Abstract
The combination of quinupristin-dalfopristin (Q-D) and gentamicin was tested against two strains of gentamicin- and dalfopristin-susceptible methicillin-resistant Staphylococcus aureus (MRSA). One strain was susceptible to macrolides, lincosamides, and streptogramin B type antibiotics (MLSB), and the other was constitutively resistant to these antibiotics by virtue of the ermA gene. The checkerboard method and time-kill curves showed that the combination of Q-D and gentamicin was indifferent. A rabbit endocarditis model simulated the pharmacokinetics achieved in humans receiving intravenous injections of Q-D (7.5 mg/kg of body weight three times a day) and gentamicin (3 mg/kg once daily). For the MLSB-susceptible strain, a 4-day regimen reduced mean bacterial titers (MBT) in vegetations from 8.5 ± 0.8 log CFU/g (control group) to 4.1 ± 2.6 (gentamicin), 3.0 ± 0.9 (Q-D), and 2.6 ± 0.5 log CFU/g (Q-D plus gentamicin). For the strain constitutively resistant to MLSB, a 4-day regimen reduced MBT in vegetations from 8.7 ± 0.9 log CFU/g (control group) to 5.0 ± 2.2 (gentamicin), 5.2 ± 2.2 (Q-D), and 5.1 ± 2.4 log CFU/g (Q-D plus gentamicin). The differences between control and treatment groups were significant for both strains (P < 0.0001), although there was no significant difference between treatment groups. No resistant variant was isolated from vegetations, and no significant difference in MBT in vegetations of treatment groups after 1-day regimens was observed. This experimental study found no additive benefit in combining Q-D and gentamicin against dalfopristin- and gentamicin-susceptible MRSA.
Infections caused by methicillin-resistant Staphylococcus aureus (MRSA) are usually treated by glycopeptides, especially vancomycin (23). The emergence of glycopeptide-intermediate MRSA in Japan, Europe, and North America constitutes a risk of clinical failure of glycopeptide therapy, and studies of experimental infections in animals have confirmed this apparent risk (4, 8). Thus, it seems essential to evaluate new antistaphylococcal drugs and regimens.
Quinupristin and dalfopristin are water-soluble injectable streptogramin B (SB) and SA antibiotics, respectively, whose combination in a 30:70 (wt/wt) ratio acts synergistically on gram-positive bacteria (5, 14). Streptogramins inhibit protein synthesis by binding to the ribosomal 50S subunit, and resistance is a concern mainly for the SB type (9, 20). The most frequent mechanism of quinupristin resistance encountered in recent years is target modification by methylation of an adenine residue in 23S rRNA, encoded in S. aureus by the ermA, ermB, or ermC gene. Constitutively expressed erm genes confer in vitro cross-resistance to macrolides, lincosamides, and SB. Some strains harbor inducible erm genes, whose expression is triggered only by 14- and 15-member macrolides (18). The msrA gene is believed to encode an efflux system, and vgb and vgbB genes encode a hydrolase that inactivates SB type antibiotics (22). Recently, mutations in the L22 ribosomal protein have been associated with resistance to quinupristin in S. aureus (B. Malbruny, A. Canu, B. Bozdogan, V. Zarrouk, B. Fantin, and R. Leclercq, Abstr. 40th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 1928, 2000). Constitutive resistance to quinupristin has led to a relative loss of the bactericidal effect of quinupristin-dalfopristin (Q-D) in S. aureus rat and rabbit experimental endocarditis models (13, 15).
Previous experimental studies showed a potential benefit in combining Q-D with other antibiotics such as β-lactams and rifampin against MRSA (26, 28). Gentamicin, like other aminoglycosides, is considered to exert its antibacterial effect via inhibition of protein synthesis after binding to the 30S ribosomal subunit (21). Its use, in association with other antibiotics, in the first days of treatment of serious S. aureus infections is usually recommended. However, the combination of gentamicin and Q-D was only studied in vitro, and conflicting results, including possible antagonism, were obtained (16, 17).
This study tested the combination of Q-D with gentamicin both in vitro and in vivo against dalfopristin- and gentamicin-susceptible S. aureus strains with various susceptibilities to quinupristin.
