Abstract
Most Helicobacter pylori strains are susceptible to amoxicillin, an important component of combination therapies for H. pylori eradication. The isolation and initial characterization of the first reported stable amoxicillin-resistant clinical H. pylori isolate (the Hardenberg strain) have been published previously, but the underlying resistance mechanism was not described. Here we present evidence that the β-lactam resistance of the Hardenberg strain results from a single amino acid substitution in HP0597, a penicillin-binding protein 1A (PBP1A) homolog of Escherichia coli. Replacement of the wild-type HP0597 (pbp1A) gene of the amoxicillin-sensitive (Amxs) H. pylori strain 1061 by the Hardenberg pbp1A gene resulted in a 100-fold increase in the MIC of amoxicillin. Sequence analysis of pbp1A of the Hardenberg strain, the Amxs H. pylori strain 1061, and four amoxicillin-resistant (Amxr) 1061 transformants revealed a few amino acid substitutions, of which only a single Ser414→Arg substitution was involved in amoxicillin resistance. Although we cannot exclude that mutations in other genes are required for high-level amoxicillin resistance of the Hardenberg strain, this amino acid substitution in PBP1A resulted in an increased MIC of amoxicillin that was almost identical to that for the original Hardenberg strain.
Helicobacter pylori, a spiral-shaped gram-negative bacterium which colonizes the human stomach, is the causative agent of chronic active gastritis and peptic ulcer disease and is associated with increased risk for gastric cancer and gastric lymphoma (3, 9, 21). Anti-H. pylori therapy often consists of the β-lactam antibiotic amoxicillin in combination with one or more antimicrobial drugs, a bismuth component, and/or a proton pump inhibitor (9, 12). H. pylori is usually sensitive to amoxicillin, but occasionally strains for which an increased MIC is observed have been reported. The first reported amoxicillin-resistant (Amxr) strains (MIC > 256 mg/liter) were isolated from dyspeptic patients in Italy and the United States (10), but they all rapidly lost their amoxicillin resistance in vitro (8). Subsequently the isolation of a stable amoxicillin-resistant clinical H. pylori isolate, the Hardenberg strain, from an 82-year-old Dutch dyspeptic patient was reported (27).
Bacterial resistance against β-lactam antibiotics results mostly from either the production of β-lactamase (16), structural alterations in one of the penicillin-binding proteins (PBPs) (24), or changes in other proteins involved in cell wall synthesis (4, 17, 29). Initial studies with H. pylori indicated the presence of three high-molecular-weight PBPs, designated PBP1A, PBP2, and PBP3 (5). Later a fourth, low-molecular-weight PBP, PBP4, was identified (14), and subsequently five other potential PBPs (13) and an H. pylori-specific β-lactamase (19) have been described. Amoxicillin resistance in H. pylori was found not to rely on the acquisition or expression of β-lactamase (8, 27). The nonstable amoxicillin resistance of H. pylori (8) is probably due to a decreased expression of the penicillin-binding protein PBPD (7). In the recently described in vitro-selected Amxr H. pylori strains, amoxicillin resistance was suggested to result from alterations in PBP1A (6, 22).
In this study, we describe the molecular mechanism of amoxicillin resistance of the Hardenberg strain. Ten genes from the published H. pylori genomes were selected as potential candidates based on their putative role in cell wall synthesis (Table 1). After genetic transformation of the amoxicillin-sensitive (Amxs) strain 1061 with PCR products of the selected candidate genes of the Amxr Hardenberg strain, the minimal PCR fragment able to transfer amoxicillin resistance was sequenced and the amino acid alterations responsible for amoxicillin resistance were identified.
TABLE 1.
