Abstract
The sequences of the ftsI gene, encoding the transpeptidase domain of penicillin binding protein (PBP) 3A and/or PBP 3B, which are involved in septal peptidoglycan synthesis, were determined for 108 clinical strains of Haemophilus influenzae with reduced susceptibility to β-lactam antibiotics with or without β-lactamase production and were compared to those of the ampicillin-susceptible Rd strain and ampicillin-susceptible clinical isolates. The sequences have 18 different mutation patterns and were classified into two groups on the basis of amino acid substitutions deduced from the nucleotide sequences located between bp 960 and 1618 of the ftsI gene. In group I strains (n = 7), His-517 was substituted for Arg-517. In group II strains (n = 101), Lys-526 was substituted for Asn-526. In subgroup IIa (n = 5; H. influenzae ATCC 49247), the only observed substitution was Lys-526 for Asn-526; in subgroup IIb (n = 56), Val-502 was substituted for Ala-502 (n = 13), along with several other substitutions: Asn-350 for Asp-350 (n = 15), Asn-350 for Asp-350 and Glu-490 for Gly-490 (n = 14), and Asn-350 for Asp-350 and Ser-437 for Ala-437 (n = 5). In subgroup IIc (n = 25), Thr-502 was substituted for Ala-502. In subgroup IId, Val-449 was substituted for Ile-449 (n = 15). The MICs of β-lactam antibiotics for the 108 strains were to 8 to 16 times the MICs for susceptible strains. The strains, isolated from both adults and children, were analyzed for genetic relationship by pulsed-field gel electrophoresis and by determination of ftsI sequence phylogeny. Both analyses revealed the lack of clonality and the heterogeneity of the strains, but some clusters suggest the spread and/or persistence of a limited number of strains of the same pulsotype and pattern of amino acid substitutions. Reduced susceptibility to β-lactam, brought about by mutations of the ftsI gene, is becoming a frequent phenomenon, affecting both strains that produce β-lactamase and those that do not. The level of resistance remains low but opens the way to greater resistance in the future.
In spite of the widespread use of the anti-Haemophilus b vaccine in the industrialized countries and the decreased incidence of invasive diseases (18), Haemophilus influenzae remains a key species in bronchopulmonary and ear, nose, and throat (ENT) infections in both adults and children. These diseases are most often caused by noncapsulated strains (10, 14). Treatment of such infections can be severely affected by antibiotic resistance. In H. influenzae resistance to antibiotics, especially to β-lactams, has, for a number of years, become a serious problem (6, 15, 30).
Two main mechanisms are at the origin of resistance to aminopenicillins: enzymatic hydrolysis of the antibiotic and a change in penicillin-binding proteins (PBPs). By far the more frequent is the production of β-lactamase, usually of the TEM-1 type but sometimes of the ROB-1 type (15, 32). In certain countries, the incidence of strains producing β-lactamase is particularly high, reaching 50% in type b strains, which are responsible for invasive manifestations (before anti-Haemophilus b vaccination), and 20 to 30% in noncapsulated strains that lead to bronchopulmonary and ENT infections (5, 12, 32).
Resistance by mechanisms other than β-lactamase production is based on decreased affinity of the PBPs involved in septal peptidoglycan synthesis (4). The first observations of ampicillin-resistant non-β-lactamase producing (BLNAR) strains were reported in the early 1980s and concerned type b capsulated (20, 27) and noncapsulated strains (2, 25). In contrast to the incidence of β-lactamase-producing strains, the incidence of BLNAR strains remains low in various countries (4, 5, 8, 32), but in Japan the proportion of BLNAR clinical isolates is more than 25% and seems to be increasing (33, 36).
In H. influenzae, ampicillin resistance unrelated to β-lactamase production was shown to be chromosomally mediated and was correlated with alterations in PBPs 3A and 3B (4, 22, 29, 34). Recently, Ubukata et al. (36) demonstrated that mutations in the ftsI gene, which is involved in septal peptidoglycan synthesis, are the most important for the development of resistance to β-lactams in BLNAR strains. In clinical isolates for which the ampicillin MIC was ≥1 μg/ml and in transformants into which the ftsI gene from BLNAR strains was introduced, PBP 3A and PBP 3B showed decreased affinity for β-lactams. In BLNAR strains, the amino acid sequences, deduced from the sequences of the ftsI gene encoding the transpeptidase domain of PBP 3A and/or PBP 3B, show several common amino acid substitutions. On the basis of amino acid substitutions deduced from the ftsI gene, the BLNAR strains were classified into three groups (36).
We studied a series of strains with BLNAR characteristics (ampicillin MIC ≥ 1 μg/ml and reduced susceptibility to cephalosporins) and β-lactamase-producing strains with reduced susceptibility to cephalosporins. The sequence of the part of the ftsI gene coding for the transpeptidase domain was determined and compared to those of fts1 genes of susceptible strains and of the ampicillin-susceptible Rd strain. We report here the diversity of amino acid substitutions observed among French clinical isolates and describe new amino acid substitutions arising from alterations of the ftsI gene.
MATERIALS AND METHODS
Strains.
The strains used in this study are listed in Table 1. They were selected from clinical isolates received at the French National Haemophilus influenzae Reference Center. The strains were identified with the usual techniques and required β-NAD+ (V factor) and hemin (X factor) for growth. The production of β-lactamase was assessed by a chromogenic cephalosporin test (nitrocefin and cefinase; bioMérieux, Marcy l'Etoile, France). The capsular type was determined by slide agglutination with specific a to f antisera (Difco, BD, Le Pont de Claix, France). The biotype was determined as described by Kilian (17). The strains were stored at −80°C in 15% glycerol brain heart infusion broth. Strains H. influenzae ATCC 10211 and ATCC 49247 were used as controls. Strain ME870 was kindly provided by K. Ubukata (36). Nine strains that were susceptible to β-lactam antibiotics or that were β-lactamase producers were used as controls.
TABLE 1.
