Abstract
The activities of ampicillin, meropenem, azithromycin, gentamicin, ciprofloxacin, and moxifloxacin against intracellular hemolysin-positive Listeria monocytogenes were measured in human THP-1 macrophages and were compared with the extracellular activities observed in broth. All extracellular concentrations were adjusted to explore ranges that are clinically achievable in human serum upon conventional therapy. In broth, ampicillin, meropenem, and azithromycin were only bacteriostatic, whereas gentamicin, ciprofloxacin, and moxifloxacin were strongly bactericidal in a concentration-dependent manner. In cells, ampicillin, meropenem, azithromycin, and ciprofloxacin were slightly bactericidal (0.3- to 0.8-log CFU reductions), moxifloxacin was strongly bactericidal (2.1-log CFU reduction), and gentamicin was virtually inactive. The difference in the efficacies of moxifloxacin and ciprofloxacin in cells did not result from a difference in levels of accumulation in cells (6.96 ± 1.05 versus 7.75 ± 1.03) and was only partially explainable by the difference in the MICs (0.58 ± 0.04 versus 1.40 ± 0.17 mg/liter). Further analysis showed that intracellular moxifloxacin expressed only approximately 1/7 of the activity demonstrated against extracellular bacteria and ciprofloxacin expressed only 1/15 of the activity demonstrated against extracellular bacteria. Gentamicin did not increase the intracellular activities of the other antibiotics tested. The data suggest (i) that moxifloxacin could be of potential interest for eradication of the intracellular forms of L. monocytogenes, (ii) that the cellular accumulation of an antibiotic is not the only determinant of its intracellular activity (for fluoroquinolones, it is actually a self-defeating process as far as activity is concerned), and (iii) that pharmacodynamics (activity-to-concentration relationships) need to be considered for the establishment of efficacy against intracellular bacteria, just as they are for the establishment of efficacy against extracellular infections.
The treatment of listeriosis with conventional antibiotic therapy remains a challenge, and the intracellular forms of Listeria monocytogenes have proved difficult to eradicate in the absence of a fully effective immune response (4, 5, 18, 51). The classically recommended treatment for listeriosis, which is mainly based on drug sensitivities determined in broth and clinical experience, is still a combination of ampicillin (or a penem) and an aminoglycoside (28, 45). Yet, both types of drugs fail to accumulate to significant levels in cells, at least in short-term experiments (15, 34, 40). Conversely, macrolides or fluoroquinolones accumulate in cells and show activity against the intracellular forms of L. monocytogenes in cell culture models (13, 30, 32, 34, 42). Yet, their use has been disappointing in animal models (2, 24), and the use of ciprofloxacin has met with clinical failures (23).
These observations may suggest the absence of a true correlation between the cellular accumulation of antibiotics in cell models and their effective in vivo intracellular activities (perhaps because of modulation by a series of critical parameters such as subcellular drug bioavailability and intracellular expression of activity (30, 31, 49). Yet, it is also possible that the macrolides and fluoroquinolones that have been examined so far exhibit intrinsic activities that are too weak (i.e., have MICs that are too high, as determined for extracellular bacteria) and therefore cannot be used in vivo at concentrations that are high enough.
The availability of new fluoroquinolones with improved activities against gram-positive bacteria such as moxifloxacin has triggered us to reexamine the potential activity of this class of antimicrobials against intracellular L. monocytogenes. In sharp contrast to β-lactams and macrolides, fluoroquinolones indeed display marked concentration-dependent activities (8, 27). We reasoned that efficient intracellular bacterial killing could be obtained with clinically meaningful extracellular concentrations (Ce) by the combination of this improved activity and the intracellular accumulation of fluoroquinolones. It must be pointed out, however, that the basic pharmacodynamic properties of most antibiotics, including fluoroquinolones, β-lactams, and macrolides, have so far been examined only in extracellular infections. The present work therefore aimed to examine which pharmacodynamic parameter governs the activities of these antibiotics against intracellular bacteria and to determine how critical the parameter is for effective intracellular chemotherapy.
In the present study we used a model of L. monocytogenes-infected human THP-1 macrophages with which we previously demonstrated that bacterial multiplication occurs predominantly in the cytosol (34). Two β-lactams (ampicillin and meropenem), two fluoroquinolones (ciprofloxacin and moxifloxacin), a macrolide (azithromycin), and an aminoglycoside (gentamicin) were systematically examined for their levels of intracellular accumulation and quantitative assessment of bacterial killing at Ces covering the range of concentrations seen in the serum of humans after administration by conventional means.
MATERIALS AND METHODS
Materials.
N,N-Diacetyl-l-Lys-d-Ala-d-Ala (the substrate of the dd-carboxypeptidase) and the purified Streptomyces R39 dd-carboxypeptidase were kindly prepared and donated by J.-M. Frère (Centre d'Ingénierie des Protéines, Université de Liège, Liège, Belgium). Azithromycin was obtained from Pfizer Inc. (Groton, Conn.), and ciprofloxacin and moxifloxacin were from Bayer AG (Wuppertal, Germany). These products were obtained as standard powders for microbiological studies with the following potencies: azithromycin, 94.4%; ciprofloxacin, 85.0%; moxifloxacin, 98.6%. All concentrations were corrected for 100% potency. Gentamicin and meropenem, procured as Geomycin and Meronem, respectively (the brand names of the products registered for clinical use in Belgium), were kindly donated by their corresponding Belgian marketing authorization holders (Shering-Plough Belgium [Brussels, Belgium] and Astra-Zeneca [Astra Pharmaceuticals, Brussels, Belgium], respectively). Ampicillin (sodium salt) was purchased from Sigma-Aldrich Co. (St. Louis, Mo.). Cell culture media and serum were purchased from Gibco Biocult (a subsidiary of Life Science Technologies), Paisley, Scotland. Unless stated otherwise, all other reagents were purchased from E. Merck AG (Darmstadt, Germany).
Bacterial strain and determination of MICs and MBCs.
Hemolysin-producing strain EGD of L. monocytogenes (serotype 1/2a) was obtained, maintained, and characterized exactly as described before (34). MICs were determined both in RPMI 1640 medium (supplemented with 10% decomplemented fetal calf serum and 2 mM glutamine) and in tryptic soy broth (TSB; Difco, Becton Dickinson & Co., Sparks, Md.) by an arithmetic dilution method (34). Minimal bactericidal concentrations (MBCs) were determined in TSB by geometric dilution, with the bacterial suspension set at 5 × 105 CFU/ml and by using as the endpoint the drug concentration that caused a 5-log decrease in the original inoculum.
Time and dose-kill curves studies (acellular system).
Cultures in the logarithmic phase of growth (≈109 bacteria/ml) were centrifuged at 14,000 rpm (Eppendorf 5415 C centrifuge; Gerätgebau Eppendorf GmbH, Engelsdorf, Germany) for 1 min at 4°C. The supernatant was removed and the pelleted bacteria were resuspended at a density of 106 CFU per ml in TSB. Antibiotics were then added at increasing concentrations, and the numbers of viable bacteria (CFU) were determined by plate assay (tryptic soy agar; Difco, Becton Dickinson & Co.) after appropriate periods of incubation. Samples were diluted to reach a mean bacterial content of 100 to 1,000 bacteria/plate (minimum value for experiments with a residual inoculum after exposure to the maximal concentration of fluoroquinolones, 60 CFU). Enumeration was done by a semiautomated method with a Gel Doc 2000 apparatus (Bio-Rad Laboratories, Hercules, Calif.) operated with Quantity One software (Bio-Rad Laboratories) for bacterial colony counting. We checked that the amount of antibiotic carried over was insufficient to impair bacterial growth (i.e., that each time its final concentration was severalfold lower than its MIC) and took into account the dilution of the samples and the amount of fluid (50 μl) plated onto the dishes (13 ml of agar).