Transformation with PCR products of putative Amxr genes
| Putative function of selected gene (gene name)a | Gene no.b | Position of fragmentc | Transformation frequencyd |
|---|---|---|---|
| Penicillin-binding protein (pbp4) | HP0160 | 168848-169882 | None |
| H. pylori cysteine-rich protein A (hcpA) | HP0211 | 218980-218291 | None |
| Lysis tolerance protein (lytB) | HP0400 | 411955-411354 | None |
| Penicillin-binding protein (pbp1A) | HP0597 | 632742-631000 | 5 × 10−6 |
| Rod shape-determining protein (rodA1) | HP0743 | 798879-797794 | None |
| Rod shape-determining protein (mreC) | HP1372 | 1436921-1436354 | None |
| Rod shape-determining protein (mreB) | HP1373 | 1438015-1437011 | None |
| Cell division protein (ftsI) | HP1556 | 1639818-1638044 | None |
| Penicillin-binding protein (pbp2) | HP1565 | 1648924-1647271 | None |
| Methicillin resistance protein (llm) | HP1581 | 1660483-1659552 | None |
Genes were selected from the published H. pylori genomes (2, 25) as potential candidates based on their putative role in cell wall synthesis.
HP gene numbers corresponding to the H. pylori 26695 genome sequence (25).
Position of duplicated fragment, corresponding to the H. pylori 26695 genome sequence (25). Primers used for the amplification of the PCR fragments started at the outside of the fragment and had each a length of 20 bp.
Determined as the number of Amxr colonies per microgram of DNA per recipient CFU. Data represent the means of three experiments.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
H. pylori strains used in this study were the Amxr Hardenberg strain (27) and the Amxs H. pylori strains 1061 (11), J99 (2), 26695 (25), and SS1 (15). Bacteria were routinely grown on Columbia agar plates (Becton Dickinson, Cockeysville, Md.) supplemented with 7% lysed horse blood (BioTrading, Mijdrecht, The Netherlands) and H. pylori-selective supplement (Oxoid, Basingstoke, United Kingdom) for 48 to 72 h at 37°C in an atmosphere of 5% O2, 10% CO2, and 85% N2. Escherichia coli strain DH5α MCR (Life Technologies BV, Paisley, United Kingdom) was grown on Luria-Bertani agar plates (23) for 24 h at 37°C in an aerobic environment. Selection of E. coli DH5α MCR transformed with pGEM-T Easy clones was performed on ampicillin-containing (100 mg/liter) Luria-Bertani agar plates.
Determination of MIC.
The MIC was routinely determined by E-test (AB Biodisk, Solna, Sweden) (10) or by agar dilution (8).
DNA manipulation.
Recombinant DNA techniques were performed according to standard protocols (23). PCR fragments and pGEM-T Easy clones were sequenced on an Amersham Vistra 725 DNA sequencer using Thermo-Sequenase premixed cycle sequencing kit (Amersham, Buckinghamshire, United Kingdom).
Natural transformation of H. pylori.
Bacteria were transformed with approximately 1 μg of total DNA or 200 to 500 ng of PCR product essentially as described by Wang et al. (28). Transformants were selected on Dent plates containing 0.2 to 2 mg of amoxicillin (Sigma Aldrich Chemie, Zwijndrecht, The Netherlands) per liter. As a control, bacteria were transformed with Tris-EDTA (TE) (1 mM Tris-HCl [pH 8.0], 0.1 mM EDTA) or DNA from the Amxs strain 1061.
PCR.
The oligonucleotide primers (Isogen, Maarsen, The Netherlands) used in this study are indicated in Table 1 and Fig. 1. PCR was performed in an automated thermal cycler (GeneAmp PCR system 9700; Perkin-Elmer), using the PCR-core system I (Promega, Madison, Wis.).
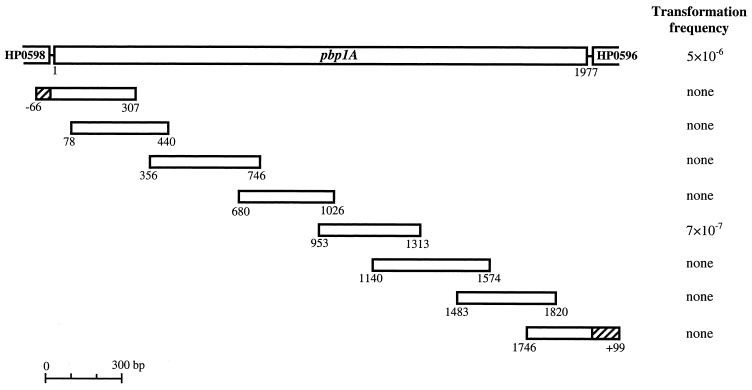
FIG. 1.