Characteristics of the H. influenzae strains studieda
| Group | Strain no.b | Specimen, specimen source, or illness | Biotype | MIC (μg/ml) of:
|
PCR result for:
|
Areac | Age | Amino acidsd | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AMX | AMC | CEC | CXM | CTX | CFM | CPD | CRO | TEM | J1-J2 | |||||||||||||||||||||||
| Control | ATCC 10211 | NK | NK | ND | ND | ND | ND | ND | ND | ND | ND | − | + | NK | NK | DSAMSLAIGARN | ||||||||||||||||
| 99 126 | Otitis | I | 0.5 | 0.5 | 2 | 1 | 0.015 | 0.03 | 0.06 | 0.003 | − | + | 68 | 9 mo | DSAMSLAIGARN | |||||||||||||||||
| 99 051 | Otitis | I | 0.25 | 0.25 | 2 | 0.5 | 0.015 | 0.03 | 0.06 | 0.003 | − | + | 44 | NK | DSAMSLAIGARN | |||||||||||||||||
| 99 169 | Br sec | I | 0.5 | 0.25 | 2 | 0.5 | 0.015 | 0.03 | 0.06 | 0.003 | − | + | 13 | 41 yr | DSAMSLAIGARN | |||||||||||||||||
| 00 051 | Otitis | II | 0.5 | 0.25 | 4 | 1 | 0.015 | 0.06 | 0.06 | 0.003 | − | + | 13 | 6 mo | DSAMSLAIGARN | |||||||||||||||||
| I 89 187 | Conj | II | 0.5 | 0.5 | 8 | 1 | 0.015 | 0.06 | 0.06 | 0.015 | − | + | 35 | 3 days | DSAMSLAIGARN | |||||||||||||||||
| 99 072 | Otitis | II | 8 | 0.25 | 2 | 0.5 | 0.007 | 0.03 | 0.03 | 0.003 | + | + | 13 | 16 mo | DSAMSLAIGARN | |||||||||||||||||
| 99 114 | Br sec | II | 16 | 0.25 | 2 | 1 | 0.015 | 0.03 | 0.03 | 0.007 | + | + | 44 | 67 yr | DSAMSLAIGARN | |||||||||||||||||
| 99 157 | Br sec | IV | 1 | 0.12 | 2 | 0.5 | 0.015 | 0.03 | 0.03 | 0.003 | + | + | 12 | 85 yr | DSAMSLAIGARN | |||||||||||||||||
| 99 907 | Otitis | II | 4 | 0.25 | 4 | 1 | 0.015 | 0.06 | 0.06 | 0.003 | + | + | 91 | 11 mo | DSAMSLAIGARN | |||||||||||||||||
| I | 99 702 | Rhino | I | 1 | 0.5 | 8 | 1 | 0.03 | 0.25 | 0.12 | 0.007 | − | + | 59 | <5 yr | DSAMSLAIGAHN | ||||||||||||||||
| 99 211 | Br sec | III | 1 | 1 | 4 | 1 | 0.03 | 0.25 | 0.12 | 0.007 | − | + | 44 | 65 yr | DSAMSLAIGAHN | |||||||||||||||||
| 00 068 | Br sec | II | 2 | 2 | 16 | 1 | 0.12 | 0.25 | 0.25 | ND | − | + | 93 | 61 yr | DSAMSLAIGAHN | |||||||||||||||||
| 00 100 | Br sec | III | 1 | 0.5 | 16 | 1 | 0.03 | 0.25 | 0.12 | ND | − | + | 44 | 63 yr | DSAMSLAIGAHN | |||||||||||||||||
| 92 008 | Br sec, CF | I | 1 | 0.5 | 8 | 2 | 0.03 | 0.5 | 0.12 | 0.007 | − | + | 12 | 7 mo | DSAMSLAIGATHN | |||||||||||||||||
| III 88 095 | Br sec | III | 2 | 2 | 16 | 1 | 0.03 | 0.5 | 0.25 | 0.007 | − | + | 54 | 50 yr | DNAMSLAIGAHN | |||||||||||||||||
| 00 700 | Br sec | I | 2 | 2 | ND | ND | 0.03 | ND | ND | ND | − | + | 44 | 60 yr | NSAMSLAIGTHN | |||||||||||||||||
| IIa | II 88 176 | Br sec | I | 4 | 2 | 16 | 2 | 0.12 | 0.25 | 0.25 | 0.03 | − | − | 31 | 55 yr | DSAMSLAIGARK | ||||||||||||||||
| 93 349 | Conj | III | 1 | 0.5 | 8 | 1 | 0.03 | 0.06 | 0.12 | 0.007 | − | − | 31 | 21 mo | DSAMSLAIGARK | |||||||||||||||||
| ATCC 49247 | NK | III | 2 | 2 | 8 | 2 | 0.12 | 0.12 | 0.25 | 0.06 | − | − | NK | NK | DSAMSLAIGARK | |||||||||||||||||
| 92 275 | Sinus | VI | 2 | 2 | 8 | 2 | 0.06 | 0.12 | 0.25 | 0.015 | − | − | 44 | 7 yr | DSAMSLAIGARK | |||||||||||||||||
| 93 921 | Br sec | I | 1 | 1 | 8 | 1 | 0.03 | 0.06 | 0.06 | 0.007 | − | − | 83 | 8 yr | DSAMSLAIGARK | |||||||||||||||||
| 99 024 | Otitis | II | 32 | 1 | 4 | 1 | 0.03 | 0.06 | 0.12 | 0.007 | + | − | 31 | 7 yr | DSAMSLAIGARK | |||||||||||||||||
| IIb | 98 244 | Sinus | III | 1 | 1 | 8 | 2 | 0.03 | 0.06 | 0.03 | 0.007 | − | − | 13 | 56 yr | DSAMSLAIGVRK | ||||||||||||||||
| III 87 064 | Br sec, CF | I | 8 | 4 | 32 | 4 | 0.12 | 0.12 | 0.5 | 0.03 | − | − | 33 | 17 yr | DSAMSLAIGVRK | |||||||||||||||||
| IV 89 219 | Br sec | I | 1 | 1 | 4 | 2 | 0.03 | 0.03 | 0.12 | 0.003 | − | − | 44 | 47 yr | DSAMSLAIGVRK | |||||||||||||||||
| 99 444 | Otitis | II | 1 | 1 | 8 | 1 | 0.03 | 0.03 | 0.12 | 0.007 | − | − | 91 | 6 yr | DSAMSLAIGVRK | |||||||||||||||||
| II 88 194 | Br sec, CF | I | 8 | 8 | 32 | 8 | 0.12 | 0.25 | 0.5 | 0.03 | − | − | 33 | 18 yr | DSAMSLAIGVRK | |||||||||||||||||
