Abstract
In experiments to assess the in vitro impact of the candidate microbicides nonoxynol 9 (N-9), C31G, and sodium dodecyl sulfate (SDS) on human immune and epithelial cell viability, cell lines and primary cell populations of lymphocytic and monocytic origin were generally shown to be equally sensitive to exposures ranging from 10 min to 48 h. However, U-937 cells were more sensitive to N-9 and C31G after 48 h than were primary monocyte-derived macrophages. Cytokine activation of monocytes and lymphocytes had no effect on cell viability following exposure to these microbicidal compounds. Primary and passaged vaginal epithelial cultures and cell lines differed in sensitivity to N-9 and C31G but not SDS. These studies provide a foundation for in vitro experiments in which cell lines of human immune and epithelial origin can be used as suitable surrogates for primary cells to further investigate the effects of microbicides on cell metabolism, membrane composition, and integrity and the effects of cell type, proliferation, and differentiation on microbicide sensitivity.
The increase in human immunodeficiency virus type 1 (HIV-1) transmission, particularly in developing countries of sub-Saharan Africa and Asia (30), continues to challenge the global efforts to control the AIDS epidemic. Although male and female condoms can lower the risk of transmission (3, 31), universal acceptance of these mechanical barrier methods may be difficult to achieve (11, 32). An alternative strategy for controlling heterosexual HIV-1 transmission is the development and deployment of broad-spectrum, low-toxicity, female-controlled, low-cost microbicidal agents for topical vaginal use against infection by HIV-1 and other sexually transmitted disease (STD) pathogens. Nonoxynol 9 (N-9), a spermicidal ingredient in common over-the-counter contraceptive products, has been tested as a microbicide in phase III clinical trials. However, its future as a microbicide is in question, due to its dose-dependent toxicity (27, 28), proinflammatory effects (13), and apparent lack of in vivo efficacy against HIV-1 transmission (8, 27). As a consequence, investigations are being directed toward discovery and development of numerous second-generation microbicidal agents. Experimental efforts reported herein have focused primarily on characterization of two potential microbicides, C31G (6, 10, 20, 29) and sodium dodecyl sulfate (SDS), which have in vitro activity against STD pathogens, including HIV-1 and herpes simplex virus type 2 (16, 21, 22, 25). In addition, SDS is an attractive candidate microbicide due to its lower cytotoxicity (16, 20, 21) and ability to inactivate papillomaviruses (16, 17, 22).
The ideal vaginal microbicide must have in vivo activity against HIV-1 and other STD pathogens as well as a differential effect on the viability of human cells and tissues encountered during use as a topical agent. On the one hand, the ideal agent should be effective against incoming cells infected by STD pathogens. Specifically, microbicides that effectively reduce or eliminate the risk of HIV-1 transmission must kill HIV-1-infected immune cells (T cells, monocytes, and macrophages) as well as inactivate cell-free virions. On the other hand, topical microbicides must have minimal or no impact on the viability, function, and structural integrity of the vaginal and cervical epithelium. Although preclinical, in vitro assays of immune and epithelial cell sensitivity to candidate microbicides are necessary steps in the development of a potential microbicide, in vitro assays may fail to predict a compound's in vivo activity (4, 33). One approach previously thought to be a necessary prerequisite for defining the fidelity of in vitro assays was the use of tissues or cells of primary human origin to test candidate microbicides (1, 15). However, compared to available human immune and epithelial cell lines, primary tissues and cells are more difficult to acquire and isolate, less convenient and more expensive to maintain, potentially contaminated with unwanted cell populations, and prone to donor-specific variation. These studies compare the sensitivity of primary cells and cell lines of human immune and vaginal epithelial origins to three vaginal microbicide candidates: N-9, C31G, and SDS. The investigations reported herein demonstrate that primary cells and cell lines of immune origin are generally similar with respect to their in vitro sensitivity to each agent. In contrast, primary vaginal keratinocyte cultures and cells of an immortalized vaginal epithelial cell line differ with respect to their sensitivity to N-9 and C31G but not to SDS.
Cells of the SupT1 T-cell line and primary human T lymphocytes are similar with respect to short- and long-term sensitivity to candidate microbicides.