Identification of the region of pbp1A required for amoxicillin resistance. Transformants were obtained by transformation of overlapping PCR fragments of the Amxr Hardenberg strain to the Amxs strain 1061. The transformation frequency was determined as the number of Amxr colonies per microgram of DNA per recipient CFU. Data represent the means of three experiments. Primers used for the amplification of the smaller overlapping PCR fragments started at the outside of the fragment and had each a length of 20 bp. The first and last fragments (hatched areas) partially consist of the flanking genes of pbp1A, namely, HP0596 and HP0598.
Site-directed mutagenesis.
Four different 135-bp oligonucleotides (Isogen) were designed based on the sequence of pbp1A of H. pylori strain 1061, except that they contained mismatches resulting in none, one, or two amino acid substitutions (Glu406→Ala and/or Ser414→Arg). For amplification, 5 pmol of the 135-bp oligonucleotides was used as template, with 25 pmol of each terminal primer (5′-GCTATTCCACGACTTCTAAA-3′ and 5′-GCAAGGTTACAAGCCCTAAA-3′).
Nucleotide sequence accession numbers.
The pbp1A sequences of the H. pylori Hardenberg and 1061 strains have been deposited into the GenBank database under accession no. AF479617 and AF479618, respectively.
RESULTS AND DISCUSSION
Determination of the MICs of various antibiotics.
The MICs of the four antibiotics commonly used in anti-H. pylori therapy, as well as several β-lactam antibiotics, are presented in Table 2. For the Hardenberg strain, the MIC of amoxicillin was 8 mg/liter, while the MIC for strain 1061 was well below 0.016 mg/liter. The MICs of the other β-lactam antibiotics were significantly higher for the Hardenberg strain than for H. pylori strain 1061. MICs of clarithromycin and tetracycline were identical for both strains, but the Hardenberg strain was found to be sensitive to metronidazole, while strain 1061 is known to be resistant to that drug (11).
TABLE 2.
MICs for the Hardenberg strain, H. pylori strain 1061, and Amxr 1061 transformants determined by E-test
| Antibiotic | MIC (mg/liter)a
|
||
|---|---|---|---|
| Hardenberg | 1061 | Amxr 1061 transformantsb | |
| Amoxicillin | 8 | <0.016 | 0.75 |
| Amoxicillin-clavulanate | 2 | <0.016 | 0.25 |
| Cefotaxime | 0.75 | 0.023 | 0.75 |
| Ceftazidime | 4 | 0.25 | 3 |
| Penicillin | 2 | 0.032 | 0.25 |
| Piperacillin | 4 | <0.016 | 0.38 |
| Clarithromycin | <0.016 | <0.016 | <0.016 |
| Metronidazole | 0.023 | 192 | 192 |
| Tetracycline | <0.016 | 0.023 | 0.032 |
Data shown are the averages of three separate experiments.
MICs are the averages of 10 randomly selected transformants (obtained after transformation with total DNA of the Hardenberg strain) derived from three independent transformation experiments.
As the E-test was reported to generate discrepant results when compared to the agar dilution method (1, 20, 26), and as the E-test does not allow determination of amoxicillin MICs below 0.016 mg/liter, the susceptibility to amoxicillin was also tested by agar dilution. The MIC of amoxicillin for the Hardenberg strain by agar dilution was slightly higher (10 mg/liter) than by E-test. For H. pylori strain 1061, the MIC of amoxicillin was 0.01 mg/liter.
Transfer of amoxicillin resistance by natural transformation.