(This work was presented in part at the 40th Interscience Conference on Antimicrobial Agents and Chemotherapy, Toronto, Canada, 17 to 20 September 2000.)
MATERIALS AND METHODS
Microorganisms.
Two strains of gentamicin-susceptible MRSA were used. Strain NA8 was a kind gift from Roland Bismuth (Paris, France), and strain BCB8 was isolated from a blood culture. Strain NA8 is susceptible to macrolides, lincosamides, and streptogramins (MLSB-S phenotype), whereas strain BCB8 is constitutively resistant to macrolides, lincosamides, and SB (MLSB-CR phenotype). Both strains were resistant to kanamycin and tobramycin, suggesting the production of an ANT(4′) modifying enzyme.
Antibiotics and media.
Gentamicin and Q-D were provided, respectively, by Schering-Plough (Hérouville-Saint-Clair, France), and Aventis (Paris, France). Unless otherwise stated, bacteria were grown overnight at 37°C either in cation-supplemented Mueller-Hinton broth (Sanofi Diagnostic Pasteur, Marne-la-Coquette, France) or on Mueller-Hinton agar (Difco, Becton-Dickinson France SA, Le Pont de Claix, France).
Search for quinupristin resistance determinants.
Quinupristin resistance determinants were searched for by PCR. Genomic DNA was extracted from culture using the Qiagen DNeasy tissue kit. The sequences of primers for ermA, ermB, ermC, and msrAB are shown in Table 1. The 50-μl reaction mixture for PCR contained 10 ng of total bacterial DNA, 20 pmol (each) oligodeoxynucleotide, 200 μM deoxynucleoside triphosphates, 2 mM MgCl2, 1× PCR buffer, and 1 U of Taq DNA polymerase (Eurobio). PCR experiments were carried out in a Perkin-Elmer 4600 thermal cycler with a denaturation step (94°C for 5 min), followed by 35 amplification cycles (30 s of denaturation at 94°C, 45 s of annealing at 47°C, and 1 min of elongation at 72°C) and a final elongation step (72°C for 10 min). PCR products were separated by electrophoresis through a 2% agarose gel under a 100-V gradient.
TABLE 1.
Sequences of primers for genes encoding resistance to quinupristin
| Gene(s) | Primer sequence (25) (5′-3′) | Predicted length (bp) | Positive control strain (2) |
|---|---|---|---|
| ermA | TCTAAAAAGCATGTAAAAGAA | 645 | S. aureus HM1051 |
| CTTCGATAGTTTATTAATATTAGT | |||
| ermB | GAAAAGGTACTCAACCAAATA | 639 | Streptococcus pneumoniae HM28 |
| AGTAACGGTACTTAAATTGTTTAC | |||
| ermC | TCAAAACATAATATAGATAAA | 642 | S. aureus HM1054R |
| GCTAATATTGTTTAAATCGTCAAT | |||
| msrAB | CAAATGGTGTAGGTAAGACAACT | 399 | Staphylococcus epidermidis HM1053 |
| ATCATGTGATGTAAACAAAAT |
In vitro susceptibilities to antibiotics.
MICs of quinupristin, dalfopristin, Q-D, and gentamicin were determined by the agar dilution method (1).
Checkerboard method.
Interactions between Q-D and gentamicin were assessed by the checkerboard method in 96-well microtiter plates (Nalge Nunc International, Roskilde, Denmark), with an inoculum of 106 CFU/ml. Plates were incubated for 18 h at 37°C. Fractional inhibitory concentration indices were interpreted as follows: ≤0.5, synergy; >4, antagonism; >0.5 and ≤4, indifference (1).
Time-kill curves.
Time-kill curves were plotted on the basis of results obtained with an inoculum of 5 × 106 CFU/ml in glass tubes containing Mueller-Hinton broth and gentamicin and/or Q-D at concentrations equal to their MICs. Control tubes contained no antibiotics. After 0, 2, 6, and 24 h of incubation at 37°C, viable counts were determined by subculturing 50-μl serial dilutions of samples on agar plates with a spiral plater (Spiral Système, Saint-Nom-la-Bretèche, France). Colonies were counted after 48-h of incubation at 37°C. Antibiotic carryover was ruled out after the plating of a 100-CFU/ml suspension in the presence or absence of antibiotics. The lower limit of detection for the spiral plater was 2 log10 CFU/ml. Synergy was defined as a decrease of ≥2 log10 CFU/ml between the combination (Q-D and gentamicin) and its most active constituent after 24 h. Antagonism was defined as an increase of ≥2 log10 CFU/ml between the combination and its most active constituent. The interaction was otherwise considered indifferent.