Cells, cell infection, and assessment of intracellular activities of antibiotics.
All experiments were performed with THP-1 cells, a human myelomonocytic cell line displaying macrophagelike activity (47). The cells were maintained as described earlier (34). In brief, the cells were cultured as a loose suspension in RPMI 1640 medium supplemented with 10% decomplemented fetal calf serum and 2 mM glutamine in an atmosphere of 95% air-5% CO2. The cells (5 × 105 cells/ml) were infected by using a fresh inoculum of L. monocytogenes (2.5 × 106 CFU/ml) that had been incubated for 1 h at 37°C and washed extensively to remove nonphagocytosed and non-firmly adherent bacteria (four successive sedimentations at 1,300 rpm [5810R Centrifuge; Gerateban Eppendorf, GmbH, Engeldorf, Germany] followed by gentle resuspension in prewarmed sterile phosphate-buffered saline), yielding an average infection index of one bacterium per four macrophages (as determined by counting the numbers of CFU). The cells were thereafter incubated in fresh medium (with or without antibiotics) for up to 5 h. To ensure the absence of extracellular bacteria, the culture medium that contained cells with phagocytosed L. monocytogenes and that had not been exposed to antibiotics after the washing procedure described here were incubated at 37°C for 48 h; no bacterial growth was detected. At suitable intervals, the cells were collected by centrifugation, washed with ice-cold sterile phosphate-buffered saline, and lysed in distilled water. The lysates were then plated on tryptic soy agar at appropriate dilutions for determination of the number of viable bacteria by counting of the colonies (determination of the numbers of CFU) and used for total cell protein measurement (29). All results are expressed as the number of CFU per milligram of cell protein.
Determination of cellular antibiotic accumulation.
Ciprofloxacin and moxifloxacin concentrations were measured by a fluorimetric assay by previously described procedures (32, 39) by use of excitation and emission λ readings of 275 and 450, respectively, for ciprofloxacin and 298 and 504 nm, respectively, for moxifloxacin. A systematic correction for nonspecific fluorescence due to cell protein was performed by subtracting the values obtained for samples from cells not exposed to fluoroquinolones and using protein determination for normalization of the values between samples with different protein contents. This correction amounted to approximately 30 and 10% of the raw readings for ciprofloxacin and moxifloxacin, respectively (due to the conditions of the assay). We checked that the infection of the cells did not cause significant changes in the levels of nonspecific fluorescence. The lowest limits of detection for ciprofloxacin and moxifloxacin (in lysis medium, which consisted of 0.1 M glycine-HCl [pH 3]) were 10 and 25 ng/ml, respectively, with linearity (R2 = 0.997 and 0.999, respectively) at concentrations up to 200 and 400 ng/ml, respectively. The ampicillin concentration was determined by fluorimetry of the 3,6-disubstituted piperazine formed in acid solution in the presence of formaldehyde, as described by Jusko (26) (excitation λ, 346 nm; emission λ, 422 nm; lowest limit of detection, 50 ng/ml; linearity [R2 = 0.992], concentrations up to 500 ng/ml). Meropenem was assayed by an enzymatic method based on the ability of β-lactams to bind to and irreversibly inhibit the bacterial dd-carboxypeptidase (from Streptomyces R39), as described by Frère et al. (19) (lowest limit of detection, 1 ng/ml; linearity [R2 = 0.906], concentrations up to 30 ng/ml). Azithromycin concentrations were measured by microbiological assay (by the disk diffusion method) with Microccocus luteus ATCC 9341 and antibiotic medium 2 (Difco, Becton Dickinson & Co.) adjusted to pH 8 (lowest limit of detection, 0.5 μg/ml; linearity [R2 = 0.998], concentrations up to 8 μg/ml). Gentamicin concentrations were also measured by microbiological assay, but Bacillus subtilis was used as the test organism (lowest limit of detection, 1 μg/ml; linearity [R2 = 0.934], concentrations up to 256 μg/ml). The drug contents of the cells were systematically expressed by reference to the corresponding protein contents of the cells, and the apparent intracellular concentration (Cc) was determined by using a conversion factor of 5 μl of cell volume per mg of cell protein, as discussed in previous publications (43, 48).
Statistical analyses.
Curve-fitting analyses were done with GraphPad Prism software (version 2.01; GraphPad Software, San Diego, Calif.). Other analyses were performed with XLSTAT software (version 4.2; Thierry Famhy, 1995 to 1999; AddinSoft SARL, Paris, France). Comparisons between groups were done by Student's t test, and comparisons between curves (for data presented in Fig. 6) were done by analysis of covariance.
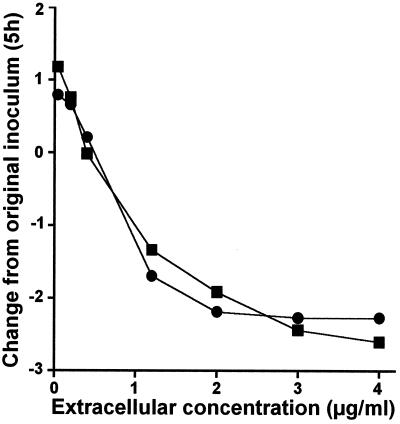
FIG. 6.
Combined data for moxifloxacin from Fig. 2 and 4. The values on the abscissa indicate the actual drug concentration in broth or the macrophage culture fluid. The values on the ordinate indicate the variations in the numbers of CFU per milliliter of broth (closed squares) or per milligram of cell protein (closed circles) after 5 h of incubation. Statistical analysis (analysis of covariance) shows that there were no significant difference between the two sets of data (P = 0.6457).
RESULTS
MICs and MBCs.
The MICs and MBCs of the antibiotics used in the present study are shown in Table 1. No constant, major differences were observed between the MICs determined in broth and those determined in the macrophage culture medium. Apart from meropenem, which displayed a remarkably low MIC, all MICs were between 0.3 and 1.4 μg/ml, i.e., within an approximately twofold geometric dilution range. Determination of MBCs showed that both β-lactams and azithromycin were essentially bacteriostatic over a clinically meaningful range of concentrations. Conversely, both fluoroquinolones and gentamicin were bactericidal within that range.
TABLE 1.
MICs and MBCs of the antibiotics used in the present study for L. monocytogenes EGD serotype 1/2a
| Antibiotic | MICa (mg/liter) in:
|
MBCb (mg/liter) in TSB | |
|---|---|---|---|
| TSB | Culture medium | ||
| Ampicillin | 0.37 ± 0.23 (8) | 0.30 ± 0.12 (8) | >64 |
| Meropenem | 0.050 ± 0.000 (4) | 0.072 ± 0.005 (4) | >64 |
| Ciprofloxacin | 1.36 ± 0.06 (3) | 1.40 ± 0.17 (3) | 4 |
| Moxifloxacin | 0.54 ± 0.05 (5) | 0.58 ± 0.04 (5) | 2 |
| Azithromycin | 0.37 ± 0.11 (3) | 0.43 ± 0.06 (3) | >64 |
| Gentamicin | 0.77 ± 0.06 (3) | 0.83 ± 0.06 (3) | 4 |
Arithmetic dilutions (0.1-μg/ml increments were used for all antibiotics except meropenem (for which [0.01-μg/ml increments were used]). Values are means ± standard deviations the number of independent determinations is given in parentheses).