| 91 477 | Br sec | III | 1 | 1 | 8 | 2 | 0.03 | 0.06 | 0.12 | 0.015 | − | − | 44 | 41 yr | DSAMSLAIGVRK | |||||||||||||||||
| 99 343 | Rhino | V | 1 | 0.5 | 8 | 2 | 0.03 | 0.06 | 0.12 | 0.007 | − | − | 6 | <5 yr | DSAMSLAIGVRK | |||||||||||||||||
| 00 043 | Conj | II | 32 | 1 | 8 | 2 | 0.03 | 0.06 | 0.12 | 0.007 | + | − | 13 | 18 mo | DSAMSLAIGVRK | |||||||||||||||||
| 99 356 | Rhino | V | 1 | 0.5 | 8 | 2 | 0.03 | 0.06 | 0.12 | 0.007 | − | − | 6 | <5 yr | DSAMSLAIGVRK | |||||||||||||||||
| 96 192 | Br sec | III | 1 | 1 | 16 | 2 | 0.03 | 0.12 | 0.12 | 0.007 | − | − | 12 | 87 yr | DSAMSLAIGVRK | |||||||||||||||||
| I 88 073 | Br sec, CF | IV | 4 | 4 | 32 | 2 | 0.03 | 0.12 | 0.5 | 0.015 | − | − | 33 | 18 yr | DSAMSLAIGVRK | |||||||||||||||||
| II 88 198 | Br sec, CF | I | 8 | 8 | 32 | 4 | 0.12 | 0.25 | 0.5 | 0.03 | − | − | 33 | 18 yr | DSAMSLAIGVRK | |||||||||||||||||
| II 88 196 | Br sec, CF | I | 8 | 8 | 32 | 8 | 0.12 | 0.25 | 0.5 | 0.03 | − | − | 33 | 5 yr | DSAMSLAIGVRK | |||||||||||||||||
| 98 086 | Br sec, CF | II | 1 | 0.5 | 16 | 1 | 0.03 | 0.06 | 0.12 | 0.007 | − | − | 44 | 5 yr | DSAMSLAIEVRK | |||||||||||||||||
| 00 088 | Rhino | II | 1 | 1 | 4 | 1 | 0.03 | 0.06 | 0.12 | 0.007 | − | − | 6 | <5 yr | DSAMSLAIEVRK | |||||||||||||||||
| 97 765 | Otitis | II | 1 | 1 | 16 | 2 | 0.03 | 0.12 | 0.25 | 0.015 | − | − | 44 | 4 yr | DSAMSLAIEVRK | |||||||||||||||||
| 00 285 | Br sec | II | 1 | 1 | 16 | 1 | 0.03 | 0.06 | 0.25 | ND | − | − | 44 | 59 yr | DSAMSLAIEVRK | |||||||||||||||||
| 00 374 | Conj | II | 2 | 2 | 16 | 4 | 0.06 | 0.06 | 0.25 | ND | − | − | 68 | 3 mo | DSAMSLAIEVRK | |||||||||||||||||
| I 90 190 | Br sec | II | 1 | 0.5 | 8 | 1 | 0.12 | 0.12 | 0.25 | 0.03 | − | − | 44 | 54 yr | DNAMSLAIGVRK | |||||||||||||||||
| 98 386 | Conj | II | 1 | 0.5 | 8 | 2 | 0.12 | 0.25 | 0.5 | 0.03 | − | − | 91 | 1 mo | NNAMSLAIGVRK | |||||||||||||||||
| 00 084 | Br sec | II | 2 | 1 | 16 | 2 | 0.06 | 0.12 | 0.25 | 0.015 | − | − | 44 | 9 mo | NSALSLAIGVRK | |||||||||||||||||
| 99 761 | Rhino | III | 2 | 1 | 8 | 4 | 0.03 | 0.06 | 0.25 | 0.015 | − | − | 59 | <5 yr | NSALSLAIGVRK | |||||||||||||||||
| 99 008 | Rhino | II | 2 | 2 | 8 | 2 | 0.06 | 0.12 | 0.25 | 0.015 | − | − | 22 | 56 yr | NSALSLAIGVRK | |||||||||||||||||
| 99 431 | Rhino | II | 2 | 2 | 8 | 2 | 0.06 | 0.12 | 0.25 | 0.015 | − | − | 6 | <5 yr | NSALSLAIGVRK | |||||||||||||||||
| 00 722 | Blood c | II | 1 | 1 | 16 | 2 | 0.06 | 0.12 | 0.25 | ND | − | − | 13 | 82 yr | NSALSLAIGVRK | |||||||||||||||||
| 00 092 | Br sec | II | 2 | 2 | 16 | 2 | 0.06 | 0.06 | 0.25 | ND | − | − | 44 | 59 yr | NSALSLAIGVRK | |||||||||||||||||
| 00 547 | Blood c | II | 1 | 1 | 16 | 2 | 0.06 | 0.12 | 0.25 | ND | − | − | 31 | 40 yr | NSALSLAIGVRK | |||||||||||||||||
| 99 791 | Rhino | II | 1 | 0.5 | 8 | 2 | 0.03 | 0.12 | 0.25 | 0.015 | − | − | 59 | <5 yr | NSALSLAIGVRK | |||||||||||||||||
| 00 498 | Br sec | II | 2 | 2 | 16 | 2 | 0.06 | 0.06 | 0.25 | ND | − | − | 25 | <6 yr | NSALSLAIGVRK | |||||||||||||||||
| 99 1046 | Conj | II | 1 | 0.5 | 8 | 2 | 0.03 | 0.06 | 0.12 | 0.015 | − | − | 13 | 21 mo | NSALSLAIGVRK | |||||||||||||||||
| 99 790 | Rhino | II | 1 | 0.5 | 16 | 2 | 0.06 | 0.12 | 0.25 | 0.015 | − | − | 59 | <5 yr | NSALSLAIGVRK | |||||||||||||||||
| 99 100 | Rhino | II | 2 | 2 | 8 | 2 | 0.06 | 0.12 | 0.25 | 0.03 | − | − | 22 | 56 yr | NSALSLAIGVRK | |||||||||||||||||
| 99 780 | Rhino | III | 1 | 1 | 16 | 4 | 0.03 | 0.12 | 0.25 | 0.015 | − | − | 59 | <5 yr | NSALSLAIGVRK | |||||||||||||||||
| 00 215 | Conj | II | 1 | 1 | 16 | 2 | 0.06 | 0.06 | 0.25 | ND | − | − | 13 | 17 mo | NSALSLAIGVRK | |||||||||||||||||
| 00 269 | Br sec | II | 1 | 1 | 16 | 2 | 0.06 | 0.06 | 0.25 | ND | − | − | 44 | 43 yr | NSALSLAIGVRK | |||||||||||||||||
| 98 113 | Otitis | II | 1 | 1 | 4 | 1 | 0.03 | 0.06 | 0.12 | 0.007 | − | − | 13 | 1 mo | NSALSLAIEVRK | |||||||||||||||||
| 94 396 | Rhino | III | 2 | 2 | 16 | 4 | 0.06 | 0.12 | 0.25 | 0.015 | − | − | 57 | 9 mo | NSALSLAIEVRK | |||||||||||||||||
