Abstract
Acyclovir cream has been available for the treatment of herpes labialis in numerous countries outside the United States for over a decade. Evidence for its efficacy comes from a few small clinical trials conducted in the 1980s. To examine more comprehensively the efficacy and safety of this formulation, we conducted two independent, identical, parallel, randomized, double-blind, vehicle-controlled, large-scale multicenter clinical trials. Healthy adults with a history of frequent herpes labialis were recruited from the general population, screened for eligibility, randomized equally to 5% acyclovir cream or vehicle control, given study medication, and told to self-initiate treatment five times daily for 4 days beginning within 1 h of the onset of a recurrent episode. The number of patients who treated a lesion was 686 in study 1 and 699 in study 2. In study 1, the mean duration of episodes was 4.3 days for patients treated with acyclovir cream and 4.8 days for those treated with the vehicle control (hazards ratio [HR] = 1.23; 95% confidence interval [CI], 1.06 to 1.44; P = 0.007). In study 2, the mean duration of episodes was 4.6 days for patients treated with acyclovir cream and 5.2 days for those treated with the vehicle control (HR = 1.24; 95% CI, 1.06 to 1.44; P = 0.006). Efficacy was apparent whether therapy was initiated “early” (prodrome or erythema lesion stage) or “late” (papule or vesicle stage). There was a statistically significant reduction in the duration of lesion pain in both studies. Acyclovir cream did not prevent the development of classical lesions (progression to vesicles, ulcers, and/or crusts). Adverse events were mild and infrequent.
Acyclovir (ACV) has been found to be efficacious in the management of herpes labialis in a variety of formulations and routes of administration. ACV (5%) ointment (Zovirax ointment) accelerated healing of herpes labialis in immunocompromised patients (22). Peroral administration of 400 mg of ACV five times/day for 5 days significantly reduced virus shedding and reduced lesion healing time and the duration of lesion pain among the subgroup of patients who started treatment “early” (in the prodrome or erythema lesion stage) (19). Peroral ACV administered as prophylaxis at 400 mg two times/day significantly reduces the frequency of recurrent episodes and prevents herpes-associated erythema multiforme (4, 8, 11).
Demonstration of the clinical efficacy of topical formulations of antiviral drugs among immunocompetent patients has been difficult because of the marked variance in lesion severity that occurs, necessitating large numbers of subjects; the rapid natural healing of lesions; and the difficulty in finding a topical drug formulation that facilitates skin penetration without causing undue skin irritation (15, 16, 20). Several studies with ACV ointment in immunocompetent subjects provided sparse evidence of efficacy in this population (1, 6, 13, 17). ACV was shown to penetrate human skin more effectively in a cream than in an ointment formulation (3). Accordingly, data supporting the efficacy of ACV cream have been more readily obtained, sufficient to support licensure in European countries, even though the sizes of the studies were small (2, 7, 21) and one negative report has been published (9).
Since the original studies of ACV cream that were conducted 10 to 20 years ago, there has been considerable progress in our understanding of sample size requirements for herpes labialis studies and the most appropriate outcome measures (12). Using a more robust and modern protocol, we have reexamined the efficacy of ACV cream in two independent clinical trials.
(This research was presented in part at the 14th International Conference on Antiviral Research, Seattle, Wash., 8 to 12 April 2001.)
MATERIALS AND METHODS
Study overview.
Two independent, identical, parallel, randomized, double-blind, vehicle-controlled, large-scale multicenter clinical trials were conducted to compare the effectiveness of ACV cream versus cream vehicle for the treatment of an acute episode of herpes labialis in immunocompetent adults (ZOVA3003 and ZOVA3004). Study 1 was conducted at 22 centers and study 2 was conducted at 23 centers, all located in the United States. Study enrollment began in November 1997, and the last patient to treat a lesion was in June 1998. The protocol and statement of informed consent were reviewed and approved by a central or local institutional review board prior to each center's initiation.
Study population.