Experimental staphylococcal endocarditis.
New Zealand White rabbits (weight: 2.0 to 2.7 kg) were used in an aortic endocarditis model, as previously described (12). A polyethylene catheter was inserted into the left ventricle via the carotid artery and left in place throughout the experiment. One milliliter of an overnight bacterial culture (diluted in saline serum at 9 g/liter to obtain 106 CFU/ml) was inoculated into the ear vein 48 h after catheterization.
Twenty-four hours after inoculation, the animals were randomly assigned to groups receiving Q-D, gentamicin, Q-D and gentamicin, or no treatment (control group). Antibiotics were administered for 1 or 4 days in order to obtain serum concentrations close to those observed in humans after 1-h intravenous (i.v.) perfusions of 7.5 mg of Q-D/kg of body weight three times a day (t.i.d.) and 3 mg of gentamicin/kg once a day. For this purpose, Q-D was administered by intramuscular injection of 30 mg/kg t.i.d. (14, 15). The total daily dose of gentamicin (16.6 mg/kg) was infused i.v. at changing flow rates controlled by a computer dedicated to human pharmacokinetic simulations (6, 7).
Rabbits were sacrificed by i.v. injection of thiopental 24 h after inoculation in the control group and at the end of the last day of treatment in the other groups. Vegetations were excised, immediately placed on ice, weighed, homogenized in 0.5 ml of saline, and plated on Mueller-Hinton agar after serial dilutions. Viable counts after a 48-h incubation at 37°C were expressed as means ± standard deviations (SD) of log10 CFU per gram of vegetation. The lower limit of detection for this method is 1 CFU per 100 μl of undiluted vegetation homogenate.
To detect in vivo selection of resistant variants during treatment, undiluted vegetation homogenates were also plated on agar containing gentamicin or Q-D at a concentration corresponding to 16 times the MIC.
Determination of antibiotic concentrations in serum and pharmacokinetic analysis.
Antibiotic concentrations in serum from three healthy rabbits were determined. Samples were drawn (i) 30 min (peak) and 23.5 h (trough) after the first injection of gentamicin and (ii) 15, 30, 45, 60, 120, 240, and 360 min after the first injection of Q-D. For streptogramin assays, 1-ml blood samples were immediately placed in tubes containing 0.25 ml of 0.25 N hydrochloric acid and then gently stirred and centrifuged (10 min at 1,500 × g). The upper phase was immediately stored at −80°C. Gentamicin concentrations were assessed by immunoenzymatic assays (EMIT; Behring). The lower limit of detection for gentamicin was 0.3 mg/liter.
Quinupristin, dalfopristin, and Q-D concentrations were determined by a microbiological assay based on the bactericidal synergy between quinupristin and dalfopristin. The bactericidal activities of pH-neutralized serum samples against Micrococcus luteus DRUG 320 were assessed and compared with a standard curve constructed on the basis of results for rabbit serum. Dalfopristin was added to sera at a final bacteriostatic concentration of 2 mg/liter for determination of quinupristin activity, and quinupristin was added at a final bacteriostatic concentration of 2 mg/liter for determination of dalfopristin activity. No antibiotic was added for determination of Q-D activity. After a 4-h incubation at 37°C, surviving bacteria were counted by plating 3 μl of diluted samples on Mueller-Hinton agar containing activated charcoal (10 g/liter) to prevent antibiotic carryover. Lower limits of detection were 0.09 mg/liter for quinupristin, 0.06 mg/liter for dalfopristin, and 0.23 mg/liter for Q-D. The coefficient of variation of the assay was 16%.
Pharmacokinetic data were analyzed by regression to determine the terminal slope and β elimination half-lives. Areas under the curve of serum concentrations were determined by the trapezoidal method, with extrapolation from the last point toward infinity.