| 00 328 | Br sec | III | 1 | 1 | 16 | 2 | 0.06 | 0.06 | 0.25 | ND | − | − | 80 | 58 yr | NSALSLAIEVRK | |||||||||||||||||
| 97 640 | Conj | II | 1 | 1 | 16 | 2 | 0.03 | 0.12 | 0.25 | 0.015 | − | − | 68 | 2 mo | NSALSLAIEVRK | |||||||||||||||||
| 97 936 | Br sec | III | 2 | 2 | 16 | 4 | 0.03 | 0.5 | 0.25 | 0.015 | − | − | 44 | 49 yr | NSALSLAIEVRK | |||||||||||||||||
| 97 924 | Br sec | III | 2 | 1 | 16 | 4 | 0.06 | 0.12 | 0.25 | 0.015 | − | − | 13 | 66 yr | NSALSLAIEVRK | |||||||||||||||||
| 00 321 | Br sec | III | 1 | 1 | 16 | 2 | 0.06 | 0.06 | 0.25 | ND | − | − | 80 | 58 yr | NSALSLAIEVRK | |||||||||||||||||
| 00 268 | Br sec | I | 1 | 1 | 16 | 2 | 0.06 | 0.06 | 0.25 | ND | − | − | 44 | 42 yr | NSALSLAIEVRK | |||||||||||||||||
| 00 752 | Br sec | III | 1 | 1 | 16 | 2 | 0.06 | 0.06 | 0.12 | ND | − | − | 17 | 64 yr | NSALSLAIEVRK | |||||||||||||||||
| 00 763 | CSF | III | 2 | ND | 16 | 4 | 0.06 | 0.12 | 0.25 | ND | − | − | 75 | 11 yr | NSALSLAIEVRK | |||||||||||||||||
| 98 411 | Br sec | II | 1 | 0.5 | 8 | 2 | 0.03 | 0.06 | 0.12 | 0.015 | − | − | 31 | 11 mo | NSALSLAIEVRK | |||||||||||||||||
| 94 518 | Rhino | III | 2 | 2 | 16 | 4 | 0.06 | 0.12 | 0.25 | 0.015 | − | − | 57 | 9 mo | NSALSLAIEVRK | |||||||||||||||||
| 00 688 | Br sec | II | 1 | 1 | 8 | 1 | 0.03 | 0.06 | 0.12 | ND | − | − | 44 | 7 yr | NSALSLAIEVRK | |||||||||||||||||
| 99 671 | Otitis | II | 32 | 1 | 8 | 2 | 0.03 | 0.06 | 0.12 | 0.015 | + | − | 44 | 7 mo | NSALSLAIEVRK | |||||||||||||||||
| 93 704 | Br sec | III | 2 | 2 | 16 | 4 | 0.06 | 0.12 | 0.25 | 0.015 | − | − | 33 | 6 yr | NSAMSLAIEVRK | |||||||||||||||||
| 00 274 | Blood c | II | 1 | 1 | 16 | 1 | 0.03 | 0.06 | 0.12 | ND | − | − | 56 | 74 yr | NSAMSLSIEVRK | |||||||||||||||||
| 00 171 | Rhino | II | 1 | 1 | 4 | 1 | 0.03 | 0.06 | 0.12 | 0.003 | − | − | 59 | <5 yr | NSAMSLSIGVRK | |||||||||||||||||
| 00 519 | Br sec | II | 1 | 1 | 16 | 1 | 0.03 | 0.03 | 0.12 | ND | − | − | 44 | 46 yr | NSAMSLSIGVRK | |||||||||||||||||
| 99 010 | Br sec | II | 1 | 0.5 | 4 | 1 | 0.015 | 0.03 | 0.12 | 0.003 | − | − | 13 | 84 yr | NSAMSLSIGVRK | |||||||||||||||||
| 00 286 | Br sec, CF | II | 1 | 1 | 16 | 1 | 0.03 | 0.03 | 0.12 | ND | − | − | 44 | 19 yr | NSAMSLSIGVRK | |||||||||||||||||
| 00 699 | Br sec | II | 1 | 1 | 16 | 1 | 0.03 | 0.06 | 0.12 | ND | − | − | 44 | 57 yr | NSAMSLSIGVRK | |||||||||||||||||
| IIc | 94 075 | Rhino | II | 2 | 2 | 16 | 4 | 0.06 | 0.12 | 0.25 | 0.015 | − | − | 75 | <6 yr | DSAMSLAIGTRK | ||||||||||||||||
| 00 616 | Br sec | III | 1 | 1 | 16 | 2 | 0.03 | 0.12 | 0.12 | ND | − | − | 17 | 68 yr | DSAMSLAIGTRK | |||||||||||||||||
| I 90 172 | Sinus | II | 4 | 4 | 16 | 2 | 0.12 | 0.25 | 0.25 | 0.03 | − | − | 44 | 70 yr | DSAMSLAIGTRK | |||||||||||||||||
| 97 707 | Br sec | III | 1 | 1 | 8 | 2 | 0.03 | 0.06 | 0.12 | 0.015 | − | − | 13 | 70 yr | DSAMSLAIGTRK | |||||||||||||||||
| 97 150 | Br sec | III | 2 | 1 | 16 | 4 | 0.06 | 0.12 | 0.25 | 0.015 | − | − | 12 | 87 yr | DSAMSLAIGTRK | |||||||||||||||||
| 93 232 | Br sec | II | 2 | 4 | 16 | 4 | 0.12 | 0.25 | 0.25 | 0.03 | − | − | 31 | 29 yr | DSAMSLAIGTRK | |||||||||||||||||
| 00 216 | Br sec | II | 2 | 2 | 16 | 2 | 0.06 | 0.06 | 0.25 | ND | − | − | 12 | 68 yr | DSAMSLAIGTRK | |||||||||||||||||
| 00 399 | Conj | III | 1 | 1 | 16 | 2 | 0.06 | 0.06 | 0.25 | ND | − | − | 22 | 4 mo | DSAMSLAIGTRK | |||||||||||||||||
| 00 574 | Br sec | III | 1 | 1 | 16 | 2 | 0.06 | 0.06 | 0.12 | ND | − | − | 12 | 80 yr | DSAMSLAIGTRK | |||||||||||||||||
| 00 214 | Br sec | II | 2 | 2 | 16 | 2 | 0.06 | 0.06 | 0.25 | ND | − | − | 12 | 82 yr | DSAMSLAIGTRK | |||||||||||||||||
| 99 147 | Br sec | III | 1 | 1 | 8 | 4 | 0.06 | 0.06 | 0.12 | 0.015 | − | − | 12 | 51 yr | DSAMSLAIGTRK | |||||||||||||||||
| 95 103 | Conj | II | 1 | 0.5 | 8 | 2 | 0.03 | 0.12 | 0.25 | 0.015 | − | − | 94 | 1 mo | DSAMSLAIGTRK | |||||||||||||||||
| 00 759 | Br sec | III | 1 | 1 | 16 | 2 | 0.06 | 0.06 | 0.12 | ND | − | − | 13 | 32 yr | DSAMSLAIGTRK | |||||||||||||||||