Patients were included in these studies if they were at least 18 years of age and in good general health. Patients had a clinical history of recurrent herpes labialis with at least three episodes of typical herpes labialis lesions in the past year. Patients had a history of prodromal symptoms during more than 50% of prior herpes labialis episodes and had a history of greater than 50% of their herpes labialis episodes producing classical lesions (i.e., vesicle, ulcer, and/or hard crust). Written informed consent was obtained from each patient prior to entry into a study. Patients were not allowed to use other antiviral medications, anti-inflammatory medications, steroids, or analgesics during the treatment period, until healing occurred. The use of any topical agents in the treated area (including cosmetics, lip balms, sunscreens, etc.) during the treatment period was prohibited until healing occurred. Mechanical disruption (i.e., scrubbing, lancing, shaving, etc.) of the prodromal area or lesion was prohibited. Patients were not enrolled in the studies if they were pregnant or breast-feeding, were immunocompromised, or had received any antiviral therapy during the previous 4 weeks.
Study medication.
ACV cream, 5%, was supplied in white plastic tubes containing 2 g of medication. Vehicle cream control was prepared in identical tubes. Four tubes of cream were packaged in a single box for each clinical trial randomized medication number. ACV cream and vehicle cream were identical in appearance, odor, and consistency. The vehicle formulation contained 40% propylene glycol and 1% sodium lauryl sulfate and was identical to the product currently marketed in Europe.
Study design.
Patients were screened to ascertain herpes labialis history, demographics, and conformance with study inclusion and exclusion criteria. Following screening, patients were randomly assigned to either ACV cream or the vehicle cream treatment group according to a computer-generated randomization schedule. The random code used for treatment assignment was double-blind and maintained by the sponsor during the trial. Patients were instructed to initiate treatment within 1 h of onset of either prodromal symptoms (if prodrome occurred) or the first clinical sign of herpes labialis and to present to the clinic within 12 h of initiating treatment. Patients who had symptoms or signs of more than 1 h in duration, intraoral lesions, or lesions within the nares were instructed not to initiate therapy for the current episode but to wait for the next recurrence. Patients were instructed to apply study treatment five times daily for 4 days (20 total applications over 96 h). Patients were followed daily at the clinic until healing occurred. The study was continued until the treatment target numbers were completed.
Patients were instructed at screening and randomization on how to complete a diary card, stage herpes labialis progression, and record key milestone events important in assessing the outcomes of the treatment. Since an interval of time could elapse between instructions given at randomization and when treatment was initiated, the diary book contained photographs of herpes labialis lesion stages as well as written instructions. On the diary card, the patient was to record the date and time of onset of a herpes labialis episode, dates and times of each treatment application, lesion stage prior to each application, severity of pain and change in pain compared to the prior evaluation (starting at dose number 2), and presence or absence of tenderness. All patient assessments were completed prior to application of study medication and recorded in the diary book. In addition, study sites contacted patients every 2 to 4 weeks to remind them of study procedures and that they should initiate treatment within 1 h of the onset of prodromal symptoms and call for a clinic appointment within 12 h of onset.
Lesion stage was assessed as follows: prodrome (symptoms including itching, pain, tingling, but no physical evidence of disease by inspection or by palpation in the application area), macule (erythema in the application area), papule (any elevation of skin without fluid in the application area), vesicle (blister, fluid filled or collapsed, in the application area), crusted (soft or hard crust in the application area), or healed (return to normal skin with the cessation of signs or symptoms, including loss of hard crust if a vesicle formed; residual erythema may be present). Pain was assessed by the patient using an ordinal (0 to 10) scale, where 0 = no pain and 10 = very painful.
Databases.
At each clinic visit, the clinician and/or the study coordinator observed the lesion stage and recorded this assessment on the site source documents and the case report form (clinician-observed database), prior to reviewing the patient's entries in the diary card or any discussions with the patient. The patient independently assessed lesion stage and recorded this information in the patient diary (patient-assessed database). The clinician and/or the study coordinator then discussed the progression of the herpes labialis episode with the patient, reviewed the patient's diary book, and made a third record of lesion stage changes based on the clinical observations, the diary record, and any adjustments arising from the discussion (clinician-assessed database).
Efficacy assessments.
The primary efficacy measure was the clinician-assessed duration of herpes labialis episode. Secondary efficacy measures were the patient-assessed duration of pain, as recorded in the patient's diary card, and the proportion of patients developing a classical herpes labialis lesion (vesicles, ulcers, and/or crusts).
Safety assessments.
The safety and tolerability of ACV cream were assessed by the evaluation of any changes in the occurrence and nature of any adverse experiences reported during the treatment and follow-up period of one treated herpes labialis episode.