Statistics.
Statistical analysis was performed using StatView software (Abacus Concepts). Mean bacterial titers in vegetations were globally compared by analysis of variance (ANOVA). Scheffe's test was used to compare mean titers two by two. A P value ≤0.05 was considered significant.
RESULTS
Susceptibility tests.
MICs are shown in Table 2. Although strains NA8 and BCB8 showed quite different susceptibilities to quinupristin, the MICs of Q-D for them were similar. Both strains revealed susceptibility to dalfopristin and gentamicin.
TABLE 2.
Agar dilution MICs of quinupristin, dalfopristin, Q-D, and gentamicin for S. aureus strains NA8 and BCB8
| Strain | MIC (mg/liter) of:
|
||||
|---|---|---|---|---|---|
| Oxacillin | Quinupristin | Dalfopristin | Q-D | Gentamicin | |
| NA8 | 64 | 1 | 1 | 0.50 | 0.25 |
| BCB8 | 16 | 128 | 2 | 0.50 | 0.25 |
Search for quinupristin resistance determinants.
PCR identified the ermA gene in strain BCB8. No other genes encoding resistance to quinupristin were detected in the two strains.
Checkerboard.
Fractional inhibitory concentration indices of Q-D and gentamicin for strains NA8 and BCB8 were, respectively, 1.0 and 1.2. Therefore, the combination of the tested antibiotics was interpreted as indifferent for both strains.
Time-kill curves.
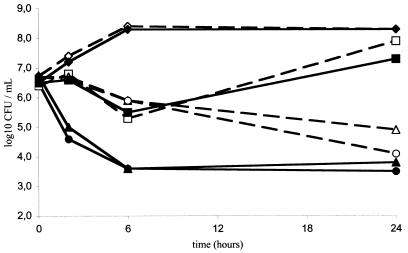
Time-kill curves for strains NA8 and BCB8 are shown in Fig. 1. At a concentration of 0.5 mg/liter, Q-D proved more bactericidal against quinupristin-susceptible S. aureus NA8 than against BCB8: the initial inoculum was reduced by 3.0 log CFU/ml for NA8 and by 1.8 log CFU/ml for BCB8 after a 24-h incubation. No antagonism or synergism between Q-D and gentamicin was observed.
FIG. 1.
Time-kill curves for S. aureus NA8 and BCB8. Results are shown for the control group (diamonds) and groups of rabbits given gentamicin at 0.25 mg/liter (squares), Q-D at 0.5 mg/liter (triangles), or gentamicin at 0.25 mg/liter in combination with Q-D at 0.5 mg/liter (circles). Solid lines, strain NA8; dashed lines, strain BCB8.
Serum antibiotic levels.
Peak and trough mean serum gentamicin concentrations were, respectively, 15.8 ± 1.2 and 1.2 ± 0.1 mg/liter. The pharmacokinetics of quinupristin, dalfopristin, and Q-D are shown in Table 3.
TABLE 3.
Pharmacokinetic serum parameters of quinupristin, dalfopristin, and Q-D in three healthy rabbits after a single intramuscular injection of Q-D (30 mg/kg)a
| Drug | Cmax (mg · liter−1) | AUC (h · mg · liter−1) | β half-life (h) |
|---|---|---|---|
| Quinupristin | 1.5 ± 0.4 | 4.0 ± 2.3 | 1.5 ± 1.5 |
| Dalfopristin | 6.9 ± 0.5 | 6.8 ± 3.3 | 0.7 ± 0.3 |
| Q-D | 5.4 ± 1.8 | 6.0 ± 2.6 | 0.6 ± 0.3 |
Mean values ± SD of peak concentrations (Cmax), areas under the curve (AUC), and β half-lives are shown. Peak concentrations were observed 15 min after injection for dalfopristin and 30 min after injection for quinupristin and Q-D.
Experimental endocarditis.
Activities of drugs in 4-day regimens are reported in Table 4. For both strains, gentamicin, Q-D, and the combination of both drugs provided significant bactericidal effects. There was no significant difference in either strain among the mean bacterial titers in vegetations induced by these three regimens. Although Q-D seemed somewhat less effective against quinupristin-resistant BCB8 than against quinupristin-susceptible NA8, the Q-D regimen reduced the mean bacterial titer by 3.5 log CFU/g of vegetation for the quinupristin-resistant strain.