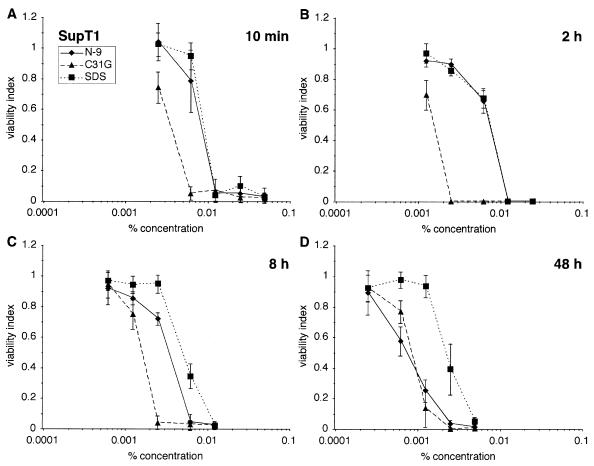
Cells of the SupT1 T-cell line (ATCC CRL-1942) were assessed for their sensitivity to N-9, C31G, or SDS exposure (Fig. 1). Immediately following in vitro exposure to each agent for 10 min, 2 h, 8 h, or 48 h, cell viability was determined using an MTT assay (24) as described earlier (20, 21). After a 10-min exposure (Fig. 1A), SupT1 cells were equally sensitive to N-9 (CC50 = 0.0087%, where CC50 is the microbicide concentration at which cell viability is reduced by 50% relative to that of mock-exposed cells) and SDS (CC50 = 0.0093%, P = 0.1152 in comparison of N-9 and SDS; statistical analyses were performed using the PROC REG procedure in SAS [SAS, Cary, N.C.] and P values were calculated based on the Wald statistic) but were more sensitive to C31G (CC50 = 0.0038%; N-9 versus C31G, P < 0.0001; and SDS versus C31G, P < 0.0001). C31G cytotoxicity increased after a 2-h exposure (CC50 = 0.0016%), in contrast to the cytotoxic effects of N-9 or SDS, which changed little during this interval (CC50 ≈ 0.0078%) relative to results obtained after a 10-min exposure (Fig. 1B). Following an 8-h exposure, cells were clearly more sensitive to N-9 than to SDS (Fig. 1C). By 48 h (Fig. 1D), C31G and N-9 were similarly cytotoxic (CC50 = 0.0009 and 0.0008%, respectively; P = 0.0401 in comparison of C31G and N-9) and more cytotoxic than SDS (CC50 = 0.002%; N-9 versus SDS, P < 0.0001; and C31G versus SDS, P < 0.0001).
FIG. 1.
SupT1 cells are more sensitive to C31G than to N-9 or to SDS. SupT1 cells were exposed to N-9, C31G, or SDS for 10 min (A), 2 h (B), 8 h (C), or 48 h (D) and were assayed for viability immediately after the exposure period. In experiments represented by this and subsequent figures, cell viability after exposure, determined by MTT assay, is expressed relative to that of mock-exposed cells. Unless otherwise indicated, each graph illustrates data collected in two independent experiments in which each concentration was examined in triplicate wells. Error bars indicate standard deviations on mean data.
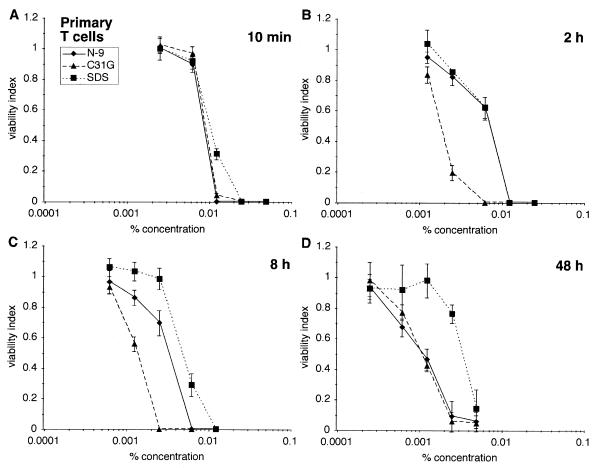
Similar experiments were performed using primary T lymphocytes (Fig. 2) (5). Results obtained after each exposure were almost identical to those obtained after experiments using SupT1 cells. The single exception to this observation was that N-9, C31G, and SDS resulted in similar, concentration-dependent reductions in primary T-cell viability after a 10-min exposure (Fig. 2A compared to Fig. 1A). Collectively, results of experiments using human cells of T-lymphocyte origin indicate that primary T cells and T-cell lines are very similar in their sensitivity to N-9, C31G, or SDS.