Sample size.
A sample size of 652 patients (with two treatment arms: 326 treated with ACV cream and 326 treated with vehicle cream) was estimated for each study to achieve a statistical power of 0.80 at a two-sided significance level of 0.05 to detect a 0.5-day difference in the duration of an episode between the ACV cream and vehicle treatment arms, with a standard deviation of 2.25 days. An iterative procedure was used to estimate a sample size for these studies. The iterative procedure reached stability at an estimated sample size of 320 patients per treatment arm. An additional six patients were added to each treatment arm to allow blocking of treatments during randomization, resulting in a sample size of 326 each for the ACV cream and vehicle cream treatment arms. It was estimated that at least 1,000 subjects would need to be screened and randomized to a treatment group to ensure that a total of 652 subjects completed treatment for a single episode of herpes labialis in each study. This overrandomization was necessary due to the episodic nature of herpes labialis.
Statistical methods.
If no vesicle formed, the duration of episode was measured from the initiation of treatment to the return to normal skin with cessation of signs or symptoms. If a vesicle formed, the duration of episode was measured from the initiation of treatment to the loss of hard crust (residual erythema could be present after loss of hard crust). Patient-assessed duration of pain was defined as the period from the onset of pain to the final cessation of pain. Final cessation of pain was the point in time after which no pain score was greater than 1 throughout the remainder of dosing. For both measurements, univariate summaries (means, standard errors of the mean, medians, minima, and maxima) defined in the protocol and analysis plan were computed and tabulated in each treatment group. A two-sample t test was performed to compare the mean durations (10). For supportive analysis, a time-to-event analysis (“survival analysis”) was completed for herpes labialis episode duration following Kaplan-Meier methodology and a Cox proportional hazards model (5).
RESULTS
Characteristics of study population.
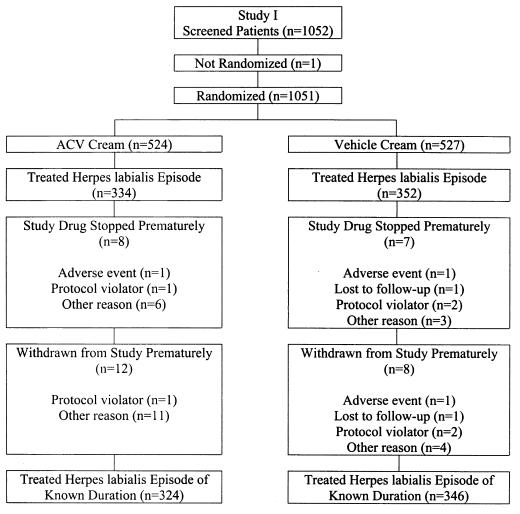
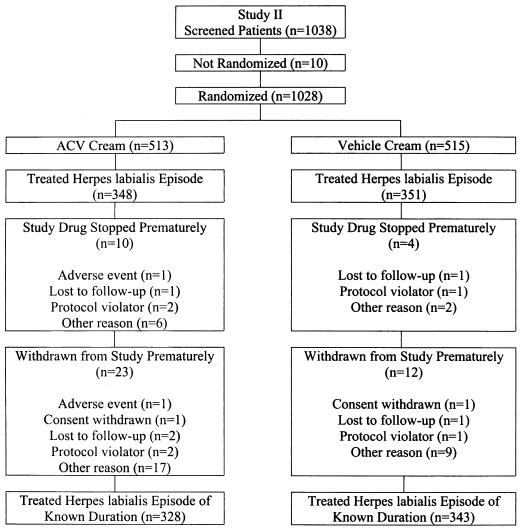
A total of 1,051 patients were randomized in study 1 and a total of 1,028 patients were randomized in study 2 (Fig. 1 and 2). Patients who treated a herpes labialis episode of known duration during the studies were included in efficacy analyses. A total of 324 acyclovir-treated patients and 346 vehicle-treated patients were included in study 1, and 328 acyclovir-treated patients and 343 vehicle-treated patients were included in study 2.
FIG. 1.
Randomization and disposition of patients in study 1.
FIG. 2.
Randomization and disposition of patients in study 2.
The majority of patients in each treatment group were female (71 to 76%) and Caucasian (94 to 95%). Disease characteristics were similar across treatment groups for both studies (Table 1).