TABLE 4.
Activity of drugs in 4-day regimensb
| Regimen | Log10 CFU/g of vegetation (no. of animals) with S. aureus strain:
|
|
|---|---|---|
| NA8 | BCB8 | |
| Control (untreated) | 8.5 ± 0.8 (9) | 8.7 ± 0.9 (13) |
| Gentamicin | 4.1 ± 2.6 (8)a | 5.0 ± 2.2 (8)a |
| Q-D | 3.0 ± 0.9 (6)a | 5.2 ± 2.2 (7)a |
| Gentamicin + Q-D | 2.6 ± 0.5 (6)a | 5.1 ± 2.4 (12)a |
P < 0.0001 versus controls by Scheffe's test after ANOVA.
Values are mean bacterial titers in vegetations ± SD.
No increase in bactericidal effect for the combination of gentamicin and Q-D versus either antibiotic alone was apparent. However, to determine whether bactericidal activity was accelerated by the combination, the treatment groups were compared at the end of the first day of therapy (Table 5). The results showed that gentamicin, Q-D, and the combination of both drugs reduced mean bacterial titers significantly in vegetations, except for gentamicin against S. aureus BCB8.
TABLE 5.
Activity of drugs in 1-day regimensc
| Regimen | Log10 CFU/g of vegetation (no. of animals) with S. aureus strain:
|
|
|---|---|---|
| NA8 | BCB8 | |
| Control (untreated) | 8.5 ± 0.8 (9) | 8.7 ± 0.9 (13) |
| Gentamicin | 4.2 ± 1.3 (6)a | 7.2 ± 2.0 (5) |
| Q-D | 4.0 ± 1.1 (8)a | 5.3 ± 1.7 (7)b |
| Gentamicin + Q-D | 3.8 ± 0.4 (4)a | 5.5 ± 2.8 (8)b |
P < 0.001 versus controls.
P < 0.01 versus controls.
Values are mean bacterial titers in vegetations ± SD. Statistical significance was determined by Scheffe's test after ANOVA.
Incubation of vegetations in agar plates containing gentamicin or Q-D (depending on the regimen for the animal before sacrifice) yielded no S. aureus colony.
DISCUSSION
It has been reported that Q-D MICs for MLSB-CR and MLSB-S S. aureus strains, in the absence of resistance to dalfopristin, were indistinguishable, despite a lack of in vitro bactericidal activity against certain MLSB-CR strains (15, 19). Moreover, in experimental (rabbit and rat) endocarditis studies, Q-D exerted constant bactericidal activity against MLSB-S S. aureus strains (13, 15, 26). In vivo efficacy of Q-D against MLSB-CR strains is more difficult to interpret. The lack of bactericidal activity of Q-D in rat endocarditis studies may be due to lower doses than those recommended for treatment of severe infections in humans; activity was restored by increasing the total daily dose of dalfopristin in order to maintain a serum dalfopristin concentration of 2 mg/liter for 12 h a day (13). With the same rabbit endocarditis model that we subsequently used, Fantin et al. observed variable results for the same MLSB-CR strain, with a maximal reduction in vegetation bacterial titers of 2.9 log CFU/g (15, 28; J. Pavie, V. Zarrouk, A. Lefort, L. Garry, R. Leclercq, C. Carbon, and B. Fantin, Abstr. 40th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 1006, 2000). In our model, Q-D proved bactericidal for the MLSB-CR S. aureus strain by the end of the first day of treatment: there was a mean reduction of the bacterial titer in vegetations of 3.4 log CFU/g. These data seem therefore to call into question the presumed global lack of efficacy of Q-D against MLSB-CR S. aureus in severe infections. Many differences between study strains could explain the diversity of in vivo responses to Q-D. Interstrain differences in susceptibility of S. aureus to platelet factors, such as thrombin-induced platelet microbicidal protein 1, could explain variability in the in vivo efficacy of Q-D, as previously reported for oxacillin and vancomycin (10, 11).
