.60 days, with 50% survival), with deaths occurring from days 2 to 10 in both cases. All mice receiving LAMB at any dose or AMB at 2.5 mg/kg/day survived to the end of the experiment (day 11) and had a significantly higher MST (P = 0.035) than those in the control group.
FIG. 1.
Survival curve for groups of 10 mice infected with F. verticillioides and treated with AMB at 1.5 mg/kg/day (▾), AMB at 2.5 mg/kg/day (•), LAMB at 3 mg/kg/day (▿), LAMB at 5 mg/kg/day (♦), LAMB at 10 mg/kg/day (▵), or LAMB at 20 mg/kg/day (○) or untreated as a control (▪).
Liver, spleen, and kidneys of mice from the control group sacrificed on day 11 showed high concentrations of CFU per gram (Table 1). None of the treatments was able to reduce colony counts in kidneys. AMB at 1.5 mg/kg/day was not able to reduce colony counts in any of the studied organs. In the liver, AMB at 2.5 mg/kg/day reduced the tissue burden significantly compared to the control (P = 0.039). Mice in groups treated with LAMB at 3, 5, 10, or 20 mg/kg/day all showed significantly lower counts than those treated with the control or AMB at either dosage (P ≤ 0.001). In the spleen, only the highest dosages of LAMB (10 and 20 mg/kg/day) showed a significant reduction in colony counts (P ≤ 0.011).
TABLE 1.
Effects of treatment on colony counts of F. verticillioides in liver, spleen, and kidney
| Treatment (concn [mg/kg]) | Mean log10 CFU/g of tissue (95% CIa)
|
||
|---|---|---|---|
| Liver | Spleen | Kidney | |
| Control | 4.8 (4.5-5.0) | 5.7 (4.9-6.4) | 3.5 (2.9-4.1) |
| AMB (1.5) | 4.2 (3.7-4.7) | 5.4 (5.1-5.7) | 3.7 (3.4-4.0) |
| AMB (2.5) | 3.9 (3.2-4.7)b | 5.3 (4.6-6.0) | 3.7 (3.5-4.0) |
| LAMB (3) | 2.5 (1.9-3.2)c | 4.5 (3.4-5.6) | 4.0 (3.1-4.8) |
| LAMB (5) | 2.7 (2.3-3.1)c | 4.5 (3.5-5.4) | 3.7 (3.3-4.0) |
| LAMB (10) | 2.3 (1.4-3.1)c | 3.2 (2.4-4.0)d | 3.4 (2.9-4.0) |
| LAMB (20) | 2.4 (1.9-2.8)c | 3.3 (2.6-4.1)d | 3.4 (3.1-3.6) |
CI, confidence interval.
P < 0.05 versus control; P < 0.001 versus LAMB at 3, 5, 10, or 20 mg/kg.
P < 0.001 versus control or AMB at 1.5 or 2.5 mg/kg.
P < 0.01 versus control or AMB at 1.5 or 2.5 mg/kg.
In our study, AMB at the usual dosage was clearly ineffective, a finding which agrees with those of other animal studies performed previously (1, 11). Only at 2.5 mg/kg/day did it improve mouse survival and reduce colony counts in liver. However, this high dosage is not recommended in the clinical setting due to its remarkable nephrotoxicity. The high dosages of LAMB used in our study showed no apparent signs of toxicity for the animals. This agrees with the results of Walsh et al., who administered high doses of LAMB (7.5 to 15 mg/kg/day) to patients with proven or suspected fungal infection for a long period (up to 100 days) with no dosage-related increase in toxicity (26). These high dosages, even though they can increase the economic cost of the treatment, are an option worth considering because of their therapeutic effectiveness and the absence of adverse effects. Although more studies are needed to confirm these aspects and to recommend discontinuing the use of AMB at high dosages because of its toxicity, LAMB at high dosages might be a good option in the treatment of refractory fusariosis caused by this species.
Acknowledgments
This work was supported in part by a grant from Gilead Sciences, S.A., Madrid, Spain.