| 00 349 | Rhino | II | 2 | 2 | 16 | 2 | 0.06 | 0.06 | 0.25 | ND | − | − | 76 | 1 mo | NSAMSLAIGTRK | |||||||||||||||||
| II 89 205 | Br sec | II | 2 | 2 | 16 | 4 | 0.06 | 0.12 | 0.25 | 0.015 | − | − | 31 | 12 yr | NSAMSLAIGTRK | |||||||||||||||||
| 00 348 | Rhino | II | 2 | 2 | 16 | 2 | 0.06 | 0.06 | 0.25 | ND | − | − | 76 | 1 mo | NSAMSLAIGTRK | |||||||||||||||||
| 00 690 | Br sec | II | 1 | 1 | 16 | 4 | 0.06 | 0.12 | 0.25 | ND | − | − | 44 | 28 yr | NSAMSLAIGTRK | |||||||||||||||||
| 00 587 | Br sec | III | 1 | 1 | 16 | 2 | 0.06 | 0.12 | 0.12 | ND | − | − | 47 | 80 yr | NSAMSLAIGTRK | |||||||||||||||||
| 00 067 | Br sec | II | 8 | 1 | 8 | 2 | 0.03 | 0.12 | 0.25 | 0.015 | + | − | 93 | 74 yr | NSAMSLAIGTRK | |||||||||||||||||
| 00 025 | Br sec | II | 8 | 0.5 | 8 | 2 | 0.06 | 0.12 | 0.25 | 0.015 | + | − | 93 | 64 yr | NSAMSLAIGTRK | |||||||||||||||||
| 94 414 | Conj | III | 1 | 0.5 | 8 | 2 | 0.03 | 0.06 | 0.12 | 0.015 | − | − | 44 | 2 yr | DSTMSLAIGTRK | |||||||||||||||||
| 94 416 | Rhino | III | 1 | 0.5 | 8 | 2 | 0.03 | 0.06 | 0.12 | 0.015 | − | − | 44 | 2 yr | DSTMSLAIGTRK | |||||||||||||||||
| 98 123 | Br sec | III | 1 | 1 | 8 | 2 | 0.03 | 0.06 | 0.12 | 0.015 | − | − | 80 | 3 yr | DSTMSLAIGTRK | |||||||||||||||||
| 99 484 | Br sec | II | 32 | 1 | 8 | 2 | 0.06 | 0.06 | 0.25 | 0.015 | + | − | 68 | 62 yr | DSTMSLAIGTRK | |||||||||||||||||
| 98 264 | CSF | III | 1 | 1 | 4 | 1 | 0.03 | 0.03 | 0.12 | 0.007 | − | − | 19 | 51 yr | DSTMSLAIGTRK | |||||||||||||||||
| IId | 91 392 | Otitis | III | 1 | 0.5 | 16 | 2 | 0.03 | 0.06 | 0.25 | 0.015 | − | − | 72 | 4 yr | DSAMSLAVGARK | ||||||||||||||||
| 92 158 | Conj | III | 1 | 1 | 8 | 2 | 0.03 | 0.06 | 0.12 | 0.015 | − | − | 13 | 11 mo | DSAMSLAVGARK | |||||||||||||||||
| 00 530 | Conj | III | 1 | 1 | 16 | 1 | 0.06 | 0.06 | 0.12 | ND | − | − | 68 | 10 mo | DSAMSLAVGARK | |||||||||||||||||
| 99 013 | Br sec | III | 2 | 0.5 | 4 | 2 | 0.03 | 0.03 | 0.12 | 0.007 | + | − | 44 | 72 yr | DSAMSLAVGARK | |||||||||||||||||
| 99 012 | Br sec | III | 8 | 0.12 | 2 | 1 | 0.007 | 0.015 | 0.06 | 0.001 | + | − | 68 | 64 yr | DSAMSLAVGARK | |||||||||||||||||
| 99 227 | Br sec | IV | 8 | 0.12 | 4 | 1 | 0.015 | 0.03 | 0.12 | 0.003 | + | − | 31 | 60 yr | DSAMSLAVGARK | |||||||||||||||||
| 99 091 | Br sec | III | 16 | 0.5 | 4 | 1 | 0.015 | 0.03 | 0.12 | 0.003 | + | − | 12 | 77 yr | DSAMSLAVGARK | |||||||||||||||||
| 94 013 | Rhino | III | 2 | 2 | 8 | 2 | 0.03 | 0.06 | 0.12 | 0.015 | − | − | 13 | <6 yr | DSAMSLAVGARK | |||||||||||||||||
| 91 453 | Conj | IV | 1 | 0.25 | 8 | 2 | 0.03 | 0.06 | 0.12 | 0.015 | − | − | 31 | 32 yr | DSAMSLAVGARK | |||||||||||||||||
| 99 657 | Br sec | II | 4 | 0.5 | 4 | 1 | 0.015 | 0.03 | 0.12 | 0.003 | + | − | 93 | 17 yr | DSAMSLAVGARK | |||||||||||||||||
| 99 220 | Br sec | III | 4 | 0.12 | 2 | 0.25 | 0.015 | 0.03 | 0.03 | 0.003 | + | − | 12 | 86 yr | DSAMSLAVGARK | |||||||||||||||||
| 00 071 | Otitis | III | 32 | 0.5 | 8 | 1 | 0.015 | 0.06 | 0.12 | 0.003 | + | − | 13 | 9 mo | DSAMSLAVGARK | |||||||||||||||||
| 93 662 | Urine | III | 1 | 0.5 | 8 | 2 | 0.03 | 0.06 | 0.12 | 0.007 | − | − | 33 | 1 day | DSAMSLAVGARK | 99 524 | Br sec | III | 16 | 2 | 8 | 1 | 0.03 | 0.06 | 0.12 | 0.015 | + | − | 17 | 57 yr | DSAMSLAVGARK | |
| 99 352 | Br sec | III | 16 | 1 | 16 | 2 | 0.03 | 0.06 | 0.12 | 0.007 | + | − | 44 | 47 yr | DSAMSLAVGARK | |||||||||||||||||
| III | ME870 | Rhino | NK | 4 | 4 | ND | ND | 0.5 | ND | ND | ND | − | − | NK | NK | NNALTFAIGARK | ||||||||||||||||
Abbreviations: Br sec, bronchial secretions; CF, cystic fibrosis; Conj, conjunctivitis; Blood c, blood culture; CSF, cerebrospinal fluid; Rhino, rhinopharynx infection; Sinus, sinusitis; ND, not done; NK, not known; AMX, amoxicillin; AMC, amoxicillin-clavulanic acid; CEC, cefaclor; CXM, cefuroxime; CTX, cefotaxime; CFM, cefixime; CPD, cefpodoxime; CRO, ceftriaxone.
The two first Arabic numerals of the strain number indicate the year of isolation.