Geometric dilutions.
General experimental design.
Whether the experiments dealt with extracellular (in broth) or phagocytosed bacteria, all experiments were conducted for a maximum of 5 h. Typical bacterial growth in the absence of antibiotic was approximately 1.2 log CFU in broth and approximately 0.8 log CFU in cells. Antibiotic concentrations were systematically adjusted to concentrations ranging from 0.5 to 100% of the peak concentration (Cmax) usually observed in the serum of humans after administration of conventional doses of each drug (Table 2).
TABLE 2.
Cmax of the antibiotics useda and corresponding Cmax/MIC ratios
| Antibiotic | Cmax (mg/liter) | Human dosage yielding a similar Cmax in serumb (reference) | Cmax/MICc |
|---|---|---|---|
| Ampicillin | 50 | 1 g i.v. (17) | 167 |
| Meropenem | 50 | 1 g i.v. (9) | 694 |
| Ciprofloxacin | 4.3 | 750 mg p.o. (Bayer Corp.d) | 3 |
| Moxifloxacin | 4 | 400 mg p.o. (44) | 7 |
| Azithromycin | 0.4 | 500 mg p.o. (16) | 0.9 |
| Gentamicin | 18 | 5 mg/kg i.v.e (21) | 22 |
The Cmax were meant to mimic the maximal concentrations obtainable in serum in vivo after the administration of commonly accepted dosages to humans.
i.v., intravenously; p.o., orally.
Based on the MICs observed in macrophage culture medium (see Table 1).
Bayer Corporation (U.S. ciprofloxacin package insert, 2000).
Once-daily dosing.
Activity against extracellular L. monocytogenes.
In a first series of experiments, we measured the rate of killing of L. monocytogenes in broth when the organism was exposed to a fixed antibiotic concentration corresponding to the Cmax of each drug. The data presented in Fig. 1 show that the extent and rates of killing were very different among the different antibiotic classes. Thus, the two β-lactams (ampicillin and meropenem) and azithromycin were essentially bacteriostatic. On the contrary, gentamicin and the two fluoroquinolones (ciprofloxacin and moxifloxacin) achieved considerable bacterial killing, and this killing progressed almost linearly with time. Of interest, however, was the fact that gentamicin was immediately bactericidal, whereas a lag period of about 1 h was observed for ciprofloxacin and moxifloxacin.
FIG. 1.
Influences of antibiotics on L. monocytogenes survival (numbers of CFU) in broth upon incubation at a fixed drug concentration for up to 5 h. Closed diamonds, test antibiotics; open squares, controls (to which no antibiotic was added). Values are given as arithmetic means ± standard deviations (n = 3), but most of the corresponding error bars are smaller than the symbols. The values at the bottom of the panel for gentamicin show the number of CFU recorded at the corresponding times.
To further explore the influence of the drug concentration on bacterial growth, the bacteria were then exposed for 5 h to drug concentrations equivalent to 1, 30, and 100% of their Cmax (Fig. 2). It appears that meropenem achieves all of its effect at the lowest concentration tested (0.5 mg/liter; 1% of its Cmax), with no improvement at higher concentrations. Ampicillin was maximally effective at 15 mg/liter (30% of its Cmax). Azithromycin was considerably less potent than ampicillin or meropenem at low concentrations but eventually was as potent as ampicillin or meropenem when it was used at its Cmax (0.4 mg/liter). All these antibiotics, however, remained bacteriostatic. In contrast, both ciprofloxacin and moxifloxacin became bactericidal once their concentrations exceeded approximately 15 to 20% of their Cmax (i.e., concentrations close to their MICs), and this effect was clearly concentration dependent.
FIG. 2.
Influences of antibiotics on L. monocytogenes survival (numbers of CFU) in broth upon incubation for a fixed period of 5 h with increasing concentrations of antibiotics (as a percentage of their corresponding Cmaxs [Table 2]). Large open diamond, value observed in controls (no antibiotic was added); closed diamonds, ampicillin (Cmax, 50 mg/liter); closed squares, meropenem (Cmax, 50 mg/liter); open circles, azithromycin (Cmax, 0.4 mg/liter); open squares, ciprofloxacin (Cmax, 4.3 mg/liter); closed circles, moxifloxacin (Cmax, 4 mg/liter). Values are given as arithmetic means ± standard deviations (n = 3), but some of the corresponding error bars are smaller than the symbols.
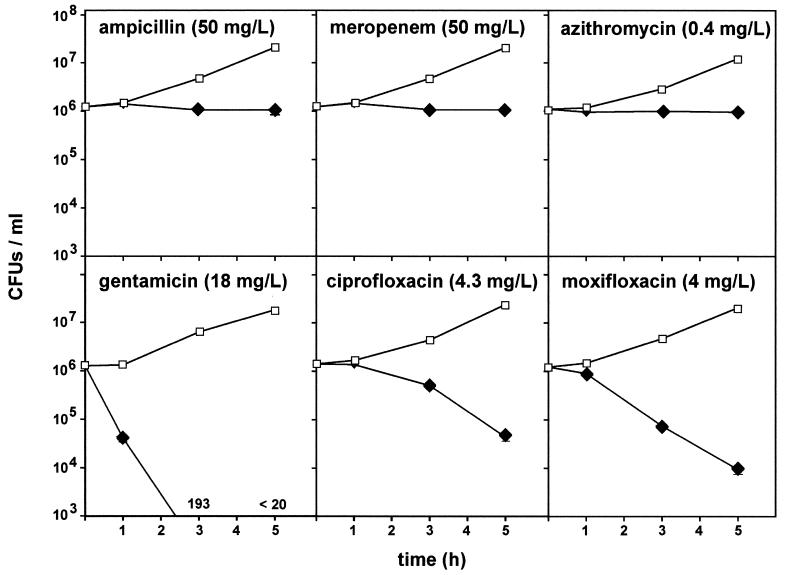
Activity against intracellular bacteria.
In a series of experiments, we measured the activities of antibiotics against L. monocytogenes that had been phagocytosed by THP-1 macrophages. We first examined the influence of time using for each antibiotic a fixed Ce corresponding to its Cmax. The results are shown in Fig. 3. Ampicillin and meropenem were slowly bactericidal, but the reduction in the numbers of CFU seen at 5 h did not exceed 0.5 to 0.7 log. Azithromycin was only very modestly bactericidal (causing a reduction of approximately 0.3 log). Gentamicin caused an almost immediate reduction of approximately 0.3 log but did not further prevent bacterial growth, which actually occurred at the same rate as it did in the controls (to which no antibiotic was added). Ciprofloxacin was slightly bactericidal over time (and actually showed a behavior essentially similar to that of meropenem). In contrast to all other antibiotics tested, moxifloxacin achieved a marked bactericidal effect which progressed in a time-dependent fashion after a lag period of 1 h and caused a reduction of 2.3 logs from the original inoculum at 5 h.