Transformation of H. pylori strain 1061 with total DNA of the Hardenberg strain resulted in Amxr colonies at a frequency of 10−5. The MICs of amoxicillin for 10 randomly selected transformants (obtained from three independent transformation experiments) as determined by E-test (Table 2) and agar dilution were 0.75 and 1.0 mg/liter, respectively. In the agar dilution method, for these transformants the MIC of amoxicillin was 100-fold higher than that for the 1061 acceptor strain, and it was only slightly lower than that of the Hardenberg donor strain. For the Amxr transformants, the MICs of the other β-lactam antibiotics also displayed increases (Table 2).
Transformation with PCR products of cell wall synthesis-encoding genes.
Based on their putative role in cell wall synthesis, 10 genes from the published H. pylori genome sequences of strain 26695 (25) and strain J99 (2) were selected as potential Amxr genes (Table 1). Amxs strain 1061 was transformed with PCR-amplified products of the selected genes of the Amxr Hardenberg strain. Only transformation with pbp1A resulted in Amxr colonies with a frequency of 5 × 10−6 (Table 1). PCR products of the other putative amoxicillin resistance genes, TE, and DNA from strain 1061 were each unable to transfer amoxicillin resistance. The MIC of amoxicillin for 10 randomly selected Amxr colonies (obtained from three independent transformation experiments) was identical to that of strain 1061 transformed with total DNA of the Hardenberg strain. Repeated transformation of these transformants with either the total DNA or the other nine PCR products of the Hardenberg strain did not result in an increase of the MIC (data not shown). The MIC for these transformants could be increased to the same level as the Hardenberg strain only by continued exposure to amoxicillin, but this additional increase of the resistance was not stable. This indicated that the additional increase in the MIC of amoxicillin for the Amxr 1061 transformants was due to a transient physiological change. Apparently a stable increase of amoxicillin resistance to the level of the Hardenberg strain requires mutations in more than one locus, and the likelihood that these are all transferred by transformation is relatively small.
Identification of pbp1A mutations involved in amoxicillin resistance.
To determine the mutations of pbp1A responsible for amoxicillin resistance, natural transformation experiments were performed by using small overlapping PCR fragments of pbp1A (Fig. 1). Amxr 1061 transformants (7 × 10−7) were observed only with the 361-bp DNA fragment that spanned nucleotides 953 to 1313 (numbering according to pbp1A of H. pylori strain 26695). No Amxr colonies were observed after transformation with the seven other pbp1A fragments, TE, or DNA from strain 1061. The MIC of amoxicillin for 10 randomly selected transformants (obtained from three independent transformation experiments) was 1.0 mg/liter.
Analysis of PBP1A sequences obtained from the Hardenberg strain, the Amxs 1061 strain, and four Amxr 1061 transformants (obtained from three independent transformation experiments with total DNA from the Hardenberg strain) showed several amino acid substitutions in the Hardenberg strain as well as in the transformants that did not occur in the Amxs strain. Three transformants had incorporated the complete Hardenberg pbp1A sequence, while the fourth transformant contained only the second half of the gene. For this fourth transformant the DNA exchange apparently occurred between nucleotides 793 and 854. This was independent proof that the first 792 bp of pbp1A of the Hardenberg strain are not involved in amoxicillin resistance, as was already indicated by transformation with the pbp1A PCR fragments.
Amoxicillin resistance of pbp1A is due to the Ser414→Arg substitution.