FIG. 2.
Primary human T lymphocytes are similar to cell lines in sensitivity to N-9, C31G, or SDS. Primary human T lymphocytes were exposed to N-9, C31G, or SDS for 10 min (A), 2 h (B), 8 h (C), or 48 h (D) and subsequently assayed for viability as described.
U-937 monocytic cells and primary human monocyte-derived macrophages (MDMs) exhibit little difference in sensitivity to microbicide exposure.
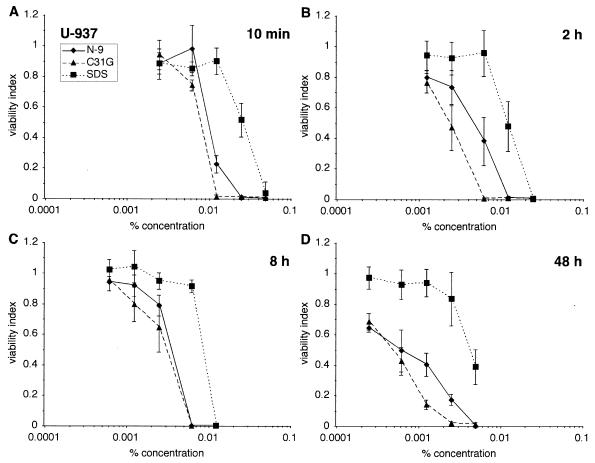
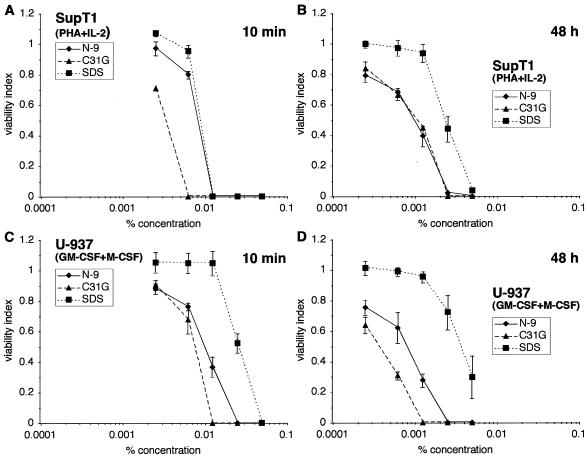
In vitro sensitivity to N-9, C31G, or SDS was also examined using cells of the U-937 human monocytic cell line (ATCC CRL-1593.2). In these experiments, the cytotoxic activity of N-9 was more similar to C31G than to SDS after all four exposure durations (Fig. 3). The greater cytotoxicity of C31G (CC50 = 0.0005%) and N-9 (CC50 = 0.0006%) than of SDS (CC50 = 0.0043%; N-9 versus SDS, P < 0.0001; and C31G versus SDS, P < 0.0001) was most pronounced after a 48-h exposure (Fig. 3D).
FIG. 3.
U-937 cells are more sensitive to C31G than to N-9 or to SDS. U-937 cells were exposed to N-9, C31G, or SDS for 10 min (A), 2 h (B), 8 h (C), or 48 h (D) and subsequently assayed for viability.
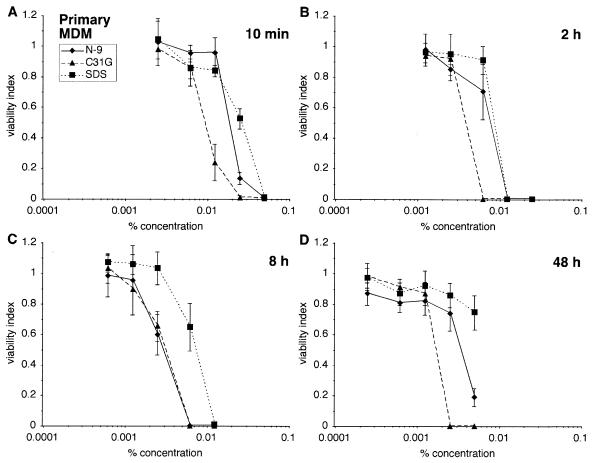
Similar experiments were used to test the sensitivity of primary human MDM cultures to the three vaginal microbicide candidates (Fig. 4). These cells were isolated from Ficoll-fractionated donor peripheral blood mononuclear cells by differential adhesion and size sedimentation and were subsequently cultured for 14 days before use (14). Differences in the viability of primary MDMs exposed to N-9, C31G, or SDS were not as great as those observed with the U-937 cells. However, a difference in sensitivity was observed between MDMs and U-937 cells after a 48-h exposure. MDMs (Fig. 4D) were almost 1 log less sensitive to N-9 (CC50 = 0.0036%, P < 0.0001) and to C31G (CC50 = 0.0018%, P < 0.0001) than were U-937 cells after the same exposure, suggesting differences between monocytic cell lines and primary cells relevant to their sensitivity to N-9 and C31G.