According to international guidelines, gentamicin was administered in our study by simulating a human i.v. regimen of a once-daily dose of 3 mg/kg (23). A previous study showed that this regimen was bactericidal against S. aureus NA8 after 48 h (3). Our data indicate that the in vivo activity of gentamicin administered as a once-daily dose was bactericidal against NA8 at the end of the first day of treatment, but required 4 days of treatment to be effective against BCB8. Sande and Courtney showed that gentamicin, in a t.i.d. regimen yielding a mean peak concentration of 5.0 mg/liter, had no bactericidal activity in a rabbit experimental staphylococcal endocarditis model after 3 and 5 days of treatment (24). Nevertheless we cannot exclude the possibility of synergy between Q-D and a multiple-dose regimen of gentamicin.
The combination of Q-D and gentamicin proved no more beneficial than Q-D or gentamicin alone against S. aureus in this study in terms of either in vitro and in vivo bactericidal activity or in vivo selection of a resistant variant. This lack of benefit may have been due to the high potency of the monotherapies, each of which reduced mean bacterial titers in vegetations by ≥3.5 log CFU/g after a 4-day regimen and selected no resistant variant. However, we have possibly underestimated the selection of such variant clones by using high antibiotic concentrations in solid media (i.e., 16 times the MIC). Furthermore we did not search variant clones showing resistance or increased resistance specifically to quinupristin or to dalfopristin. Such resistant variants were not selected in previous experimental endocarditis studies using S. aureus strains susceptible to dalfopristin but have been isolated when one dalfopristin-resistant strain was used (15, 27). Moreover, the combination of another antibiotic with Q-D might help prevent the emergence of constitutively resistant mutants during treatment of infections caused by S. aureus strains with inducible resistance to MLSB (MLSB-IR). This situation has been a matter of concern for Q-D and for other non-erm-inducing MLS antibiotics, by analogy with clindamycin, which is thought to select MLSB-CR mutants from MLSB-IR strains (18). Because of the low in vitro frequency of mutation from inducible to constitutive MLSB resistance (≈10−7 to 10−8), this hypothesis needs to be tested by using experimental models yielding larger inocula than the rabbit endocarditis model (18).
Serum streptogramin concentrations were determined with a new bioassay based on the measurement of the bactericidal activity of the serum, but not its bacteriostatic activity, as in microbiological methods developed previously (13). This new assay uses one unique test strain without a streptogramin resistance mechanism, which prevents the instability due to formerly used strains (13). To measure the concentration of one compound, the other one was added at a high concentration, allowing for control of the synergy between quinupristin and dalfopristin. Conversely, for the global assay of Q-D, concentrations of separate compounds in test sera were not controlled, whereas control sera had a fixed 30:70 (wt/wt) ratio of quinupristin to dalfopristin (Q/D ratio). Although the bacteriostatic activities of Q-D against S. aureus, as assessed by determining MICs, are similar for Q/D ratios ranging from 90:10 to 10:90, no data about the influence of the Q/D ratio on Q-D in vitro bactericidal activity have been published (5). In our work, the sum of activities for separate compounds exceeded the activity from the global assay when the Q/D ratio was less than 0.6 (data not shown). The global Q-D assay should therefore be considered a functional one. Even if its result is expressed as a mass concentration, it yields nearly the amount of Q-D in serum with a 30:70 (wt/wt) Q/D ratio which has the same bactericidal activity as the test serum.
In conclusion, our experiments showed no additive benefit from the combination of Q-D and gentamicin against dalfopristin- and gentamicin-susceptible S. aureus, whether susceptible or resistant to quinupristin. Contrary to previous studies, Q-D showed in vivo bactericidal activity against the quinupristin-resistant strain. Further investigations are needed to determine the role of Q-D, with regard to vancomycin, in the management of MRSA infections.
Acknowledgments
We are grateful to Anne-Françoise Miègeville and Virginie Le Mabecque for technical assistance.
Eric Batard was the recipient of a grant from the Direction Régionale des Affaires Sanitaires et Sociales des Pays de la Loire (France). This work was supported by Aventis Laboratories, France.
REFERENCES
- 1.Acar, J. F., and F. W. Goldstein. 1996. Disk susceptibility test, p. 1-51. In V. Lorian (ed.), Antibiotics in laboratory medicine, 4th ed. Williams & Wilkins, Baltimore, Md.