Geographic origin by French department number.
Deduced amino acids at positions 350, 357, 368, 377, 385, 389, 437, 449, 490, 502, 517, and 526 (see also Table 2); amino acid substitutions are in boldface.
Media, antibiotics, and antimicrobial susceptibility testing.
Chocolate agar plates were used routinely for growth (bioMérieux). The medium for determination of MIC was Haemophilus test medium agar (HTM; Oxoid, Dardilly, France) (16). The β-lactams used in this study were amoxicillin, clavulanic acid at a constant concentration of 2 μg/ml with amoxicillin, and cefuroxime (GlaxoSmithKline, Nanterre, France); cefaclor (Eli Lilly (Saint-Cloud, France); cefotaxime, cefixime, and cefpodoxime (Aventis, Paris, France); and ceftriaxone (Roche, Neuilly, France). MICs were determined by an agar dilution method using HTM agar with an inoculum of 104 cells per spot (http:/www.sfm.asso.fr).
DNA extraction for PCR.
Strains were grown overnight on chocolate agar, and two to five colonies were resuspended in 1 ml of sterile saline. Following centrifugation of 300 μl of suspension at 6,700 × g for 10 min, supernatants were removed and the resulting bacterial pellet was resuspended in 300 μl of sterile saline; this was followed by the addition of 300 μl of lysis buffer (100 mM Tris, 1% sodium dodecyl sulfate, 20 mM EDTA). Genomic DNA was obtained by phenol-chloroform extraction with ethanol precipitation.
PCR.
PCR amplification of the part of the ftsI gene encoding the transpeptidase domain of PBP 3A and/or PBP 3B was carried out with primers J1 (5′ GAT ACT ACG TCC TTT AAA TTA AG 3′) and J2 (5′ GCA GTA AAT GCC ACA TAC TTA 3′), nucleotides 1048 to 1598 (K. Ubukata, N. Chiba, K. Hasegawa, Y. Shibasaki, and H. Shiro. Abstr. 40th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 894, 2000), and primers F1 (5′ GTT AAT GCG TAA CCG TGC AAT TAC C 3′) and F2 (5′ ACC ACT AAT GCA TAA CGA GGA TC 3′), nucleotides 936 to 1640 (based on the published ftsI sequence of H. influenzae Rd; accession no. L42023). When the J1-J2 primer set is used, a lack of response is expected for some BLNAR strains. PCR products obtained with primers F1 and F2 were used for sequencing.
Primer sets TEM (5′ TGG GTG CAC GAG TGG GTT AC 3′ and 5′ TTA TCC GCC TCC ATC CAG TC 3′), nucleotides 321 to 846, and ROB (5′ ATC AGC CAC ACA AGC CAC CT 3′ and 5′ GTT TGC GAT TTG GTA TGC GA 3′), nucleotides 419 to 1110, were used to detect the presence of blaTEM and blaROB in the strains giving a positive cefinase test (35).
Amplifications with primers J1 and J2, TEM, and ROB were carried out in a total volume 10 μl containing 50 μM (each) primer, 10 mM deoxynucleoside triphosphates, 25 mM MgCl2, 1 μl of 10× reaction buffer, 0.5 U of Taq DNA polymerase (Qbiogène, Illkirch, France), and 0.2 μl of template DNA. Amplification with primers F1 and F2 for sequencing was carried out in a total volume of 60 μl.
PCR cycling was carried out in a GeneAmp PCR system 2400 (Perkin-Elmer, Nieuwerkerk ad Yssel, The Netherlands). After an initial denaturation step at 94°C for 5 min, 25 amplification cycles of denaturation at 94°C for 30 s, annealing of primers at 55°C for 30 s, and primer extension at 72°C for 30 s were carried out, followed by a final primer extension step at 72°C for 7 min. PCR products were visualized by 2% agarose gel electrophoresis and ethidium bromide staining.
DNA sequencing and analysis of sequence data.
Both strands of the DNA fragments obtained by PCR with primers F1 and F2 were used for sequencing. Sequences were determined by MWG Biotech (Ebersberg, Germany) in a DNA sequencer (ABI Prism 3700 automatic DNA sequencer; Applied Biosystems).
Multiple sequence alignments were done with Sequence Navigator (Applied Biosystems) and CLUSTAL W, version 1.7 (http://ftp.ebi.ac.uk/pub/software/mac/clustalw/), software. The Rd strain sequence was included in sequence alignment as a reference (GenBank accession no. L42023). The alignment was adjusted by hand before phylogenetic analysis with version 3.572 c of the Phylogeny Inference Package (PHYLIP) (http://evolution.genetics.washington.edu/phylip.html) software. Phylogenetic distances between sequences were calculated by the two-parameter Kimura method (DNADIST from PHYLIP) with a transition-to-transversion ratio of 2.0. Dendrograms were created by the neighbor-joining and maximum-likelihood methods with CLUSTAL W and PHYLIP programs. Tree diagrams were plotted with the TREEVIEW, version 1.6, program (http://taxonomy.zoology.gla.ac.uk/rod/treeview.html). Bootstrapping was performed on 1,000 replicates by means of the neighbor-joining tree with CLUSTAL W, version 1.7.
Pulsed-field gel electrophoresis (PFGE) analysis.
Genomic DNA from a culture grown for 18 h on chocolate agar was prepared. Bacterial cells were suspended in phosphate-buffered saline at 109 CFU/ml; 150 μl of suspension was mixed with 150 μl of 1.0% low-melting-point agarose (Gibco-BRL, Cergy Pontoise, France) to form plugs. These were incubated in 1 ml of ES buffer (0.5 M EDTA [pH 9.0], 1% sodium dodecyl sulfate)-25 μl of proteinase K (5 mg/ml) for 24 h at 50°C. The lysis was stopped with 40 μl of phenylmethanesulfonyl fluoride (0.25 M) for 1 h at room temperature. The plugs were washed four times at room temperature for 45 min with TE buffer (10 mM Tris, 1 mM EDTA, pH 7.6) with gentle shaking.
Washed plug slices were digested for 18 h at 25°C with 25 U of SmaI (Quantum-Appligene)-300 μl of SmaI enzyme buffer. The plug slices were placed on the wall comb, and tempered 1% agarose (Sigma) was poured into the gel mold. The gels were run at 6.0 V/cm with an angle of 120° and with an initial switch time of 5.3 s to a final switch time of 34.9 s at 14°C in 0.5× Tris-borate-EDTA running buffer for 20 h by using a contour-clamped homogenous electric field system (CHEF DR III; Bio-Rad Laboratories, Marnes la Coquette, France). A concatemer ladder of lambda phage DNA (48.5 kb; Bio-Rad) was used as a size marker.