FIG. 4.
Primary human MDMs are similar to a monocytic cell line in sensitivity to N-9, C31G, or SDS. Primary human MDMs were exposed to N-9, C31G, or SDS for 10 min (A), 2 h (B), 8 h (C), or 48 h (D) and subsequently assayed for viability.
Cytokine stimulation has no apparent impact on T-cell and monocytic cell line sensitivity.
Primary T cells and MDMs used in the preceding studies were cultured in the presence of interleukin 2 (20 U/ml) and phytohemagglutinin (PHA, 2.5 μg/ml) or granulocyte-macrophage colony-stimulating factor (GM-CSF, 40 U/ml) and M-CSF (M-CSF, 100 U/ml), respectively. In studies of HIV-1 infection, these extracellular factors have been used to activate primary immune cells and enhance viral replication (19). To determine if cytokine stimulation influenced primary cell sensitivity to microbicide exposure and affected the comparisons between cell lines and primary cells, SupT1 and U-937 cells were cultured under conditions identical to those for their respective primary cell counterparts and were assessed for microbicide sensitivity after a 10-min or 48-h exposure (Fig. 5). Comparisons of these results with those obtained using unstimulated cells (Fig. 1 and 3) demonstrated that stimulation of either cell line had no effect on the sensitivity to N-9, C31G, or SDS exposure.
FIG. 5.
Stimulation of immune cell lines does not appreciably change their sensitivity to N-9, C31G, or SDS. SupT1 cells stimulated with PHA (2.5 μg/ml) and interleukin 2 (20 U/ml) for 24 h were exposed to N-9, C31G, or SDS for 10 min (A) or 48 h (B) and were subsequently assayed for viability as described. Similarly, U-937 cells were treated with GM-CSF (40 U/ml) and M-CSF (100 U/ml) for 24 h and were exposed to N-9, C31G, or SDS for 10 min (C) or 48 h (D).
Primary and passaged epithelial cultures and cell lines differ in sensitivity to some microbicides.
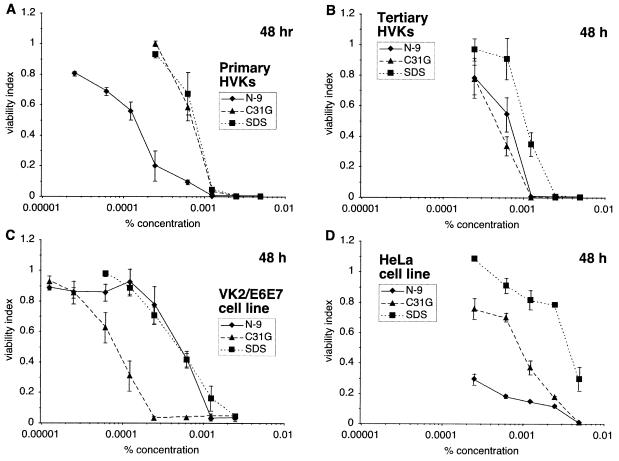
Assessments of in vitro microbicide cytotoxicity should also include experiments using vaginal and cervical epithelial cells. Human vaginal keratinocyte (HVK) cultures, described in previous studies (20), were compared to immortalized VK2/E6E7 vaginal epithelial cells (12) and HeLa cells (human cervical carcinoma; ATCC CCL-2) in experiments using N-9, C31G, and SDS (Fig. 6). After 48 h (Fig. 6A), primary HVKs were considerably more sensitive to N-9 (CC50 = 0.0002%) than to C31G (CC50 = 0.0007%, P < 0.0001) or to SDS (CC50 = 0.0008%, P < 0.0001). In similar experiments using tertiary HVKs (passaged twice after isolation), the differences between N-9, C31G, and SDS were reduced (Fig. 6B). In contrast to the primary HVKs, VK2/E6E7 cells (Fig. 6C) were more sensitive to C31G (CC50 = 0.0001%) than to N-9 (CC50 = 0.0005%, P < 0.0001) or to SDS (CC50 = 0.0005%, P < 0.0001). HeLa cells were also considerably more sensitive to N-9 than to SDS (Fig. 6D). However, unlike the HVKs or vaginal cell line, HeLa cells demonstrated a level of C31G cytotoxicity intermediate between those observed for N-9 and SDS.