FIG. 3.
Influences of antibiotics on the survival of L. monocytogenes (numbers of CFU) phagocytosed by THP-1 macrophages upon subsequent incubation of cells in the presence of a fixed extracellular drug concentration Closed diamonds, test antibiotics; open squares, controls (to which no antibiotic was added). Values are given as the arithmetic the mean ± standard deviation (n = 3) number of CFU per milligram of protein, but most of the corresponding error bars are smaller than the symbols.
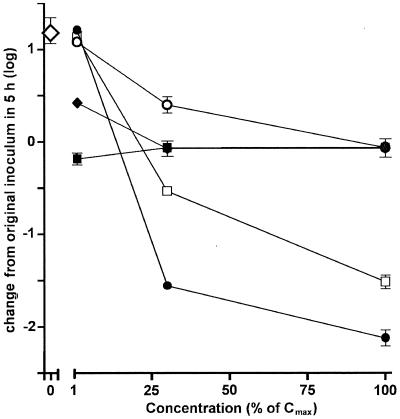
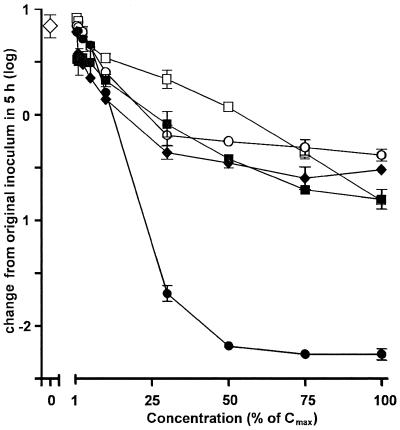
As for the experiment performed in broth, we then systematically investigated the influences of the Ce of the antibiotics for a fixed period of 5 h. As shown in Fig. 4, all antibiotics displayed concentration-dependent effects, but the magnitudes of the effects varied very markedly among the drugs. Thus, ampicillin, meropenem, and azithromycin achieved only a fair bactericidal effect (causing reductions of 0.3 to 0.7 log) at their maximal Ce, with most of the effect of the Ce already obtained at about 15 to 25% of their Cmax. Ciprofloxacin was clearly less potent than all other antibiotics at low concentrations, but it eventually achieved the same fair bactericidal effect as meropenem at its Cmax (a reduction of 0.8 log). In sharp contrast to the results for all other antibiotics, moxifloxacin caused a marked bactericidal effect, causing an approximately 2-log reduction of the inoculum when it was used at a concentration about 50% of its Cmax. No further decrease in bacterial counts was observed, however, at higher concentrations.
FIG. 4.
Influences of antibiotics on the survival of L. monocytogenes (numbers of CFU) phagocytosed by THP-1 macrophages upon a subsequent incubation for 5 h at increasing drug Ce (as a percentage of their corresponding Cmax [Table 2]). Large open diamond, value observed in controls (no antibiotic); closed diamonds, ampicillin (Cmax, 50 mg/liter); closed squares, meropenem (Cmax, 50 mg/liter); open circles, azithromycin (Cmax, 0.4 mg/liter); open squares, ciprofloxacin (Cmax, 4.3 mg/liter); closed circles, moxifloxacin (Cmax, 4 mg/liter). Values are given as the arithmetic mean ± standard deviation (n = 3) number of CFU per milligram of protein.
Because the latter data suggested an intrinsic limitation of the intracellular activities of fluoroquinolones, we examined the effect of ciprofloxacin at 15 mg/liter (i.e., a concentration that was greater than three times its Cmax). A 1.4-log reduction of the inoculum was seen (data not shown), suggesting some sort of intracellular limitation for this fluoroquinolone as well. A linear concentration-dependent effect would indeed have yielded an approximately 4.8-log reduction (all raw counts were at least 60 to 100 CFU per plate, ruling out the possibility that we had simply reached the lowest limit of detection by our assay). Additional studies showed that these persistent bacteria did not grow in broth in the presence of 1.2 mg of moxifloxacin per liter (two times the MIC) for at least 7 days, also ruling out a potential population distribution phenomenon or the mere contamination of our cultures by resistant organisms.
Cellular accumulation of antibiotics.
Table 3 shows the levels of accumulation of ampicillin, meropenem, ciprofloxacin, and moxifloxacin in cells incubated for 5 h in the presence of the drugs at fixed Ce corresponding to their Cmax. As anticipated, the Cc of the two β-lactams remained inferior to their Ce. The two fluoroquinolones showed a fair level of accumulation (approximately eightfold). Infection did not affect these values for any of the drugs except moxifloxacin, for which an approximately 15% reduction of the level of accumulation was seen. For gentamicin and azithromycin, the microbiological assay did not prove sensitive enough for accurate determination of the intracellular content after incubation in the presence of their corresponding Cmax. Experiments were therefore performed with uninfected cells at larger concentrations (200 mg/liter for gentamicin, 20 mg/liter for azithromycin). These studies showed a lack of accumulation of gentamicin (<1-fold), which is in contrast to the marked accumulation of azithromycin (∼100-fold), which is consistent with the results of several previous studies with these antibiotics (6, 34, 48).
TABLE 3.
Accumulation of antibiotics in macrophages and calculated Cc-to-MIC ratiosa
| Antibiotic |
Cc/Ceb
|
Cc/MICc | |
|---|---|---|---|
| Noninfected cells | Infected cells | ||
| Ampicillin | 0.93 ± 0.20 (12) | 0.92 ± 0.19 (12) | 153 |
| Meropenem | 0.96 ± 0.19 (12) | 1.00 ± 0.36 (12) | 694 |
| Ciprofloxacin | 7.36 ± 0.66 (6) | 7.75 ± 1.03 (6) | 24 |
| Moxifloxacin | 8.22 ± 0.87 (6)d | 6.96 ± 1.05 (6)e | 48 |
The cells were incubated in the presence of the maximum extracellular concentrations tested in this study (ampicillin, 50 mg/liter; meropenem, 50 mg/liter; ciprofloxacin, 4.3 mg/liter; moxifloxacin, 4 mg/liter), and the levels of accumulation were determined after 5 h of incubation.
The Cc-to-Ce ratio was calculated from the drug contents per milligram of cell protein by using a conversion factor of 5 μl per mg of cell protein the numbers of independent determinations are given in parentheses.
Ratio of apparent Cc to the MIC (Table 1).
Fluorescence assay; an independent assay with [14C]moxifloxacin gave a value of 8.45 ± 1.56 (n = 6).
P < 0.05 by statistical analysis by Student's t test (none of the other differences between uninfected and infected cells was significant).
Effects of concentration on the extracellular and intracellular activities of fluoroquinolones.