Sequence analysis of PBP1A showed two amino acid differences in the region between nucleotides 953 and 1313, a glutamic acid by an alanine (Glu406→Ala) and a serine by an arginine (Ser414→Arg). In order to identify which of these amino acid changes was responsible for the increase in amoxicillin resistance, site-directed mutagenesis was used. Strain 1061 was transformed with four different 135-bp fragments coding for amino acids 381 to 426 (numbering according to pbp1A of H. pylori strain 26695 [25]) containing either no, one (Glu406→Ala or Ser414→Arg), or both substitutions. Amxr colonies were observed only after natural transformation with the two fragments that contained the Ser414→Arg substitution with transformation frequencies of 7 × 10−8 and 4 × 10−8 respectively. Sequence analysis of PBP1A of four randomly selected transformants, obtained from two independent transformation experiments with the 135-bp Ser414→Arg fragment, confirmed that only this substitution was present in the Amxr transformants. The MIC of amoxicillin for these transformants was 1.0 mg/liter. In addition to the MICs of amoxicillin for these organisms, the growth rates (optical density at 600 nm measured at regular intervals during a time period of 48 h), sizes, and shapes of the bacteria were determined. In the absence of amoxicillin, no differences between the Hardenberg strain, the four Amxr transformants, and strain 1061 were found in growth rates, sizes, or shapes of the bacteria. Under amoxicillin pressure (1 mg/liter), the bacteria became a little bit shorter and thicker, but in spite of this morphological change there was no significant change in growth rates (data not shown).
Site-directed mutagenesis indicated that the Ser414→Arg substitution in PBP1A represents the main factor in amoxicillin resistance of the Hardenberg strain. Recently, other investigators (22) reported that amino acid substitutions Tyr484→Cys, Thr541→Ile, and Pro600→Thr in PBP1A are responsible for amoxicillin resistance of an in vitro-selected Amxr H. pylori strain. None of these amino acid substitutions play a role in amoxicillin resistance of the Hardenberg strain, since these substitutions were not present in the Amxr Hardenberg strain. Further, the Ser414→Arg substitution in PBP1A in the Hardenberg strain and Amxr 1061 transformants had no influence on the expressed amount of PBP1A molecules compared to the wild-type PBP1A of H. pylori strain 1061 (data not shown). Probably the amino acid substitutions in PBP1A of the Hardenberg strain and the in vitro-selected Amxr strain (22) affect the binding affinity of PBP1A for penicillins, but this was not tested.
Role of pbp1A in amoxicillin resistance of other H. pylori strains.
To test the generality of our findings, we also transformed H. pylori strains J99, 26695, and SS1 (MICs of amoxicillin for these strains, 0.01, 0.01, and 0.2 mg/liter, respectively) either with the PCR product of pbp1A or with total DNA from the Hardenberg strain. With all strains, Amxr transformants were isolated at high frequency. Ten Amxr transformants of each strain (obtained from two independent transformation experiments) were selected at random, and the MICs of amoxicillin were determined. All transformants had a MIC of 1.0 mg/liter, which was identical to the MIC of the Amxr H. pylori 1061 transformants but lower than the MIC of the Amxr Hardenberg strain. These results indicated that for strains other than 1061, the introduction of the pbp1A from the Hardenberg strain also increased the MIC of amoxicillin.
The results of our study showed that a single serine-to-arginine substitution in PBP1A induces high-level amoxicillin resistance in H. pylori. Since the existence of H. pylori strains with a moderate resistance level against amoxicillin already has been reported (18, 30) and apparently a single mutation is sufficient to increase the MIC of amoxicillin, an increase of naturally occurring H. pylori strains with a high level of amoxicillin resistance can be expected in the near future.
Acknowledgments
We thank R. G. J. Pot, J. Stoof, and T. Verboom for DNA sequence analysis. We thank S. H. Phadnis and A. H. M. van Vliet for helpful comments and discussions.