We thank A. Moreno and C. Sanmartí for their technical assistance.
REFERENCES
- 1.Anaissie, E. J., R. Hachem, C. Legrand, P. Legenne, P. Nelson, and G. P. Bodey. 1992. Lack of activity of amphotericin B in systemic murine fusarial infection. J. Infect. Dis. 165:1155-1157. [PubMed] [Google Scholar]
- 2.Arikan, S., M. Lozano-Chiu, V. Paetznick, and J. H. Rex. 2001. In vitro susceptibility testing methods for caspofungin against Aspergillus and Fusarium isolates. Antimicrob. Agents Chemother. 45:327-330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boutati, E. I., and E. J. Anaissie. 1997. Fusarium, a significant emerging pathogen in patients with hematologic malignancy: ten years' experience at a cancer center and implications for management. Blood 90:999-1008. [PubMed] [Google Scholar]
- 4.Cofrancesco, E., C. Boschetti, M. A. Viviani, C. Bargiggia, A. M. Tortorano, M. Cortellaro, and C. Zanussi. 1992. Efficacy of liposomal amphotericin B (AmBisome) in the eradication of Fusarium infection in a leukaemic patient. Haematologica 77:280-283. [PubMed] [Google Scholar]
- 5.Datry, A., V. Leblond, C. Feger, J. Gabarre, E. Gueho, G. Lesco, M. Danis, J. L. Binet, and M. Gentilini. 1988. A propos de 3 cas de mycose a Fusarium sp. Bull. Soc. Fr. Mycol. Méd. 17:137-142. [Google Scholar]
- 6.Espinel-Ingroff, A. 1998. Comparison of in vitro activities of the new triazole SCH56592 and the echinocandins MK-0991 (L-743,872) and LY303366 against opportunistic filamentous and dimorphic fungi and yeasts. J. Clin. Microbiol. 36:2950-2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Freidank, H. 1995. Hyalohyphomycoses due to Fusarium spp.—two case reports and review of the literature. Mycoses 38:69-74. [DOI] [PubMed] [Google Scholar]
- 8.Gamis, A. S., T. Gudnason, G. S. Giebink, and N. K. C. Ramsay. 1991. Disseminated infection with Fusarium in recipients of bone marrow transplants. Rev. Infect. Dis. 13:1077-1088. [DOI] [PubMed] [Google Scholar]
- 9.Guarro, J., and J. Gené. 1995. Opportunistic fusarial infections in humans. Eur. J. Clin. Microbiol. Infect. Dis. 14:741-754. [DOI] [PubMed] [Google Scholar]
- 10.Guarro, J., M. Nucci, T. Akiti, and J. Gene. 2000. Mixed infection caused by two species of Fusarium in a human immunodeficiency virus-positive patient. J. Clin. Microbiol. 38:3460-3462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guarro, J., I. Pujol, and E. Mayayo. 1999. In vitro and in vivo experimental activities of antifungal agents against Fusarium solani. Antimicrob. Agents Chemother. 43:1256-1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hay, R. J. 1994. Liposomal amphotericin B, AmBisome. J. Infect. 28(Suppl. 1):35-43. [DOI] [PubMed] [Google Scholar]
- 13.June, C. H., P. G. Beatty, H. M. Shulman, and M. G. Rinaldi. 1986. Disseminated Fusarium moniliforme infection after allogeneic marrow transplantation. South. Med. J. 79:513-515. [DOI] [PubMed] [Google Scholar]
- 14.Li, R. K., and M. G. Rinaldi. 1999. In vitro antifungal activity of nikkomycin Z in combination with fluconazole or itraconazole. Antimicrob. Agents Chemother. 43:1401-1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lopez-Berestein, G., G. P. Bodey, V. Fainstein, M. Keating, L. S. Frankel, B. Zeluff, L. Gentry, and K. Mehta. 1989. Treatment of systemic fungal infections with liposomal amphotericin B. Arch. Intern. Med. 149:2533-2536. [PubMed] [Google Scholar]