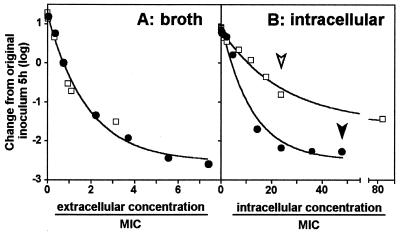
To better examine whether fluoroquinolones showed the same concentration-dependent activities against intracellular bacteria that they showed against the extracellular ones, we systematically compared the changes in the inocula observed in broth and in cells over increasing Ce. The results are shown in Fig. 5 and demonstrate (i) that concentration dependency is indeed observed in both cases; (ii) that the concentration dependencies and maximal efficacies of ciprofloxacin and moxifloxacin in broth cannot be distinguished when the results for both drugs at the same multiples of their MICs are compared; and (iii) that, in contrast to the previous point, the concentration dependency slopes and the maximal intensities of the effects of the two drugs are quite different when they are examined against intracellular bacteria. Also quite interesting was the fact that the data show that the activities of intracellular fluoroquinolones are considerably weaker than their extracellular activities if one takes into account the fact that the drugs are concentrated in cells. To illustrate this, Fig. 6 directly compares the activities of moxifloxacin at the same Ce against extracellular and intracellular bacteria (in broth and in culture medium, respectively). The two responses are almost perfectly superimposable, as if the cellular accumulation of the drug did not result in any enhancement of activity (or as if the accumulation process is self-defeating in terms of activity). Similar data were obtained for ciprofloxacin at the same Ce (data not shown), but in that case, the intracellular activity was about two times lower than the extracellular activity.
FIG. 5.
Comparative extracellular (A) and intracellular (B) activities of ciprofloxacin (open squares) and moxifloxacin (closed circles) as a function of the concentration. Experiments were conducted as described in the legends to Fig. 2 and 4, except that a higher concentration of ciprofloxacin was used to achieve a maximal effect against intracellular bacteria. (A) The values on the abscissa indicate the actual drug concentration in broth; (B) the values on the abscissa indicate the calculated Cc (based on the accumulation data shown in Table 3). All values on the abscissas have been normalized with respect to the MIC of the corresponding drug in broth (A) or in the macrophage culture medium (B). Curves were generated by fitting one-phase exponential decay equations to the data (A, R2 = 0.9875; B, R2 = 0.9793 and 0.9858 for ciprofloxacin and moxifloxacin, respectively). The open and closed arrowheads in panel B indicate the Cc/MIC ratio reached at the Cmax of ciprofloxacin and moxifloxacin used in all other experiments, respectively.
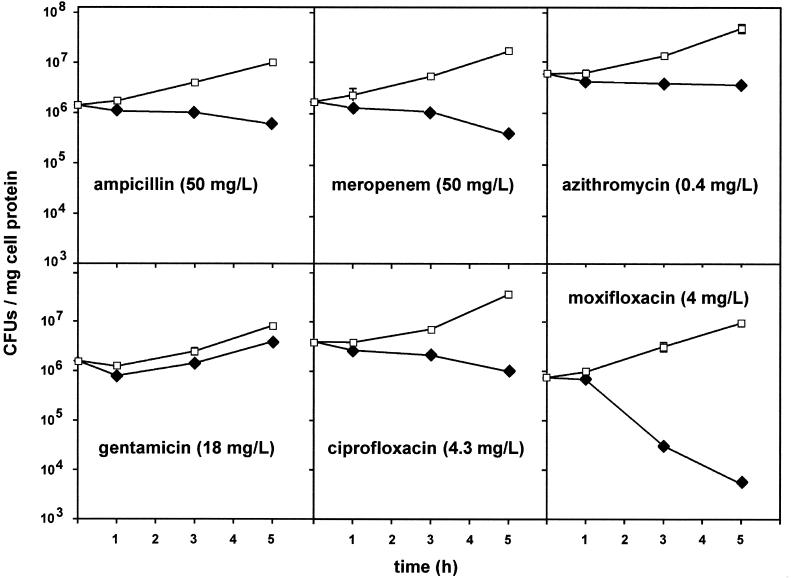
Influence of gentamicin on activities of other antibiotics.
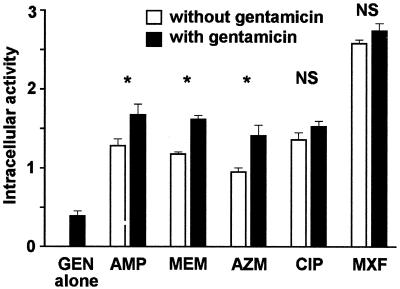
Because gentamicin is often combined with ampicillin or meropenem for the treatment of listeriosis, we examined whether its presence could modulate the intracellular activities of these antibiotics. The experiments were then extended to the other antibiotics used in this study (Fig. 7). As was shown earlier (Fig. 3) and as shown again for the sake of comparison, gentamicin alone at a concentration corresponding to its Cmax caused only a modest decrease in bacterial counts (approximately 0.3 log CFU). When gentamicin was combined with ampicillin, meropenem, and azithromycin, it also increased the overall activity by about 0.3 log (additive effect). However, no significant increases in activity were seen for ciprofloxacin or moxifloxacin.
FIG. 7.
Influence of gentamicin on the activities of antibiotics against intracellular L. monocytogenes. The cells were incubated for 5 h with the Ce of gentamicin and concentrations of the other antibiotics corresponding to their Cmax, as follows: gentamicin (GEN), Cmax = 18 mg/liter; ampicillin (AMP), Cmax = 50 mg/liter); meropenem (MEM), Cmax = 50 mg/liter; azithromycin (AZM), Cmax = 0.4 mg/liter; ciprofloxacin (CIP), Cmax = 4.3 mg/liter; moxifloxacin (MXF), Cmax = 4 mg/liter. Intracellular activity (ordinate) is defined as the difference in the log bacterial counts (numbers of CFU) between cells incubated without antibiotic and (i) cells incubated with gentamicin alone, (ii) cells incubated with each of the other antibiotics alone, or (iii) cells incubated with the combination of gentamicin and the corresponding antibiotic. Each value used for the calculation was the arithmetic mean of three independent determinations. P values between cells exposed to an antibiotic plus gentamicin and cells exposed to an antibiotic alone, as determined by Student's t test, were not significant (NS) or <0.05 or less (asterisks).
DISCUSSION
L. monocytogenes is a facultative intracellular bacterium characterized by its ability to enter various cell types, including macrophages (13, 20, 34, 50). Once it is internalized, virulent L. monocytogenes quickly escapes from the phagosomes through membrane destabilization and disruption and thrives in the cytosol (46). All these features have been observed in the model used here (34, 42), with which we also demonstrated that the escape of L. monocytogenes to the cytosol is almost complete after 1 h. Our model therefore essentially corresponds to a cytosolic infection and should allow us to define the conditions of the activity of an antibiotic within the cytosol. The model was further validated with respect to the main parameters that may govern intracellular activity (49), namely, the intrinsic antibacterial activities of the drugs under study (i.e., their MICs and MBCs), the rates of killing, and their levels of intracellular accumulation. All values or properties were consistent with previously published data (13, 30, 31, 35, 42) or are in line with the known properties of the corresponding drugs.
When one first considers intrinsic antibacterial activity and concentrates on the MICs, it appears that meropenem should be considered the drug of choice. Yet, its lack of a bactericidal effect probably represents a major weakness in terms of eradication of the organism (note that this static effect is observed under conditions in which control bacteria are in the logarithmic phase of growth [a period of 1 to 5 h] and therefore cannot reflect only a stage of unresponsiveness following growth and dilution prior to antibiotic addition). The same applies to ampicillin, for which no bactericidal effect against extracellular bacteria could be demonstrated either.