- 2.Angot, P., M. Vergnaud, M. Auzou, R. Leclercq, and Observatoire de Normandie du Pneumocoque. 2000. Macrolide resistance phenotypes and genotypes in French clinical isolates of Streptococcus pneumoniae. Eur. J. Clin. Microbiol. Infect. Dis. 19:755-758. [DOI] [PubMed] [Google Scholar]
- 3.Asseray, N., J. Caillon, N. Roux, C. Jacqueline, R. Bismuth, M. F. Kergueris, G. Potel, and D. Bugnon. 2002. Different aminoglycoside-resistant phenotypes in a rabbit Staphylococcus aureus endocarditis infection model. Antimicrob. Agents Chemother. 46:1591-1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Backo, M., E. Gaenger, A. Burkart, Y. L. Chai, and A. S. Bayer. 1999. Treatment of experimental staphylococcal endocarditis due to a strain with reduced susceptibility in vitro to vancomycin: efficacy of ampicillin-sulbactam. Antimicrob. Agents Chemother. 43:2565-2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bouanchaud, D. H. 1992. In-vitro and in-vivo synergic activity and fractional inhibitory concentration (FIC) of the components of a semisynthetic streptogramin, RP 59500. J. Antimicrob. Chemother. 30(Suppl. A):95-99. [DOI] [PubMed] [Google Scholar]
- 6.Bugnon, D., G. Potel, Y. A. Xiong, J. Caillon, M. F. Kergueris, P. Le Comte, D. Baron, and H. Drugeon. 1996. In vivo antibacterial effects of simulated human serum profiles of once-daily versus thrice-daily dosing of amikacin in a Serratia marcescens endocarditis experimental model. Antimicrob. Agents Chemother. 40:1164-1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bugnon, D., G. Potel, J. Caillon, D. Baron, H. B. Drugeon, P. Feigel, and M. F. Kergueris. 1998. In vivo simulation of human pharmacokinetics in the rabbit. Bull. Math. Biol. 60:545-567. [DOI] [PubMed] [Google Scholar]
- 8.Climo, M. W., T. L. Patron, and G. L. Archer. 1999. Combinations of vancomycin and β-lactams are synergistic against staphylococci with reduced susceptibilities to vancomycin. Antimicrob. Agents Chemother. 43:1747-1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cocito, C., M. Di Giambattista, E. Nyssen, and P. Vannuffel. 1997. Inhibition of protein synthesis by streptogramins and related antibiotics. J. Antimicrob. Chemother. 39(Suppl. A):7-13. [DOI] [PubMed] [Google Scholar]
- 10.Dhawan, V. K., M. R. Yeaman, and A. S. Bayer. 1999. Influence of in vitro susceptibility phenotype against thrombin-induced platelet microbicidal protein on treatment and prophylaxis outcomes of experimental Staphylococcus aureus endocarditis. J. Infect. Dis. 180:1561-1568. [DOI] [PubMed] [Google Scholar]
- 11.Dhawan, V. K., A. S. Bayer, and M. R. Yeaman. 2000. Thrombin-induced platelet microbicidal protein susceptibility phenotype influences the outcome of oxacillin prophylaxis and therapy of experimental Staphylococcus aureus endocarditis. Antimicrob. Agents Chemother. 44:3206-3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Durack, D., and P. Beeson. 1972. Experimental endocarditis. I. Colonization of a sterile vegetation. Br. J. Exp. Pathol. 53:44-49. [PMC free article] [PubMed] [Google Scholar]
- 13.Entenza, J. M., H. Drugeon, M. P. Glauser, and P. Moreillon. 1995. Treatment of experimental endocarditis due to erythromycin-susceptible or -resistant methicillin-resistant Staphylococcus aureus with RP 59500. Antimicrob. Agents Chemother. 39:1419-1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fantin, B., R. Leclercq, M. Ottoviani, J. M. Vallois, B. Mazière, J. Duval, J. J. Pocidalo, and C. Carbon. 1994. In vivo activities and penetration of the two components of the streptogramin RP 59500 in cardiac vegetations of experimental endocarditis. Antimicrob. Agents Chemother. 38:432-437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fantin, B., R. Leclercq, Y. Merlé, L. Saint-Julien, C. Veyrat, J. Duval, and C. Carbon. 