PFGE fingerprints were analyzed with Bio-Profil 99 software; Bio-1D software was used for the gel analysis, and Biogene software was used for pattern comparison (Vilber Lourmat, Marne La Vallée, France). The similarity of the PFGE banding patterns was estimated with the Dice coefficient and the unweighted pair group method using the arithmetic average algorithm.
Culture deposition and comparative strain accession number. The accession number for the strain ME870 ftsI gene nucleotide sequence data is AB035740 (36). Representative strains of each group are deposited in the Pasteur Culture Collection (Institut Pasteur, Paris, France) under numbers CIP 107144 to CIP 107158.
Nucleotide sequence accession numbers.
The partial DNA sequences corresponding to the ftsI genes of H. influenzae strains determined in the present study will appear in the EMBL, DDBJ, and GenBank nucleotide sequence databases under accession no. AY055605 to AY055723.
RESULTS
The characteristics of the strains studied are presented in Table 1. Strains were isolated from ENT infections (otitis, sinusitis, rhinopharynx infection) (n = 29) and from purulent bronchial secretions (n = 60). Two strains were isolated from cerebrospinal fluid, and three strains were isolated from blood culture. None of the strains were capsulated, and 84% belonged to biotypes II and III.
The majority of the strains were isolated from children under the age of 10 (n = 48; 44.4%) and from patients more than 50 years old (n = 40; 37%). The specimens came from 24 of the 94 mainland French departments.
Antibiotic susceptibility.
All isolates were inhibited by amoxicillin at ≥1 μg/ml. β-Lactamase-negative strains were inhibited by amoxicillin at concentrations of 1 to 8 μg/ml. The decreased susceptibility to amoxicillin was accompanied by a decreased susceptibility at different levels to other β-lactam antibiotics.
Compared to modal MICs of β-lactam antibiotics for susceptible strains (data not shown), the MICs for strains with reduced susceptibility to β-lactam antibiotics were elevated as follows: amoxicillin, 4- to 32-fold (β-lactamase-nonproducing strains); amoxicillin-clavulanic acid, 1- to 32-fold; cefaclor, 1- to 16-fold; cefuroxime, 2- to 16-fold; cefotaxime, 1- to 8-fold; cefixime, 1- to 16-fold; cefpodoxime, 1- to 8-fold; ceftriaxone, 1- to 16-fold.
Mutation patterns in the ftsI gene.
The 551-bp fragment (nucleotides 1048 to 1598 of the ftsI gene encoding PBP 3) amplified using primer set J1-J2 was present in all the control strains and in seven of the strains with decreased susceptibility to β-lactam antibiotics (results not shown). This fragment was not amplified in the other isolates (n = 103, including H. influenzae ATCC 49247 and strain ME870). The 705-bp fragment (nucleotides 936 to 1640 of the ftsI gene) amplified using primer set F1-F2 was present in all the strains whatever the β-lactam-inhibitory activity.
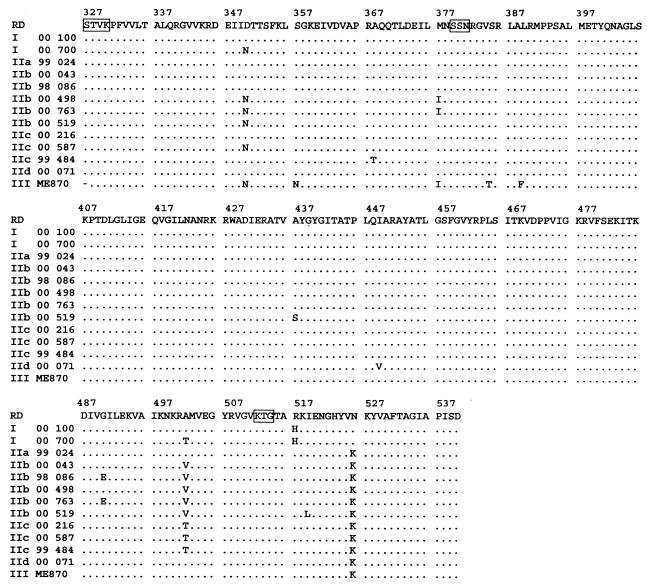
DNA sequencing was performed on F1-F2 DNA fragments. Nucleotide sequences of the ftsI gene, located from bp 960 to 1618 for the 108 clinical strains, H. influenzae ATCC 49247, strain ME870, and the control strains including H. influenzae ATCC 10211 were determined by direct sequencing (data not shown). The deduced amino acid sequences for the strains less susceptible to β-lactam antibiotics and for susceptible strains were compared with those for the standard Rd strain in the critical region between amino acids 327 and 540 (Fig. 1). Table 2 shows the amino acid substitution patterns encoded by the ftsI genes of the 108 clinical strains and H. influenzae ATCC 49247 and strain ME870.
FIG. 1.
Multiple alignment of PBP 3 amino acid sequences deduced from the sequences of the ftsI genes present in H. influenzae Rd and in representative H. influenzae strains with decreased susceptibility to β-lactam antibiotics. Conserved amino acid motifs STVK, SSN, and KTG are boxed. Dots, identical amino acids.
TABLE 2.
Deduced amino acid substitutions identified in part of PBP 3 from H. influenzae strains with reduced susceptibility to β-lactam antibiotics
| Group | No. of strains | Amino acid substitution for:
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Asp-350 | Ser-357 | Ala-368 | Met-377 | Ser-385 | Leu-389 | Ala-437 | Ile-449 | Gly-490 | Ala-502 | Arg-517 | Asn-526 | ||
| I | 1 | Asn | His | ||||||||||
| 1 | Thr | His | |||||||||||
| 4 | His | ||||||||||||
| 1 | Asn | Thr | His | ||||||||||
| IIa | 5 | Lys | |||||||||||
| 1a | Lys | ||||||||||||
| IIb | 13 | Val | Lys | ||||||||||
| 5 | Glu | Val | Lys | ||||||||||
| 1 | Asn | Val | Lys | ||||||||||
| 15 | Asn | Ile | Val | Lys | |||||||||
| 1 | Asn | Asn | Val | Lys | |||||||||
| 14 | Asn | Ile | Glu | Val | Lys | ||||||||
| 1 | Asn | Glu | Val | Lys | |||||||||
| 1 | Asn | Ser | Glu | Val | Lys | ||||||||
| 5 | Asn | Ser | Val | Lys | |||||||||
| IIc | 13 | Thr | Lys | ||||||||||
| 7 | Asn | Thr | Lys | ||||||||||
| 5 | Thr | Thr | Lys | ||||||||||
| IId | 15 | Val | Lys | ||||||||||
| III | 1b | Asn | Asn | Ile | Thr | Phe | Lys | ||||||
ATCC 49247.
ME870.
In this part of the ftsI gene, various mutations revealing 18 different mutation patterns in the 108 clinical strains were identified. The mutation patterns were classified into two groups on the basis of different amino acid substitutions.