FIG. 6.
Primary and tertiary HVK cultures differ from continuous human vaginal cells in their sensitivity to N-9 or to C31G. Primary HVKs (a single, representative experiment) (A), tertiary HVKs (passed twice after isolation) (B), immortalized VK2/E6E7 human vaginal epithelial cells (C), and HeLa cells (D) were exposed to N-9, C31G, or SDS for 48 h and subsequently assessed for viability.
Concluding comments.
These studies demonstrate that there are more similarities than differences when primary cells and cell lines of immune origin are compared with respect to in vitro sensitivity to N-9, C31G, or SDS. Primary T cells and SupT1 cells were particularly similar in assays of microbicide cytotoxicity ranging from 10 min to 48 h. Stimulation of lymphocytic cells with activation agents did not appear to affect their sensitivity to microbicide exposure. Similarly, the sensitivity of U-937 cells after stimulation by GM-CSF and M-CSF was unaltered relative to either unstimulated U-937s or primary MDMs. However, the experiments did pinpoint some interesting differences between the monocytic U-937 cells and MDMs. Although primary MDMs and U-937 cells were similarly sensitive to N-9, C31G, or SDS during exposures up to 8 h, there was a substantial difference between these two cell populations after longer exposure. Interestingly, after 48 h, U-937 cells were more sensitive than their primary counterparts to N-9 or C31G. Sensitivity to SDS also appeared to be affected (albeit to a lesser extent). Differences in microbicide sensitivity between these two cell populations may be attributed to changes that accompany the process of differentiation from monocytes to macrophages, particularly those associated with the structure and function of the cellular membrane (including altered morphology, cessation of proliferation, increased capacity for phagocytosis and adherence to plastic, changes in the expression of proteins involved in lipid metabolism, and alterations in membrane-associated protein expression) (2).
Differentiation and cell growth rates, as well as culture heterogeneity and the extent of cellular keratinization, may have also impacted observed epithelial cell sensitivity, as demonstrated by experiments using primary HVK cultures and the VK2/E6E7 cell line. The results presented in Fig. 6 suggest that epithelial cells become less sensitive to N-9 and more sensitive to C31G with the transition from primary cell to passaged cell to immortalized cell line. Similar changes in sensitivity accompanying cellular differentiation and extensive passaging were observed in studies of 7 β-hydroxycholesterol cytotoxicity, in which primary rat liver epithelial cells and early-passage cells were less sensitive to 7 β-hydroxycholesterol than the high-passage rat liver cell line (23). Since VK2/E6E7 cells divide at a higher rate than cells in the primary (or tertiary) HVK cultures (data not shown), results observed in long-term assays, such as those illustrated in Fig. 6, might also be influenced by altered sensitivity during progress through the cell cycle. However, the rank order of VK2/E6E7 cell microbicidal sensitivity to N-9, C31G, and SDS after a 2-h exposure (data not shown) was similar to that observed after 48 h (Fig. 6C), suggesting that the relative cytotoxicity of N-9, C31G, and SDS was not influenced by differences in cell division rates between the primary HVK cultures and the VK2/E6E7 cells. Finally, primary HVK cultures, which contain fibroblasts as well as epithelial cells, may differ in sensitivity from the VK2/E6E7 cell line because of nonepithelial cell types in the heterogeneous primary cell population. Tertiary HVKs may be more similar in sensitivity to the VK2/E6E7 cells since the HVK cultures are enriched for epithelial cells by successive passages in serum-free media (data not shown). To address concerns that the MTT assay may have enhanced or attenuated the observed differences in epithelial cell sensitivity, HeLa cell sensitivity to C31G exposure was assessed by MTT assay and was compared to results obtained using the CellTiter 96 proliferation assay (Promega; uses the tetrazolium salt MTS) and the CellTiter-Glo viability assay (Promega; chemiluminescently detects ATP released from viable cells). Results using all three assays were identical (data not shown), suggesting that differences between primary epithelial cells and cell lines were unrelated to the assay used to assess sensitivity. Studies are now under way to assess the impact of candidate microbicides on cell viability and function using other measures, including the induction and release of cytokines following microbicide exposure.