REFERENCES
- 1.Alarcon, T., D. Domingo, and M. Lopez-Brea. 1998. Discrepancies between E-test and agar dilution methods for testing metronidazole susceptibility of Helicobacter pylori. J. Clin. Microbiol. 36:1165-1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alm, R. A., L. S. Ling, D. T. Moir, B. L. King, E. D. Brown, P. C. Doig, D. R. Smith, B. Noonan, B. C. Guild, B. L. deJonge, G. Carmel, P. J. Tummino, A. Caruso, M. Uria-Nickelsen, D. M. Mills, C. Ives, R. Gibson, D. Merberg, S. D. Mills, Q. Jiang, D. E. Taylor, G. F. Vovis, and T. J. Trust. 1999. Genomic-sequence comparison of two unrelated isolates of the human gastric pathogen Helicobacter pylori. Nature 397:176-180. [DOI] [PubMed] [Google Scholar]
- 3.Blaser, M. J. 1997. Ecology of Helicobacter pylori in the human stomach. J. Clin. Investig. 100:759-762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Costa, C. S., and D. N. Anton. 1993. Round-cell mutants of Salmonella typhimurium produced by transposition mutagenesis: lethality of rodA and mre mutations. Mol. Gen. Genet. 236:387-394. [DOI] [PubMed] [Google Scholar]
- 5.DeLoney, C. R., and N. L. Schiller. 1999. Competition of various beta-lactam antibiotics for the major penicillin-binding proteins of Helicobacter pylori: antibacterial activity and effects on bacterial morphology. Antimicrob. Agents Chemother. 43:2702-2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DeLoney, C. R., and N. L. Schiller. 2000. Characterization of an in vitro-selected amoxicillin-resistant strain of Helicobacter pylori. Antimicrob. Agents Chemother. 44:3368-3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dore, M. P., D. Y. Graham, and A. R. Sepulveda. 1999. Different penicillin-binding protein profiles in amoxicillin-resistant Helicobacter pylori. Helicobacter 4:154-161. [DOI] [PubMed] [Google Scholar]
- 8.Dore, M. P., M. S. Osato, G. Realdi, I. Mura, D. Y. Graham, and A. R. Sepulveda. 1999. Amoxycillin tolerance in Helicobacter pylori. J. Antimicrob. Chemother. 43:47-54. [DOI] [PubMed] [Google Scholar]
- 9.Dunn, B. E., H. Cohen, and M. J. Blaser. 1997. Helicobacter pylori. Clin. Microbiol. Rev. 10:720-741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Glupczynski, Y., M. Labbe, W. Hansen, F. Crokaert, and E. Yourassowsky. 1991. Evaluation of the E-test for quantitative antimicrobial susceptibility testing of Helicobacter pylori. J. Clin. Microbiol. 29:2072-2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goodwin, A., D. Kersulyte, G. Sisson, S. J. Veldhuyzen van Zanten, D. E. Berg, and P. S. Hoffman. 1998. Metronidazole resistance in Helicobacter pylori is due to null mutations in a gene (rdxA) that encodes an oxygen-insensitive NADPH nitroreductase. Mol. Microbiol. 28:383-393. [DOI] [PubMed] [Google Scholar]
- 12.Goodwin, C. S. 1997. Antimicrobial treatment of Helicobacter pylori infection. Clin. Infect. Dis. 25:1023-1026. [DOI] [PubMed] [Google Scholar]
- 13.Harris, A. G., S. L. Hazell, and A. G. Netting. 2000. Use of digoxigenin-labelled ampicillin in the identification of penicillin-binding proteins in Helicobacter pylori. J. Antimicrob. Chemother. 45:591-598. [DOI] [PubMed] [Google Scholar]
- 14.Krishnamurthy, P., M. H. Parlow, J. Schneider, S. Burroughs, C. Wickland, N. B. Vakil, B. E. Dunn, and S. H. Phadnis. 1999. Identification of a novel penicillin-binding protein from Helicobacter pylori. J. Bacteriol. 181:5107-5110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee, A., J. O'Rourke, M. C. De Ungria, B. Robertson, G. Daskalopoulos, and M. F. Dixon. 1997. A standardized mouse model of Helicobacter pylori infection: introducing the Sydney strain. Gastroenterology 112:1386-1397. [DOI] [PubMed] [Google Scholar]