Intracellular accumulation and subcellular localization have also been suggested to be most critical in the modulation of the activities of antibiotics against intracellular bacteria (49). The drugs used in the present study actually had quite contrasting behaviors in this respect, which can be summarized as follows. The Cc of the β-lactams never exceed the Ce (14, 34). Aminoglycosides enter cells very slowly, so that their Ccs are also systematically lower than their Ce in short-term experiments (3, 48). Fluoroquinolones show fair levels of intracellular accumulation (typically 4- to 10-fold) in all cell models (7, 37, 38). Azithromycin accumulates to a very large extent in most cells (6, 22) and achieves Cc/Ce ratios of ∼80 in THP-1 macrophages (34). All these features have been confirmed in the present study. We have also shown here (i) that meropenem, like other β-lactams, does not accumulate in cells and (ii) that the level of accumulation of moxifloxacin in THP-1 macrophages is essentially similar to that of ciprofloxacin. Concerning the subcellular distribution of antibiotics, we know that (i) β-lactams and fluoroquinolones are mostly if not exclusively localized in the cytosol (on the basis of the results fractionation studies [7, 41]); (ii) azithromycin concentrates markedly in lysosomes, but about one-third of the cell-associated drug is also localized in the cytosol (6); and (iii) gentamicin localizes first in phagosomes (10) but is thereafter almost entirely transferred to the lysosomes (48). We see here that cells exposed to a clinically meaningful concentration of meropenem or ampicillin contain drug in an amount that should create a local concentration that largely exceeds the MIC (by a factor of ∼150 for ampicillin and by a factor of ∼700 for meropenem [Table 3]). These drugs can be considered in direct contact with their bacterial targets since both are located in the cytosol. The same reasoning applies to azithromycin, which, although it has a less favorable MIC than that of meropenem or ampicillin, largely accumulates in cells. Assuming that one-third of the cell-associated azithromycin is in the cytosol, as mentioned above, its local concentration should reach more than 30 times the MIC for L. monocytogenes. Thus, insufficient intracellular accumulation or inappropriate localization cannot explain the inabilities of β-lactams and azithromycin to eradicate intracellular L. monocytogenes. Conversely, a lack of rapid accumulation and restrictive localization are probably the factors which explain the almost complete lack of activity of gentamicin against cell-associated L. monocytogenes. The immediate but minor effect observed can most reasonably be ascribed to a direct action of the drug on adhering but not phagocytosed bacteria or on bacteria still in phagosomes (10).
At this point it becomes clear that the lack of concentration dependency in the intrinsic antibacterial activities of ampicillin, meropenem, and azithromycin (as determined in broth) is probably responsible for the weak eradication of intracellular L. monocytogenes. In other words, the reason for the failure of these drugs to eradicate intracellular L. monocytogenes is truly a pharmacodynamic one. This may have important implications for future research. It indeed suggests (i) that no improvement can be expected from maneuvers aimed at increasing the Ccs of β-lactams or from the selection of β-lactams with still lower MICs; (ii) that much of the eradication of intracellular L. monocytogenes during ampicillin or meropenem treatment is probably due to host defenses, as suggested previously for ampicillin (34) (these host defenses are probably responsible for the modest intracellular bactericidal effect seen here); and (iii) that macrolides are intrinsically unable to eradicate intracellular L. monocytogenes.
The situation is entirely different for the fluoroquinolones, with which, at least for moxifloxacin, we observed marked eradicating activities against intracellular L. monocytogenes. Several investigators have reported that fluoroquinolones are capable of killing intracellular L. monocytogenes (13, 30, 31, 34, 42). These studies showed that the fluoroquinolones had activities that were inversely proportional to their MICs and directly proportional to the Ce. The present study extends and systematizes these observations by (i) correlating activity to concentrations that can be obtained in human serum during conventional therapy; (ii) examining directly the relationship between drug activity and the intracellular drug concentration; and (iii) showing that moxifloxacin, in contrast to most other fluoroquinolones except clinafloxacin (30), may have real clinical potential in this context. The study also demonstrates unambiguously that one of the basic pharmacodynamic properties of fluoroquinolones, namely, the marked influence of the drug concentration on the intensity of the killing ability, which is seen for extracellular bacteria, also exists for intracellular L. monocytogenes. Extension of this conclusion to other intracellular infections would be of great interest, especially if one considers noncytosolic infections. In this context, it has been shown (11, 12) that ciprofloxacin is effective against Staphylococcus aureus and Mycobacterium fortuitum in macrophages and polymorphonuclear leukocytes as soon as its Ce exceeds its corresponding MIC.
Close analysis of the data suggests, however, that additional parameters need to be considered as essential determinants in the intracellular activities of fluoroquinolones. First, and quite interestingly, we see that the intracellular accumulation of moxifloxacin does not lead to any net gain in activity, suggesting that there is some sort of intracellular defeating effect on its action, the molecular or cellular mechanism(s) of which needs to be investigated further. This defeating effect, which has also recently been described in S. aureus-infected THP-1 macrophages (36), may vary among fluoroquinolones (viz. ciprofloxacin versus moxifloxacin), which suggests that we can perhaps modulate it in a positive direction. Second, we have consistently observed the maintenance of a residual inoculum (0.5 to 0.8% of the original inoculum) even under conditions with maximal concentrations of moxifloxacin (as well as under conditions with maximal concentrations of ciprofloxacin, but to an even greater extent). This phenomenon, which has already been observed with extracellular bacteria (for a review, see reference 25), will require careful analysis since it may indicate that there is an intrinsic limit in the eradication properties of fluoroquinolones, the clinical significance of which, however, remains uncertain (25).
The chemotherapeutic implications of the data presented here need to be underlined. The disappointing behaviors of ampicillin and meropenem raise critical questions concerning the high rate of use of these antibiotics to treat severe cases of listeriosis and point out their probable inabilities to rapidly achieve bacterial eradication in immunocompromised patients. A poor effect of amoxicillin (0.5-log reduction of intracellular L. monocytogenes) has been described in a HeLa cell model at the 5-h time point (31). A progression of the decline in bacterial counts (reductions up to ∼2 logs) was recorded, however, when incubation with the antibiotic was continued up to 24 h. This pattern is consistent with the known time dependency of β-lactam activities. The combination of the results of Michelet et al. (31) and the present data suggests that β-lactams will be efficient in vivo only if sustained concentrations are maintained over prolonged periods of time. These issues are now open to exploratory studies with more dynamic models. The present report also strongly suggests that azithromycin will be intrinsically poorly effective for the treatment of listeriosis, despite its intracellular accumulation to high concentrations. Available data from studies with animals suggest that this conclusion probably extends to other macrolides and even ketolides (33). We also show that gentamicin affects the intracellular growth of L. monocytogenes only in a minor fashion and that it offers no real synergy with β-lactams. This reinforces the conclusion that the use of gentamicin for the treatment of listeriosis can be justified only by its action against extracellular bacteria (the lack of an additive effect of gentamicin in combination with fluoroquinolones does not indicate antagonism; it more probably results from the fact that fluoroquinolones at their Cmax are quickly bactericidal against extracellular bacteria, so that there is not much to gain by the association in this context).
Finally, like other investigators (13, 30, 31, 34, 42), we suggest that fluoroquinolones may represent a valuable option for the treatment of listeriosis, especially even in difficult situations such as meningitis, because of their marked bactericidal activities. Although the concentrations of fluoroquinolones in cerebrospinal fluid are only 20 to 50% of the concentrations in serum (1), our data show that this level already allows almost a maximal effect both extra- and intracellularly (Fig. 5). More data, however, are needed concerning the penetration of moxifloxacin into brain tissues under conditions of inflamed meninges. Nevertheless, the present data open interesting perspectives and set up the conditions for the performance of meaningful trials of moxifloxacin with animals.