- 16.Mills, W., R. Chopra, D. C. Linch, and A. H. Goldstone. 1994. Liposomal amphotericin B in the treatment of fungal infections in neutropenic patients: a single-centre experience of 133 episodes in 116 patients. Br. J. Haematol. 86:754-760. [DOI] [PubMed] [Google Scholar]
- 17.Okada, H., S. Hatmani, M. Kondo, T. Imai, S. Itoh, K. Isobe, and S. Onishi. 2000. Successful treatment of disseminated Fusarium infection in an infant with leukemia. Int. J. Haematol. 72:494-498. [PubMed] [Google Scholar]
- 18.Ortoneda, M., J. Capilla, I. Pujol, F. J. Pastor, E. Mayayo, J. Fernández-Ballart, and J. Guarro. 2002. Liposomal amphotericin B and granulocyte colony-stimulating factor therapy in a murine model of invasive infection by Scedosporium prolificans. J. Antimicrob. Chemother. 49:525-529. [DOI] [PubMed] [Google Scholar]
- 19.Poirot, J. L., J. P. Laporte, E. Gueho, F. Tessier, A. Verny, M. Marteau, P. Roux, and G. Duhamel. 1985. A propos d'un cas de mycose profonde à Fusarium verticillioides. Med. Maladies Infect. 10:529-532. [Google Scholar]
- 20.Pujol, I., J. Guarro, J. Gene, and J. Sala. 1997. In-vitro antifungal susceptibility of clinical and environmental Fusarium spp. strains. J. Antimicrob. Chemother. 39:163-167. [DOI] [PubMed] [Google Scholar]
- 21.Rabodoniria, M., M. A. Piens, M. F. Monier, E. Guého, D. Fière, and M. Mojon. 1994. Fusarium infections in immunocompromised patients: case reports and literature review. Eur. J. Clin. Microbiol. Infect. Dis. 13:152-161. [DOI] [PubMed] [Google Scholar]
- 22.Richardson, S. E., R. M. Bannatyne, R. C. Summerbell, J. Milliken, R. Gold, and S. S. Weitzman. 1998. Disseminated fusarial infection in the immunocompromised host. Rev. Infect. Dis. 10:1171-1181. [DOI] [PubMed] [Google Scholar]
- 23.Tawara, S., F. Ikeda, K. Maki, Y. Morishita, K. Otomo, N. Teratani, T. Goto, M. Tomishima, H. Ohki, A. Yamada, K. Kawabata, H. Takasugi, K. Sakane, H. Tanaka, F. Matsumoto, and S. Kuwahara. 2000. In vitro activities of a new lipopeptide antifungal agent, FK463, against a variety of clinically important fungi. Antimicrob. Agents Chemother. 44:57-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Veglia, K. S., and V. J. Marks. 1987. Fusarium as a pathogen. A case report of Fusarium sepsis and review of the literature. J. Am. Acad. Dermatol. 16:260-263. [PubMed] [Google Scholar]
- 25.Viscoli, C., E. Castagnola, C. Moroni, A. Garaventa, G. Manno, and C. Savioli. 1990. Infection with Fusarium species in two children with neuroblastoma. Eur. J. Clin. Microbiol. Infect. Dis. 9:773-776. [DOI] [PubMed] [Google Scholar]
- 26.Walsh, T. J., J. L. Goodman, P. Pappas, I. Bekersky, D. N. Buell, M. Roden, J. Barrett, and E. J. Anaissie. 2001. Safety, tolerance, and pharmacokinetics of high-dose liposomal amphotericin B (AmBisome) in patients infected with Aspergillus and other filamentous fungi: maximum tolerated dose study. Antimicrob. Agents Chemother. 45:3487-3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Young, N. A., K. J. Kwon-Chung, T. T. Kubota, A. E. Jennings, and R. I. Fisher. 1978. Disseminated infection by Fusarium moniliforme during treatment for malignant lymphoma. J. Clin. Microbiol. 7:589-594. [DOI] [PMC free article] [PubMed] [Google Scholar]