An objective of these studies was to demonstrate the suitability of human cell lines for use in in vitro assays of microbicide cytotoxicity. Similar concerns have been raised in investigations of alternative methods to evaluate anticancer drugs. In experiments that explored the use of the MTT assay to evaluate the sensitivity of lung tumors to chemotherapeutic treatment, no differences in chemosensitivity between cancer cell lines and primary cell populations prepared from clinical lung tumor samples were observed (7).
The process of discovery and development of agents for use as microbicides effective against HIV-1 would be greatly facilitated by the availability of in vitro models and systems that more accurately predict in vivo efficacy. In vitro models should accurately predict not only the cytotoxicity of each agent relative to other microbicides but also the concentration that may be tolerated in vivo. Our investigations indicate that assays of in vitro cytotoxicity using established cell lines can be used to rank the relative cytotoxicity of vaginal microbicide products. In addition, experiments in progress suggest a correlation between rank orders of cytotoxicity demonstrated in vivo and in vitro. However, present in vitro assays using submerged cultures of single-cell populations cannot be used to accurately gauge concentrations that may be used safely in vivo, since these assays do not take into consideration physicochemical properties of the vehicle and elements of the vaginal and cervical environment, including the three-dimensional epithelial tissue structure, cervical mucus, vaginal fluid, semen, and changes in pH, that may alter microbicide cytotoxicity in vivo.
The need for better preclinical screening methods is illustrated by the historical evolution of N-9, which was shown to be an effective virucidal agent in vitro (18, 21) but appears to be ineffective against HIV-1 in vivo (8, 27) and detrimental to the user (26, 28). Although presently available in vivo models, including the tissue explant systems (1, 9, 15) and the human tissue xenograft model (16), can be used to factor the impact of the stratified vaginal epithelial structure into studies of microbicide cytotoxicity, these assays may be limited in scale by tissue sample size and availability. In vitro methodologies using readily available cell lines are better suited to reproducibly screen large numbers of agents for cytotoxicity as well as for effectiveness against HIV-1-infected cells of immune origin. These techniques could also be used to assess the effect of cervical fluid and vaginal mucus on microbicide concentrations that are tolerable in vivo. The studies described above provide a foundation for the development of in vitro screening methods using cell lines of immune and epithelial origin.
Acknowledgments
We acknowledge Patricia Welsh and Elizabeth Neely for the preparation of primary HVK cultures used in these experiments and Hung-Mo Lin for her statistical analyses of the data presented herein.
These studies were supported by Public Health Service grant P01 AI37829 (M.K.H., Program Director; B.W., Project 3C Director), funds from a Rockefeller Foundation Microbicides Basic Science Network Grant (R.F. and D.A.), and amfAR research grant no. 02671-28-RG (F.C.K.).