- 16.Livermore, D. M. 1995. beta-Lactamases in laboratory and clinical resistance. Clin. Microbiol. Rev. 8:557-584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maki, H., and K. Murakami. 1997. Formation of potent hybrid promoters of the mutant llm gene by IS256 transposition in methicillin-resistant Staphylococcus aureus. J. Bacteriol. 179:6944-6948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Megraud, F., N. Lehn, T. Lind, E. Bayerdorffer, C. O'Morain, R. Spiller, P. Unge, S. V. van Zanten, M. Wrangstadh, and C. F. Burman. 1999. Antimicrobial susceptibility testing of Helicobacter pylori in a large multicenter trial: the MACH 2 study. Antimicrob. Agents Chemother. 43:2747-2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mittl, P. R., L. Luthy, P. Hunziker, and M. G. Grutter. 2000. The cysteine-rich protein A from Helicobacter pylori is a beta-lactamase. J. Biol. Chem. 275:17693-17699. [DOI] [PubMed] [Google Scholar]
- 20.Osato, M. S., R. Reddy, S. G. Reddy, R. L. Penland, and D. Y. Graham. 2001. Comparison of the E-test and the NCCLS-approved agar dilution method to detect metronidazole and clarithromycin resistant Helicobacter pylori. Int. J. Antimicrob. Agents 17:39-44. [DOI] [PubMed] [Google Scholar]
- 21.Parsonnet, J., G. D. Friedman, D. P. Vandersteen, Y. Chang, J. H. Vogelman, N. Orentreich, and R. K. Sibley. 1991. Helicobacter pylori infection and the risk of gastric carcinoma. N. Engl. J. Med. 325:1127-1131. [DOI] [PubMed] [Google Scholar]
- 22.Paul, R., S. Postius, K. Melchers, and K. P. Schafer. 2001. Mutations of the Helicobacter pylori genes rdxA and pbp1 cause resistance against metronidazole and amoxicillin. Antimicrob. Agents Chemother. 45:962-965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning, a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 24.Spratt, B. G., and K. D. Cromie. 1988. Penicillin-binding proteins of gram-negative bacteria. Rev. Infect. Dis. 10:699-711. [DOI] [PubMed] [Google Scholar]
- 25.Tomb, J. F., O. White, A. R. Kerlavage, R. A. Clayton, G. G. Sutton, R. D. Fleischmann, K. A. Ketchum, H. P. Klenk, S. Gill, B. A. Dougherty, K. Nelson, J. Quackenbush, L. Zhou, E. F. Kirkness, S. Peterson, B. Loftus, D. Richardson, R. Dodson, H. G. Khalak, A. Glodek, K. McKenney, L. M. Fitzegerald, N. Lee, M. D. Adams, and J. C. Venter. 1997. The complete genome sequence of the gastric pathogen Helicobacter pylori. Nature 388:539-547. [DOI] [PubMed] [Google Scholar]
- 26.van der Wouden, E. J., A. de Jong, J. C. Thijs, J. H. Kleibeuker, and A. A. van Zwet. 1999. Subpopulations of Helicobacter pylori are responsible for discrepancies in the outcome of nitroimidazole susceptibility testing. Antimicrob. Agents Chemother. 43:1484-1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Zwet, A. A., C. M. Vandenbroucke-Grauls, J. C. Thijs, E. J. van der Wouden, M. M. Gerrits, and J. G. Kusters. 1998. Stable amoxicillin resistance in Helicobacter pylori. Lancet 352:1595. [DOI] [PubMed] [Google Scholar]
- 28.Wang, Y., K. P. Roos, and D. E. Taylor. 1993. Transformation of Helicobacter pylori by chromosomal metronidazole resistance and by a plasmid with a selectable chloramphenicol resistance marker. J. Gen. Microbiol. 139:2485-2493. [DOI] [PubMed] [Google Scholar]
- 29.Wosten, M. M., E. E. Ishiguro, and B. A. van der Zeijst. 1997. Cloning and characterization of the lytB gene of Campylobacter jejuni. FEMS Microbiol. Lett. 157:117-121. [DOI] [PubMed] [Google Scholar]
- 30.Wu, H., X. D. Shi, H. T. Wang, and J. X. Liu. 2000. Resistance of Helicobacter pylori to metronidazole, tetracycline and amoxycillin. J. Antimicrob. Chemother. 46:121-123. [DOI] [PubMed] [Google Scholar]