TABLE 1.
Demographic and disease characteristics by treatment group
| Demographic characteristic | Results of study 1
|
Results of study 2
|
||
|---|---|---|---|---|
| Acyclovir (n = 334) | Vehicle (n = 352) | Acyclovir (n = 348) | Vehicle (n = 351) | |
| No. (%) of females | 251 (75) | 258 (73) | 264 (76) | 248 (71) |
| Race [no. (%) of Caucasians] | 314 (94) | 334 (95) | 326 (94) | 329 (94) |
| Age in years [mean (range)] | 38.6 (18-79) | 38.5 (18-81) | 38.5 (18-87) | 38.5 (18-74) |
| History of herpes labialis infection in years [mean (range)] | 22.6 (1-65) | 22.7 (1-60) | 22.6 (1-80) | 23.8 (1-70) |
| No. of episodes of herpes labialis producing classical lesions in last 12 months [mean (range)] | 5.93 (2-24) | 6.16 (0-72) | 6.62 (2-50) | 5.95 (2-48) |
| Time between onset of current herpes labialis episode and initiation of therapy in hours [mean (range)] | 0.6 (0-12.3) | 0.5 (0-7.0) | 0.7 (0-12.5) | 0.8 (0-13.0) |
Efficacy results.
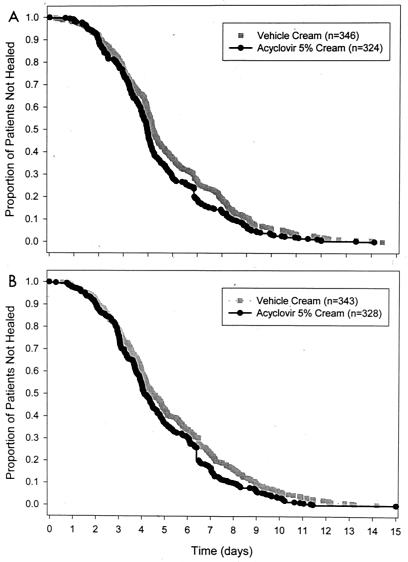
The mean clinician-assessed duration of herpes labialis episode (Table 2) was significantly shorter for patients treated with ACV cream versus patients treated with vehicle cream in each of the two clinical trials (study 1: hazards ratio = 1.23, P = 0.007; study 2: hazards ratio = 1.24, P = 0.006). Plots of the Kaplan-Meier survival curves of clinician-assessed duration of herpes labialis episode for the two studies are presented in Fig. 3. In study 1, the mean durations of herpes labialis episodes for patients with a known duration were 4.3 days for patients treated with ACV cream and 4.8 days for patients treated with vehicle cream (P = 0.010). In study 2, the mean durations of herpes labialis episodes for patients with a known duration were 4.6 days for patients treated with ACV cream and 5.2 days for patients treated with vehicle cream (P = 0.007). Analysis of the lesion course and comparison of the treatment groups according to “vesicle healing time” (time from vesicle onset to loss of crust) yielded similar findings (data not shown). There was a strong correlation between episode duration by clinician assessment compared to the clinician-observed database (r2 = 0.98 and 0.99 for the two studies). The correlation between clinician assessment and patient assessment values was not as strong (r2 = 0.91 and 0.97).
TABLE 2.
Duration of clinician-assessed herpes labialis episode in patients with known duration of episode
| Duration of episode | Study 1a
|
Study 2b
|
||
|---|---|---|---|---|
| Acyclovir (n = 324) | Vehicle (n = 346) | Acyclovir (n = 328) | Vehicle (n = 343) | |
| Mean (days) | 4.3c | 4.8 | 4.6d | 5.2 |
| Range (days) | 0.6-13.1 | 0.3-13.4 | 0.3-15.0 | 0.6-15.0 |
The hazards ratio of study 1 was 1.23 (P = 0.007), with a 95% confidence interval of 1.06 to 1.44.
The hazards ratio of study 2 was 1.24 (P = 0.006), with a 95% confidence interval of 1.06 to 1.44.
P = 0.010.
P = 0.007.
FIG. 3.
Kaplan-Meier survival curves of clinician-assessed duration of herpes labialis episode for study 1 (A) and study 2 (B).