REFERENCES
- 1.Aebischer, F., and J. K. McDougall. 1997. Organotypic raft cultures for the in vitro evaluation of vaginal microbicidal agents. Microbicides have epithelium-specific effects when repeatedly added onto organotypic human keratinocyte cultures. Sex. Transm. Dis. 24:69-76. [DOI] [PubMed] [Google Scholar]
- 2.Auwerx, J. 1991. The human leukemia cell line, THP-1: a multifaceted model for the study of monocyte-macrophage differentiation. Experientia 47:22-31. [DOI] [PubMed] [Google Scholar]
- 3.Bounds, W. 1997. Female condoms. Eur J. Contracept. Reprod. Health Care 2:113-116. [DOI] [PubMed] [Google Scholar]
- 4.Bourne, K. Z., N. Bourne, S. F. Reising, and L. R. Stanberry. 1999. Plant products as topical microbicide candidates: assessment of in vitro and in vivo activity against herpes simplex virus type 2. Antivir. Res. 42:219-226. [DOI] [PubMed] [Google Scholar]
- 5.Boyum, A. 1968. Isolation of mononuclear cells and granulocytes from human blood. Isolation of mononuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand. J. Clin. Lab. Investig. Suppl. 97:77-89. [PubMed] [Google Scholar]
- 6.Calis, S., N. Yulug, M. Sumnu, A. Ayhan, and A. A. Hincal. 1992. A non-antibiotic antimicrobial mixture (C31G): evaluation of the antimicrobial efficiency of C31G on vaginal cultures. Boll. Chim. Farm. 131:335-338. [PubMed] [Google Scholar]
- 7.Campling, B. G., J. Pym, H. M. Baker, S. P. Cole, and Y. M. Lam. 1991. Chemosensitivity testing of small cell lung cancer using the MTT assay. Br. J. Cancer 63:75-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. 2000. CDC statement on study results of product containing nonoxynol-9. JAMA 284:1376. [DOI] [PubMed] [Google Scholar]
- 9.Collins, K. B., B. K. Patterson, G. J. Naus, D. V. Landers, and P. Gupta. 2000. Development of an in vitro organ culture model to study transmission of HIV-1 in the female genital tract. Nat. Med. 6:475-479. [DOI] [PubMed] [Google Scholar]
- 10.Corner, A. M., M. M. Dolan, S. L. Yankell, and D. Malamud. 1988. C31G, a new agent for oral use with potent antimicrobial and antiadherence properties. Antimicrob. Agents Chemother. 32:350-353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feldblum, P. J., M. A. Kuyoh, J. J. Bwayo, M. Omari, E. L. Wong, K. G. Tweedy, and M. J. Welsh. 2001. Female condom introduction and sexually transmitted infection prevalence: results of a community intervention trial in Kenya. AIDS 15:1037-1044. [DOI] [PubMed] [Google Scholar]
- 12.Fichorova, R. N., J. G. Rheinwald, and D. J. Anderson. 1997. Generation of papillomavirus-immortalized cell lines from normal human ectocervical, endocervical, and vaginal epithelium that maintain expression of tissue-specific differentiation proteins. Biol. Reprod. 57:847-855. [DOI] [PubMed] [Google Scholar]
- 13.Fichorova, R. N., L. D. Tucker, and D. J. Anderson. 2001. The molecular basis of nonoxynol-9-induced vaginal inflammation and its possible relevance to human immunodeficiency virus type 1 transmission. J. Infect. Dis. 184:418-428. [DOI] [PubMed] [Google Scholar]
- 14.Gmelig-Meyling, F., and T. A. Waldmann. 1980. Separation of human blood monocytes and lymphocytes on a continuous Percoll gradient. J. Immunol. Methods 33:1-9. [DOI] [PubMed] [Google Scholar]
- 15.Greenhead, P., P. Hayes, P. S. Watts, K. G. Laing, G. E. Griffin, and R. J. Shattock. 2000. Parameters of human immunodeficiency virus infection of human cervical tissue and inhibition by vaginal virucides. J. Virol. 74:5577-5586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Howett, M. K., E. B. Neely, N. D. Christensen, B. Wigdahl, F. C. Krebs, D. Malamud, S. D. Patrick, M. D. Pickel, P. A. Welsh, C. A. Reed, M. G. Ward, L. R. Budgeon, and J. W. Kreider. 1999. A broad-spectrum microbicide with virucidal activity against sexually transmitted viruses. Antimicrob. Agents Chemother. 43:314-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Howett, M. K., B. Wigdahl, D. Malamud, N. D. Christensen, P. B. Wyrick, F. C. Krebs, and B. J. Catalone. 2000. Alkyl sulfates: a new family of broad spectrum microbicides, p. 707-712. In XIII International AIDS Conference. Monduzzi Editore, International Proceedings Division (Bologna, Italy), Durban, South Africa.