Acknowledgments
We thank J.-M. Frère and D. Klein (Centre d'Ingénierie des Protéines, Université de Liège, Liege, Belgium) for providing us with the Streptomyces R39 dd-carboxypeptidase and its substrate (bis-acetyl-l-Lys-d-Ala-d-Ala) used in this study and help with the enzymatic assay of β-lactams. F. Renoird, M. C. Cambier, and N. Aguilera provided skillful technical help. We thank the drug manufacturers for the kind gifts of the corresponding antibiotics.
S.C. is boursier of the Belgian Fonds pour la Formation à la Recherche dans l'Industrie et l'Agriculture, and F.V.B. is chercheur qualifié and M.-P.M.-L. is maître de recherches of the Belgian Fonds National de la Recherche Scientifique. This work was supported by the Belgian Fonds de la Recherche Scientifique Médicale (grant 3.4.612.00 F [3]) and by a grant-in-aid from Bayer AG, Leverkusen, Germany.
REFERENCES
- 1.Aminimanizani, A., P. Beringer, and R. Jelliffe. 2001. Comparative pharmacokinetics and pharmacodynamics of the newer fluoroquinolone antibacterials. Clin. Pharmacokinet. 40:169-187. [DOI] [PubMed] [Google Scholar]
- 2.Bauer, J., H. Hof, T. Nichterlein, and M. Wuenscher. 1993. Comparative activities of macrolide derivatives on murine listeriosis. Zentbl. Bakteriol. Parasitenkd. Infektkrankh. Hyg. Abt. 1 Orig. 278:112-119. [DOI] [PubMed] [Google Scholar]
- 3.Bonventre, P. F., R. Hayes, and J. Imhoff. 1967. Autoradiographic evidence for the impermeability of mouse peritoneal macrophages to tritiated streptomycin. J. Bacteriol. 93:445-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buchmeier, N. A., and R. D. Schreiber. 1985. Requirement of endogenous interferon-gamma production for resolution of Listeria monocytogenes infection. Proc. Natl. Acad. Sci. USA 82:7404-7408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buisman, A. M., J. A. Langermans, and R. van Furth. 1998. Effect of granulocyte-macrophage colony-stimulating factor on the number of leucocytes and course of Listeria monocytogenes infection in naive and leucocytopenic mice. Immunology 93:73-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carlier, M. B., I. Garcia-Luque, J. P. Montenez, P. M. Tulkens, and J. Piret. 1994. Accumulation, release and subcellular localization of azithromycin in phagocytic and non-phagocytic cells in culture. Int. J. Tissue React. 16:211-220. [PubMed] [Google Scholar]
- 7.Carlier, M. B., B. Scorneaux, A. Zenebergh, J. F. Desnottes, and P. M. Tulkens. 1990. Cellular uptake, localization and activity of fluoroquinolones in uninfected and infected macrophages. J. Antimicrob. Chemother. 26(Suppl. B):27-39. [DOI] [PubMed] [Google Scholar]
- 8.Craig, W. 1993. Pharmacodynamics of antimicrobial agents as a basis for determining dosage regimens. Eur. J. Clin. Microbiol. Infect. Dis. 12(Suppl. 1):S6-S8. [DOI] [PubMed] [Google Scholar]
- 9.Dreetz, M., J. Hamacher, J. Eller, K. Borner, P. Koeppe, T. Schaberg, and H. Lode. 1996. Serum bactericidal activities and comparative pharmacokinetics of meropenem and imipenem-cilastatin. Antimicrob. Agents Chemother. 40:105-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Drevets, D. A., B. P. Canono, P. J. Leenen, and P. A. Campbell. 1994. Gentamicin kills intracellular Listeria monocytogenes. Infect. Immun. 62:2222-2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Easmon, C. S., and J. P. Crane. 1985. Uptake of ciprofloxacin by macrophages. J. Clin. Pathol. 38:442-444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Easmon, C. S., J. P. Crane, and A. Blowers. 1986. Effect of ciprofloxacin on intracellular organisms: in-vitro and in-vivo studies. J. Antimicrob. Chemother. 18(Suppl. D):43-48. [DOI] [PubMed] [Google Scholar]
- 13.Facinelli, B., G. Magi, M. Prenna, S. Ripa, and P. E. Varaldo. 1997. In vitro extracellular and intracellular activity of two newer and two earlier fluoroquinolones against Listeria monocytogenes. Eur. J. Clin. Microbiol. Infect. Dis. 16:827-833. [DOI] [PubMed] [Google Scholar]
- 14.Fan, H. J., I. Paternotte, M. Vermander, K. Li, M. Beaujean, B. Scroneaux, P. Dumont, P. M. Tulkens, and E. Sonveaux. 1997. Ester prodrugs of ampicillin tailored for intracellular accumulation. Bioorg. Med. Chem. Lett. 7:3107-3112. [Google Scholar]
- 15.Forsgren, A., and A. Bellahsene. 1985. Antibiotic accumulation in human polymorphonuclear leucocytes and lymphocytes. Scand. J. Infect. Dis. Suppl. 44:16-23. [PubMed] [Google Scholar]
- 16.Foulds, G., R. M. Shepard, and R. B. Johnson. 1990. The pharmacokinetics of azithromycin in human serum and tissues. J. Antimicrob. Chemother. 25(Suppl. A):73-82. [DOI] [PubMed] [Google Scholar]
- 17.Foulds, G., J. P. Stankewich, D. C. Marshall, M. M. O'Brien, S. L. Hayes, D. J. Weidler, and F. G. McMahon. 1983. Pharmacokinetics of sulbactam in humans. Antimicrob. Agents Chemother. 23:692-699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frei, K., D. Nadal, H. W. Pfister, and A. Fontana. 1993. Listeria meningitis: identification of a cerebrospinal fluid inhibitor of macrophage listericidal function as interleukin 10. J. Exp. Med. 178:1255-1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frère, J. M., D. Klein, and J. M. Ghuysen. 1980. Enzymatic method for rapid and sensitive determination of beta-lactam antibiotics. Antimicrob. Agents Chemother. 18:506-510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gaillard, J. L., F. Jaubert, and P. Berche. 1996. The inlAB locus mediates the entry of Listeria monocytogenes into hepatocytes in vivo. J. Exp. Med. 183:359-369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gilbert, D. N. 2000. Aminoglycosides, p. 307-336. In G. L. Mandell, J. E. Bennett, and R. Dolin (ed.), Principles and practice of infectious disease. Churchill Livingstone, Philadelphia, Pa.
- 22.Gladue, R. P., G. M. Bright, R. E. Isaacson, and M. F. Newborg. 1989. In vitro and in vivo uptake of azithromycin (CP-62,993) by phagocytic cells: possible mechanism of delivery and release at sites of infection. Antimicrob. Agents Chemother. 33:277-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grumbach, N. M., E. Mylonakis, and E. J. Wing. 1999. Development of listerial meningitis during ciprofloxacin treatment. Clin. Infect. Dis. 29:1340-1341. [DOI] [PubMed] [Google Scholar]
- 24.Hof, H., and G. Waldenmeier. 1988. Therapy of experimental listeriosis—an evaluation of different antibiotics. Infection 16(Suppl. 2):S171-S174. [DOI] [PubMed] [Google Scholar]
- 25.Hooper, D. C., and J. S. Wolfson. 1993. Mechanism of quinolone action and bacterial killing, p. 53-75. In D. C. Hooper and J. S. Wolfson (ed.), Quinolone antimicrobial agents, 2nd ed. ASM Press, Washington, D.C.