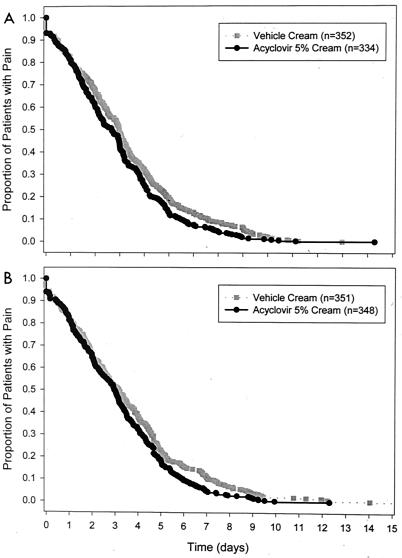
The mean patient-assessed duration of pain (Table 3) was significantly shorter for patients treated with ACV cream versus that for patients treated with vehicle cream in each of the two clinical trials (study 1: hazards ratio = 1.20, P = 0.017; study 2: hazards ratio = 1.21, P = 0.014). Plots of the Kaplan-Meier survival curves of patient-assessed duration of pain for the two studies are presented in Fig. 4. In study 1, the mean durations of pain were 2.9 days for patients treated with ACV cream and 3.2 days for patients treated with vehicle cream (P = 0.024). In study 2, the mean durations of pain were 3.1 days for patients treated with ACV cream and 3.5 days for patients treated with vehicle cream (P = 0.027).
TABLE 3.
Duration of patient-assessed pain
| Duration of pain | Study 1a
|
Study 2b
|
||
|---|---|---|---|---|
| Acyclovir (n = 334) | Vehicle (n = 352) | Acyclovir (n = 348) | Vehicle (n = 351) | |
| Mean (days) | 2.9c | 3.2 | 3.1d | 3.5 |
| Range (days) | 0.0-13.3 | 0.0-12.0 | 0.0-12.3 | 0.0-15.8 |
The hazards ratio for study 1 was 1.20 (P = 0.017), with a 95% confidence interval of 1.03 to 1.40.
The hazards ratio for study 2 was 1.21 (P = 0.014), with a 95% confidence interval of 1.04 to 1.40.
P = 0.024.
P = 0.027.
FIG. 4.
Kaplan-Meier survival curves of patient-assessed duration of pain for study 1 (A) and study 2 (B).
ACV cream did not prevent the development of classical lesions (episodes progressing to the vesicle, ulcer, and/or crust lesion stages; data not shown).
Safety results.
ACV cream was well tolerated in both studies. The overall incidence of adverse events was similar for patients treated with ACV cream and patients treated with vehicle cream (study 1: 8 and 10%, respectively; study 2: 10 and 9%, respectively). The most commonly reported adverse events in patients using either ACV cream or vehicle cream in each study were headache, cracked lips, and dry lips (Table 4). All other adverse events occurred in <1% of patients in each treatment group in each study. No serious adverse events occurred in study 1. One serious adverse event occurred in study 2: a patient treated with ACV cream was hospitalized with chest pain, and the event was considered unrelated to study drug.
TABLE 4.
Most frequently occurring adverse events in patients treated with ACV cream or vehicle cream
| Adverse event | No. in study 1 (%)
|
No. in study 2 (%)
|
||
|---|---|---|---|---|
| Acyclovir (n = 334) | Vehicle (n = 352) | Acyclovir (n = 348) | Vehicle (n = 351) | |
| Headache | 3 (<1) | 9 (3) | 5 (1) | 6 (2) |
| Flakiness of skin | 0 (0) | 0 (0) | 1 (<1) | 6 (2) |
| Desquamation | 2 (<1) | 1 (<1) | 1 (<1) | 4 (1) |
DISCUSSION
Two large-scale, randomized, placebo-controlled trials were conducted, and we have demonstrated similar and highly statistically significant effects in both studies by ACV cream on the duration of herpes labialis episodes and the duration of lesion pain. These results unequivocally establish the efficacy of ACV cream on this disease, which previously had been examined in small-scale trials (2, 7, 9, 21). In study 1, the episode duration was reduced by 0.5 days (10%; P = 0.007), and in study 2, episode duration was reduced by 0.6 days (12%; P = 0.006). The duration of lesion pain was reduced by 0.3 days in study 1 (9%; P = 0.017), and in study 2 it was reduced by 0.4 days (11%; P = 0.014). Of note, the effects were seen both in individuals who treated their lesions in an “early” lesion stage, prodrome or erythema, and in those where the lesion was more pathologically advanced, in one of the “late” stages—papule, vesicle, or ulcer.