- 18.Jennings, R., and A. Clegg. 1993. The inhibitory effect of spermicidal agents on replication of HSV-2 and HIV-1 in-vitro. J. Antimicrob. Chemother. 32:71-82. [DOI] [PubMed] [Google Scholar]
- 19.Koyanagi, Y., W. A. O'Brien, J. Q. Zhao, D. W. Golde, J. C. Gasson, and I. S. Chen. 1988. Cytokines alter production of HIV-1 from primary mononuclear phagocytes. Science 241:1673-1675. [DOI] [PubMed] [Google Scholar]
- 20.Krebs, F. C., S. R. Miller, B. J. Catalone, P. A. Welsh, D. Malamud, M. K. Howett, and B. Wigdahl. 2000. Sodium dodecyl sulfate and C31G as microbicidal alternatives to nonoxynol 9: comparative sensitivity of primary human vaginal keratinocytes. Antimicrob. Agents Chemother. 44:1954-1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krebs, F. C., S. R. Miller, D. Malamud, M. K. Howett, and B. Wigdahl. 1999. Inactivation of human immunodeficiency virus type 1 by nonoxynol-9, C31G, or an alkyl sulfate, sodium dodecyl sulfate. Antivir. Res. 43:157-173. [DOI] [PubMed] [Google Scholar]
- 22.Malamud, D., M. K. Howett, B. Wigdahl, N. D. Christensen, F. C. Krebs, J. Weisz, and J. W. Kreider. 1998. Anti-viral activity of microbicides in model systems, p. 223-227. In 12th World AIDS Conference. Monduzzi Editore, International Proceedings Division (Bologna, Italy), Geneva, Switzerland.
- 23.Nordmann, P., M. Diez-Ibanez, M. Chessebeuf-Padieu, B. Luu, G. Mack, and M. Mersel. 1989. Toxic effects of 7 beta-hydroxycholesterol on rat liver primary cultures, epithelial lines and co-cultures. Cell Biol. Toxicol. 5:261-270. [DOI] [PubMed] [Google Scholar]
- 24.Pauwels, R., J. Balzarini, M. Baba, R. Snoeck, D. Schols, P. Herdewijn, J. Desmyter, and E. De Clercq. 1988. Rapid and automated tetrazolium-based colorimetric assay for the detection of anti-HIV compounds. J. Virol. Methods 20:309-321. [DOI] [PubMed] [Google Scholar]
- 25.Piret, J., J. Lamontagne, J. Bestman-Smith, S. Roy, P. Gourde, A. Desormeaux, R. F. Omar, J. Juhasz, and M. G. Bergeron. 2000. In vitro and in vivo evaluations of sodium lauryl sulfate and dextran sulfate as microbicides against herpes simplex and human immunodeficiency viruses. J. Clin. Microbiol. 38:110-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roddy, R. E., M. Cordero, C. Cordero, and J. A. Fortney. 1993. A dosing study of nonoxynol-9 and genital irritation. Int J. STD AIDS 4:165-170. [DOI] [PubMed] [Google Scholar]
- 27.Roddy, R. E., L. Zekeng, K. A. Ryan, U. Tamoufe, S. S. Weir, and E. L. Wong. 1998. A controlled trial of nonoxynol 9 film to reduce male-to-female transmission of sexually transmitted diseases. N. Engl. J. Med. 339:504-510. [DOI] [PubMed] [Google Scholar]
- 28.Stafford, M. K., H. Ward, A. Flanagan, I. J. Rosenstein, D. Taylor-Robinson, J. R. Smith, J. Weber, and V. S. Kitchen. 1998. Safety study of nonoxynol-9 as a vaginal microbicide: evidence of adverse effects. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 17:327-331. [DOI] [PubMed] [Google Scholar]
- 29.Thompson, K. A., D. Malamud, and B. T. Storey. 1996. Assessment of the anti-microbial agent C31G as a spermicide: comparison with nonoxynol-9. Contraception 53:313-318. [PubMed] [Google Scholar]
- 30.UNAIDS/WHO 1998. Report on the global HIV/AIDS epidemic. Joint United Nations Programme on HIV/AIDS and the World Health Organization.
- 31.Weller, S. C. 1993. A meta-analysis of condom effectiveness in reducing sexually transmitted HIV. Soc. Sci. Med. 36:1635-1644. [DOI] [PubMed] [Google Scholar]
- 32.Worth, D. 1989. Sexual decision-making and AIDS: why condom promotion among vulnerable women is likely to fail. Stud. Fam. Plann. 20:297-307. [PubMed] [Google Scholar]
- 33.Zeitlin, L., K. J. Whaley, T. A. Hegarty, T. R. Moench, and R. A. Cone. 1997. Tests of vaginal microbicides in the mouse genital herpes model. Contraception 56:329-335. [DOI] [PubMed] [Google Scholar]