- 26.Jusko, W. J. 1971. Fluorometric analysis of ampicillin in biological fluids. J. Pharm. Sci. 60:728-732. [DOI] [PubMed] [Google Scholar]
- 27.Levison, M. E. 2000. Pharmacodynamics of antibacterial drugs. Infect. Dis. Clin. N. Am. 14:281-291. [DOI] [PubMed] [Google Scholar]
- 28.Lorber, B. 2000. Listeria monocytogenes, p. 2208-2214. In G. L. Mandell, J. E. Bennett, and R. Dolin (ed.), Principles and practice of infectious diseases. Churchill Livingstone, Philadelphia, Pa.
- 29.Lowry, O. H., N. J. Rosebrough, A. L. Farr, and R. J. Randall. 1951. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193:265-275. [PubMed] [Google Scholar]
- 30.Michelet, C., J. L. Avril, C. Arvieux, C. Jacquelinet, N. Vu, and F. Cartier. 1997. Comparative activities of new fluoroquinolones, alone or in combination with amoxicillin, trimethoprim-sulfamethoxazole, or rifampin, against intracellular Listeria monocytogenes. Antimicrob. Agents Chemother. 41:60-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Michelet, C., J. L. Avril, F. Cartier, and P. Berche. 1994. Inhibition of intracellular growth of Listeria monocytogenes by antibiotics. Antimicrob. Agents Chemother. 38:438-446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mortimer, P. G., and L. J. Piddock. 1991. A comparison of methods used for measuring the accumulation of quinolones by Enterobacteriaceae, Pseudomonas aeruginosa and Staphylococcus aureus. J. Antimicrob. Chemother. 28:639-653. [DOI] [PubMed] [Google Scholar]
- 33.Nichterlein, T., M. Kretschmar, and H. Hof. 1999. The ketolide antibiotic HMR 3647, a candidate substance for the treatment of systemic and intracerebral infections with Listeria monocytogenes. Zentbl. Bakteriol. Parasitenkd. Infektkrankh. Hyg. Abt. 1 Orig. 289:155-164. [DOI] [PubMed] [Google Scholar]
- 34.Ouadrhiri, Y., B. Scorneaux, Y. Sibille, and P. M. Tulkens. 1999. Mechanism of the intracellular killing and modulation of antibiotic susceptibility of Listeria monocytogenes in THP-1 macrophages activated by gamma interferon. Antimicrob. Agents Chemother. 43:1242-1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ouadrhiri, Y., Y. Sibille, and P. M. Tulkens. 1999. Modulation of intracellular growth of Listeria monocytogenes in human enterocyte Caco-2 cells by interferon-gamma and interleukin-6: role of nitric oxide and cooperation with antibiotics. J. Infect. Dis. 180:1195-1204. [DOI] [PubMed] [Google Scholar]
- 36.Paillard, D., J. Grellet, V. Dubois, M. C. Saux, and C. Quentin. 2002. Discrepancy between uptake and intracellular activity of moxifloxacin in a Staphylococcus aureus-human THP-1 monocytic cell model. Antimicrob. Agents Chemother. 46:288-293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pascual, A., I. Garcia, S. Ballesta, and E. J. Perea. 1999. Uptake and intracellular activity of moxifloxacin in human neutrophils and tissue-cultured epithelial cells. Antimicrob. Agents Chemother. 43:12-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pascual, A., I. Garcia, and E. J. Perea. 1989. Fluorometric measurement of ofloxacin uptake by human polymorphonuclear leukocytes. Antimicrob. Agents Chemother. 33:653-656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Piddock, L. J., and Y. F. Jin. 1999. Antimicrobial activity and accumulation of moxifloxacin in quinolone-susceptible bacteria. J. Antimicrob. Chemother. 43(Suppl. B):39-42. [DOI] [PubMed] [Google Scholar]
- 40.Prokesch, R. C., and W. L. Hand. 1982. Antibiotic entry into human polymorphonuclear leukocytes. Antimicrob. Agents Chemother. 21:373-380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Renard, C., H. J. Vanderhaeghe, P. J. Claes, A. Zenebergh, and P. M. Tulkens. 1987. Influence of conversion of penicillin G into a basic derivative on its accumulation and subcellular localization in cultured macrophages. Antimicrob. Agents Chemother. 31:410-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Scorneaux, B., Y. Ouadrhiri, G. Anzalone, and P. M. Tulkens. 1996. Effect of recombinant human gamma interferon on intracellular activities of antibiotics against Listeria monocytogenes in the human macrophage cell line THP-1. Antimicrob. Agents Chemother. 40:1225-1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Steinman, R. M., S. E. Brodie, and Z. A. Cohn. 1976. Membrane flow during pinocytosis. A stereologic analysis. J. Cell Biol. 68:665-687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sullivan, J. T., M. Woodruff, J. Lettieri, V. Agarwal, G. J. Krol, P. T. Leese, S. Watson, and A. H. Heller. 1999. Pharmacokinetics of a once-daily oral dose of moxifloxacin (Bay 12-8039), a new enantiomerically pure 8-methoxy quinolone. Antimicrob. Agents Chemother. 43:2793-2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Temple, M. E., and M. C. Nahata. 2000. Treatment of listeriosis. Ann. Pharmacother. 34:656-661. [DOI] [PubMed] [Google Scholar]
- 46.Tilney, L. G., and D. A. Portnoy. 1989. Actin filaments and the growth, movement, and spread of the intracellular bacterial parasite, Listeria monocytogenes. J. Cell Biol. 109:1597-1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tsuchiya, S., M. Yamabe, Y. Yamaguchi, Y. Kobayashi, T. Konno, and K. Tada. 1980. Establishment and characterization of a human acute monocytic leukemia cell line (THP-1). Int. J. Cancer 26:171-176. [DOI] [PubMed] [Google Scholar]
- 48.Tulkens, P., and A. Trouet. 1978. The uptake and intracellular accumulation of aminoglycoside antibiotics in lysosomes of cultured rat fibroblasts. Biochem. Pharmacol. 27:415-424. [DOI] [PubMed] [Google Scholar]
- 49.Tulkens, P. M. 1991. Intracellular distribution and activity of antibiotics. Eur. J. Clin. Microbiol. Infect. Dis. 10:100-106. [DOI] [PubMed] [Google Scholar]
- 50.Wadsworth, S. J., and H. Goldfine. 1999. Listeria monocytogenes phospholipase C-dependent calcium signaling modulates bacterial entry into J774 macrophage-like cells. Infect. Immun. 67:1770-1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhan, Y., G. J. Lieschke, D. Grail, A. R. Dunn, and C. Cheers. 1998. Essential roles for granulocyte-macrophage colony-stimulating factor (GM-CSF) and G-CSF in the sustained hematopoietic response of Listeria monocytogenes-infected mice. Blood 91:863-869. [PubMed] [Google Scholar]