The findings in this trial were very similar to those of a recent large study of penciclovir cream (Denavir) for the treatment of herpes labialis (16). Penciclovir cream reduced the median time to healing by 0.7 days (13%; P < 0.001) and the median time to loss of pain by 0.6 days (15%; P < 0.001). As in the present trial, drug efficacy could be seen in patients who started treatment in either the early or the late lesion stage (Table 5). The protocols in the ACV cream and penciclovir cream studies were also alike: patients were selected who historically developed classical herpes lesions (vesicle formation); treatment was patient initiated; and treatment had to be initiated within 1 h of the first sign or symptom of a recurrence. ACV cream was applied five times/day for 4 days (20 doses) while penciclovir cream was applied nine times/day for 4 days (36 doses).
TABLE 5.
Duration of clinician-assessed herpes labialis episode in patients with known duration of episode for early and late treatment initiation
| Duration of episode | Study 1
|
Study 2
|
||
|---|---|---|---|---|
| Acyclovir | Vehicle | Acyclovir | Vehicle | |
| Early treatment initiationa | ||||
| Mean (days) | 4.1b | 4.4 | 4.7c | 5.2 |
| Range (days) | 0.6-13.1 | 0.3-13.4 | 0.6-5.0 | 0.8-15.0 |
| Late treatment initiationd | ||||
| Mean (days) | 4.7e | 5.4 | 4.6f | 5.2 |
| Range (days) | 1.3-11.0 | 0.9-13.0 | 0.7-11.0 | 0.6-14.2 |
Early treatment initiation was at the prodrome or erythema stage. Study 1: acyclovir, n = 189; vehicle, n = 200. Study 2: acyclovir, n = 201; vehicle, n = 211. The hazards ratio for study 1 was 1.19 (P = 0.091), with a 95% confidence interval of 0.97 to 1.45. The hazards ratio for study 2 was 1.16 (P = 0.136), with a 95% confidence interval of 0.95 to 1.40.
P = 0.158.
P = 0.079.
Late treatment initiation was at the macule, papule, vesicle, or crusted stage. Study 1: acyclovir, n = 135; vehicle, n = 146. Study 2: acyclovir, n = 127; vehicle, n = 132. The hazards ratio for study 1 was 1.39 (P = 0.007), with a 95% confidence interval of 1.09 to 1.76. The hazards ratio for study 2 was 1.35 (P = 0.017), with a 95% confidence interval of 1.05 to 1.73.
P = 0.040.
P = 0.042.
The observation with both ACV cream and penciclovir cream that efficacy occurs independently of lesion stage at the start of treatment is paradoxical. It is intuitive that interruption of virus replication prior to the development of major pathological processes in the epidermis would be more likely to preserve the skin from virus-induced damage and abbreviate the lesion course than application of treatment at a later stage. This concept received support from two prior clinical trials with peroral ACV and topical idoxuridine in dimethyl sulfoxide which showed that antiviral nucleoside efficacy was limited to the patients who initiated treatment in the prodrome or erythema lesion stage (18, 19).
One potential explanation for the discrepancy between the peroral ACV and topical idoxuridine in dimethyl sulfoxide studies on the one hand and the penciclovir and ACV cream trials on the other is that the delivery of drug to the site of virus replication was far more efficient by the oral route of administration and from the dimethyl sulfoxide vehicle than from the cream vehicles (14). Accordingly, in the former two trials, peroral ACV and topical idoxuridine applied in the early lesion stages were immediately active with enhanced clinical activity compared to late application whereas, in contrast, the drugs in the cream formulations applied in the early lesion stages may not have been able to penetrate and reach the target site and inhibit virus replication at that time, instead becoming active at a later point when the lesion was more advanced and the stratum corneum was perforated or destroyed—effectively similar to application of treatment in a late lesion stage and hence yielding the same therapeutic benefit.