1995. Critical influence of resistance to streptogramin B-type antibiotics on activity of RP59500 (quinupristin-dalfopristin) in experimental endocarditis due to Staphylococcus aureus. Antimicrob. Agents Chemother. 39:400-405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fass, R. J. 1991. In vitro activity of RP 59500, a semisynthetic injectable pristinamycin, against staphylococci, streptococci, and enterococci. Antimicrob. Agents Chemother. 35:553-559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kang, S. L., and M. J. Rybak. 1997. In vitro bactericidal activity of quinupristin/dalfopristin alone and in combination against resistant strains of Enterococcus species and Staphylococcus aureus. J. Antimicrob. Chemother. 39(Suppl. A):33-39. [DOI] [PubMed] [Google Scholar]
- 18.Leclercq, R., and P. Courvalin. 1991. Bacterial resistance to macrolide, lincosamide, and streptogramin antibiotics by target modification. Antimicrob. Agents Chemother. 35:1267-1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leclercq, R., L. Nantas, C. J. Soussy, and J. Duval. 1992. Activity of RP 59500, a new parenteral semisynthetic streptogramin, against staphylococci with various mechanisms of resistance to macrolide-lincosamide-streptogramin antibiotics. J. Antimicrob. Chemother. 30(Suppl. A):67-75. [DOI] [PubMed] [Google Scholar]
- 20.Lina, G., A. Quaglia, M. E. Reverdy, R. Leclercq, F. Vandensech, and J. Etienne. 1999. Distribution of genes encoding resistance to macrolides, lincosamides, and streptogramins among staphylococci. Antimicrob. Agents Chemother. 43:1062-1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mingeot-Leclercq, P., Y. Glupczynski, and P. M. Tulkens. 1999. Aminoglycosides: activity and resistance. Antimicrob. Agents Chemother. 43:727-737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roberts, M. C., J. Sutcliffe, P. Courvalin, L. B. Jensen, J. Rood, and H. Seppala. 1999. Nomenclature for macrolide and macrolide-lincosamide-streptogramin B resistance determinants. Antimicrob. Agents Chemother. 43:2823-2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rubinstein, E., C. Carbon, and the Endocarditis Working Group of the International Society of Chemotherapy. 1998. Staphylococcal endocarditis—recommendations for therapy. Clin. Microbiol. Infect. 4(Suppl. 3):3S27-3S33. [PubMed] [Google Scholar]
- 24.Sande, M. A., and K. B. Courtney. 1976. Nafcillin-gentamicin synergism in experimental staphylococcal endocarditis. J. Lab. Clin. Med. 88:118-124. [PubMed] [Google Scholar]
- 25.Sutcliffe, J., T. Grebe, A. Tait-Kamradt, and L. Wondrack. 1996. Detection of erythromycin-resistant determinants by PCR. Antimicrob. Agents Chemother. 40:2562-2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vouillamoz, J., J. M. Entenza, C. Féger, M. P. Glauser, and P. Moreillon. 2000. Quinupristin-dalfopristin combined with β-lactams for treatment of experimental endocarditis due to Staphylococcus aureus constitutively resistant to macrolide-lincosamide-streptogramin B antibiotics. Antimicrob. Agents Chemother. 44:1789-1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zarrouk, V., B. Bozdogan, R. Leclercq, L. Garry, C. Carbon, and B. Fantin. 2000. Influence of resistance to streptogramin A type antibiotics on the activity of quinupristin-dalfopristin in vitro and in experimental endocarditis due to Staphylococcus aureus. Antimicrob. Agents Chemother. 44:1168-1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zarrouk, V., B. Bozdogan, R. Leclercq, L. Garry, C. Féger, C. Carbon, and B. Fantin. 2001. Activities of the combination of quinupristin-dalfopristin with rifampin in vitro and in experimental endocarditis due to Staphylococcus aureus strains with various phenotypes of resistance to macrolide-lincosamide-streptogramin antibiotics. Antimicrob. Agents Chemother. 45:1244-1248. [DOI] [PMC free article] [PubMed] [Google Scholar]