Acknowledgments
We thank Margaret Schultz and William Harris, Glaxo Wellcome Inc., for administrative support and members of the Acyclovir Cream Study Group (data acquisition): (study 1) Richard Bath, Cincinnati, Ohio; Joan Benz, Cedar Rapids, Iowa; Scott Clark, Longmont, Colo.; Alvin E. Fisher, Providence, R.I.; John Frost, Rhinelander, Wis.; Timothy Howard, Huntsville, Ala.; Irwin Kerber, Dallas, Tex.; Mark Kutcher, Chapel Hill, N.C.; William Lang, San Francisco, Calif.; Lawrence Larsen, Salt Lake City, Utah; Patricia Lee, Nassau Bay, Tex.; Lisa Marr, Portland, Oreg.; David Morris, Tulsa, Okla.; R. Lamar Parker, Jr., Winston-Salem, N.C.; Jerald Powers, Scottsdale, Ariz.; Alan Resnik, Charlotte, N.C.; Michele Reynolds, Dallas, Tex.; Robert Roth, Chico, Calif.; Gary Ruoff, Kalamazoo, Mich.; and Stephan Sharp, Nashville, Tenn.; (study 2) David Bernstein, Cincinnati, Ohio; Karl Beutner, Vallejo, Calif.; Mark Blatter, Pittsburgh, Pa.; Cathlen Collins, Champaign, Ill.; Donald England, Eugene, Oreg.; John Fling, Fort Worth, Tex.; Gary Gross, Salem, Va.; David Gubin, Memphis, Tenn.; Daniel Havlicheck, East Lansing, Mich.; W. John Henry III, Greer, S.C.; Terry Jones, Bryan, Tex.; Ronald Keeney, Raleigh, N.C.; Robert Lapidus, Wheat Ridge, Colo.; James McCarty, Fresno, Calif.; Carl Mortiz, Boulder, Colo.; John Murray, St. Petersburg, Fla.; Patrick Ottuso, Vero Beach, Fla.; Michael Siegel, Baltimore, Md.; Stephen Tyring, Nassau Bay, Tex.; Mervin Weerasinghe, Rochester, N.Y.; and Donna Zeide, West Palm Beach, Fla.
This study was funded in part by a grant from GlaxoSmithKline.
REFERENCES
- 1.Fiddian, A. P., and L. Ivanyi. 1983. Topical acyclovir in the management of recurrent herpes labialis. Br. J. Dermatol. 109:321-326. [DOI] [PubMed] [Google Scholar]
- 2.Fiddian, A. P., J. M. Yeo, R. Stubbings, and D. Dean. 1983. Successful treatment of herpes labialis with topical acyclovir. Br. Med. J. 286:1699-1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Freeman, D. J., N. V. Sheth, and S. L. Spruance. 1986. Failure of topical acyclovir (ACV) in ointment to penetrate human skin. Antimicrob. Agents Chemother. 29:730-732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Green, J. A., S. L. Spruance, G. Wenerstrom, and M. W. Piepkorn. 1985. Post-herpetic erythema multiforme prevented with prophylactic oral acyclovir. Ann. Intern. Med. 102:632-633. [DOI] [PubMed] [Google Scholar]
- 5.Harris, E. K., and A. Albert. 1991. Survivorship analysis for clinical studies. Marcel Dekker, New York, N.Y.
- 6.Raborn, G. W., W. T. McGaw, M. Grace, and L. Houle. 1989. Herpes labialis treatment with acyclovir 5 per cent ointment. Can. Dent. Assoc. J. 55:135-137. [PubMed] [Google Scholar]
- 7.Raborn, G. W., W. T. McGaw, M. Grace, J. Percy, and S. Samuels. 1989. Herpes labialis treatment with acyclovir 5% modified aqueous cream: a double-blind, randomized trial. Oral Surg. Oral Med. Oral Pathol. 67:676-679. [DOI] [PubMed] [Google Scholar]
- 8.Rooney, J. F., S. E. Straus, M. L. Mannix, C. R. Wohlenberg, D. W. Alling, J. A. Dumois, and A. L. Notkins. 1993. Oral acyclovir to suppress frequently recurrent herpes labialis: a double-blind, placebo-controlled trial. Ann. Intern. Med. 118:268-272. [DOI] [PubMed] [Google Scholar]
- 9.Shaw, M., M. King, J. M. Best, J. E. Banatvala, J. R. Gibson, and M. R. Klaber. 1985. Failure of Acyclovir cream in treatment of recurrent herpes labialis. Br. Med. J. 291:7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sokal, R. R., and J. F. Rohlf. 1995. Biometry: the principles and practice of statistics in biological research. W. H. Freeman, New York, N.Y.
- 11.Spruance, S. L. 1993. Prophylactic chemotherapy with acyclovir for recurrent herpes simplex labialis. J. Med. Virol. 41(Suppl. 1):27-32. [DOI] [PubMed] [Google Scholar]
- 12.Spruance, S. L. 1995. Herpes simplex labialis, p. 3-42. In S. L. Sacks, S. E. Straus, R. J. Whitley, and P. D. Griffiths (ed.), Clinical management of herpes viruses. IOS Press, Amsterdam, The Netherlands.
- 13.Spruance, S. L., C. S. Crumpacker, L. E. Schnipper, E. R. Kern, S. Marlow, K. A. Arndt, and J. C. Overall, Jr. 1984. Early, patient-initiated treatment of herpes labialis with topical 10% acyclovir. Antimicrob. Agents Chemother. 25:553-555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spruance, S. L., M. B. McKeough, and J. R. Cardinal. 1984. Penetration of guinea pig skin by acyclovir in different vehicles and correlation with the efficacy of topical therapy of experimental cutaneous herpes simplex virus infection. Antimicrob. Agents Chemother. 25:10-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spruance, S. L., M. B. McKeough, K. Sugibayashi, F. Robertson, P. Gaede, and D. S. Clark. 1984. Effect of azone and propylene glycol on penetration of trifluorothymidine through skin and the efficacy of different topical formulations against cutaneous herpes simplex virus infection in guinea pigs. Antimicrob. Agents Chemother. 26:819-823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spruance, S. L., T. L. Rea, C. Thoming, R. Tucker, R. Saltzman, and R. Boon. 1997. Penciclovir cream for the treatment of herpes simplex labialis. A randomized, multicenter, double-blind, placebo-controlled trial. Topical Penciclovir Collaborative Study Group. JAMA 277:1374-1379. [PubMed] [Google Scholar]
- 17.Spruance, S. L., L. E. Schnipper, J. C. Overall Jr, E. R. Kern, B. Wester, J. Modlin, G. Wenerstrom, C. Burton, K. A. Arndt, G. L. Chiu, and C. S. Crumpacker. 1982. Treatment of herpes simplex labialis with topical acyclovir in polyethylene glycol. J. Infect. Dis. 146:85-90. [DOI] [PubMed] [Google Scholar]
- 18.Spruance, S. L., J. C. B. Stewart, D. J. Freeman, V. J. Brightman, J. L. Cox, G. Wenerstrom, M. B. McKeough, and N. H. Rowe. 1990. Early application of topical 15% idoxuridine in dimethyl sulfoxide shortens the course of herpes simplex labialis: a multicenter placebo-controlled trial. J. Infect. Dis. 161:191-197. [DOI] [PubMed] [Google Scholar]
- 19.Spruance, S. L., J. C. B. Stewart, N. H. Rowe, M. B. McKeough, G. Wenerstrom, and D. J. Freeman. 1990. Treatment of recurrent herpes simplex labialis with oral acyclovir. J. Infect. Dis. 161:185-190. [DOI] [PubMed] [Google Scholar]
- 20.Spruance, S. L., and G. Wenerstrom. 1984. Pathogenesis of herpes simplex labialis. IV. Maturation of lesions within 8 hours after onset and implications for antiviral treatment. Oral Surg. Oral Med. Oral Pathol. 58:667-671. [DOI] [PubMed] [Google Scholar]
- 21.Van Vloten, W. A., R. N. J. Swart, and F. Pot. 1983. Topical acyclovir therapy in patients with recurrent orofacial herpes simplex infections. J. Antimicrob. Chemother. 12:89-93. [DOI] [PubMed] [Google Scholar]
- 22.Whitley, R. J., M. Levin, N. Barton, B. J. Hershey, G. Davis, R. E. Keeney, J. Whelchel, A. G. Diethelm, P. Kartus, and S. J. Soong. 1984. Infections caused by herpes simplex virus in the immunocompromised host: natural history and topical acyclovir therapy. J. Infect. Dis. 150:323-329. [DOI] [PubMed] [Google Scholar]