In group I (n = 7), His-517 was substituted for Arg-517. In group II (n = 102, including H. influenzae ATCC 49247), Lys-526 was substituted for Asn-526. These substitutions (at amino acids 517 and 526) were not observed simultaneously in the same strain. None of the French clinical isolates were found to belong to group III, proposed by Ubukata et al. (36), which includes strain ME870 (Table 2).
In each group various other substitutions were also observed (Tables 1 and 2). In group I, three different substitution profiles were noted in addition to that at amino acid 517. We divided group II into four parts. Subgroup IIa (n = 5) includes strains that exhibit only the substitution at amino acid 526 (H. influenzae ATCC 49247 is in subgroup IIa). Subgroup IIb (n = 56) is defined by the substitution of Val-502 for Ala-502. Additional substitutions can be observed in subgroup IIb: Asn-350 for Asp 350 and Ile-377 for Met-377 (n = 15), as well as Glu-490 for Gly-490 (n = 14). These are the most frequent patterns of substitutions in group II. Subgroup IIc (n = 25) is defined by the substitution of Thr-502 for Ala-502; additional substitutions noted were Asn-350 for Asp-350 (n = 7) and Thr-368 for Ala-368 (n = 5). Subgroup IId (n = 15) is defined by the substitution of Val-449 for Ile-449.
TEM-type β-lactamase-producing strains (n = 15) were found in all subgroups (IIa, n = 1; IIb, n = 2; IIc, n = 3; IId, n = 9).
Distribution of the amino acid substitution patterns from year to year and according to the ages of the patients.
The strains studied were isolated over a period of 14 years; most of them, though, were isolated in 1999 and 2000 (n = 65). Group I and subgroups IIa and IIb existed as early as 1988 with 1, 1, and 5 strains, respectively, in our sample. Subgroups IIc and IId (25% of the strains) have been in circulation since 1989 and 1991, respectively. Six new patterns have appeared since 1999 and concern 27 strains. One of these new patterns has become preponderant in subgroup IIb (n = 15); the others have remained in a minority.
The greatest diversity was observed in 1999, with 9 profiles for 26 strains, and in 2000, with 11 profiles for 39 strains. However, it should noted that some years only a small number of strains were studied, an indication of the low incidence of these strains during those years.
All the amino acid substitution groups and subgroups were found in the two most frequently encountered age classes, children under the age of 6 years (n = 41) and adults over the age of 50 years (n = 40). No more than three and four single-strain patterns were found in children and adults, respectively. The strains isolated in children and adults included 12 and 15 patterns, respectively.
The strains isolated from purulent bronchial secretions (n = 60) included 16 patterns, and those from rhinopharynx infections (n = 19) and conjunctivitis (n = 13) included 10 and 9 patterns, respectively.
Analysis of sequence data.
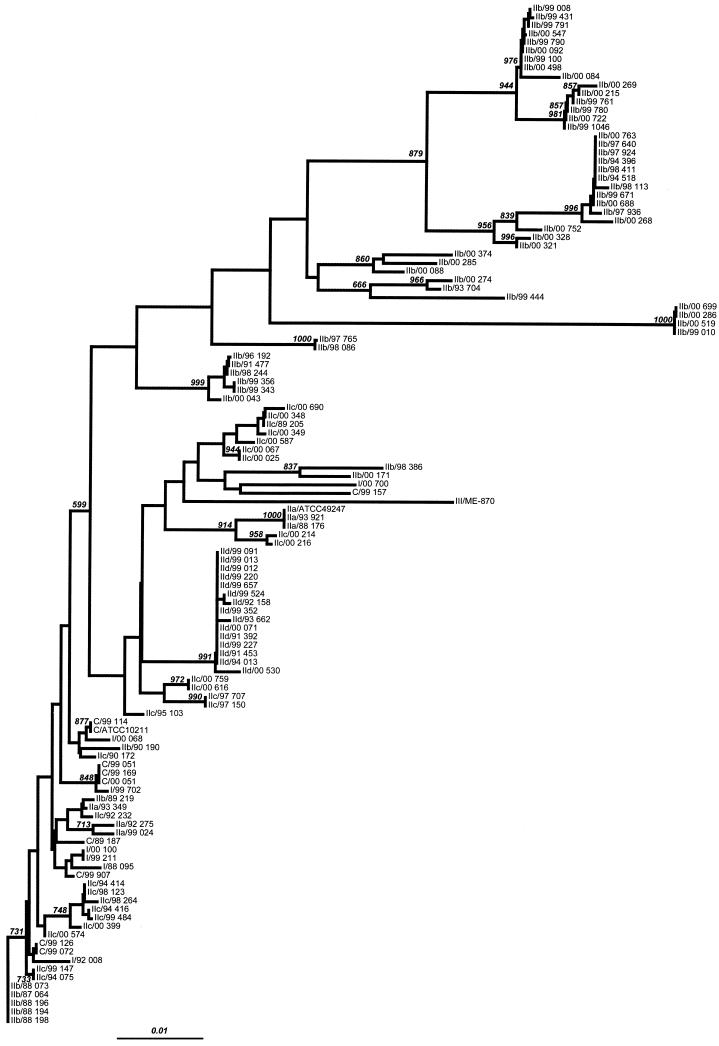
The phylogenetic tree based on partial sequences of the ftsI gene (nucleotides 960 to 1618, i.e., 659 bp) is presented in Fig. 2. Analysis of the nucleotide sequences shows high genetic diversity. Only the sequences belonging to the strains of group IId (n = 15) are significantly clustered in the same subtree. The absence of sequences belonging to other subgroups in this subtree suggests a clonal evolution of the strains.
FIG. 2.
Phylogenetic analysis of a 659-bp fragment sequence from the ftsI gene of H. influenzae. A neighbor-joining phylogenetic tree was built from all isolates and reference strain sequences. The Kimura two-parameter method of estimating genetic distance was used. Numbers next to the nodes of the tree represent bootstrap values (1,000 replicates). Bar, 0.01 genetic distance.
The group III strain (ME870) is clearly not phylogenetically associated with the strains in the other groups.
The sequences of the strains of group I and subgroups IIa, IIb, and IIc are distributed throughout the tree, indicating genetic heterogeneity in spite of the similar amino acid sequences in each group or subgroup. The sequences of subgroup IIb, which are the most frequent, are also genetically the most heterogeneous, with a polyclonal profile. Most of the sequences of the control strains (9 of 10) are found in one subtree (bootstrap 599), which also includes a homogeneous group of sequences belonging to group I and subgroups IIa, IIb, and IIc. This genetic heterogeneity suggests convergent evolution in the groups and subgroups with the exception of subgroup IId, which appears to be clonal.
PFGE analysis.
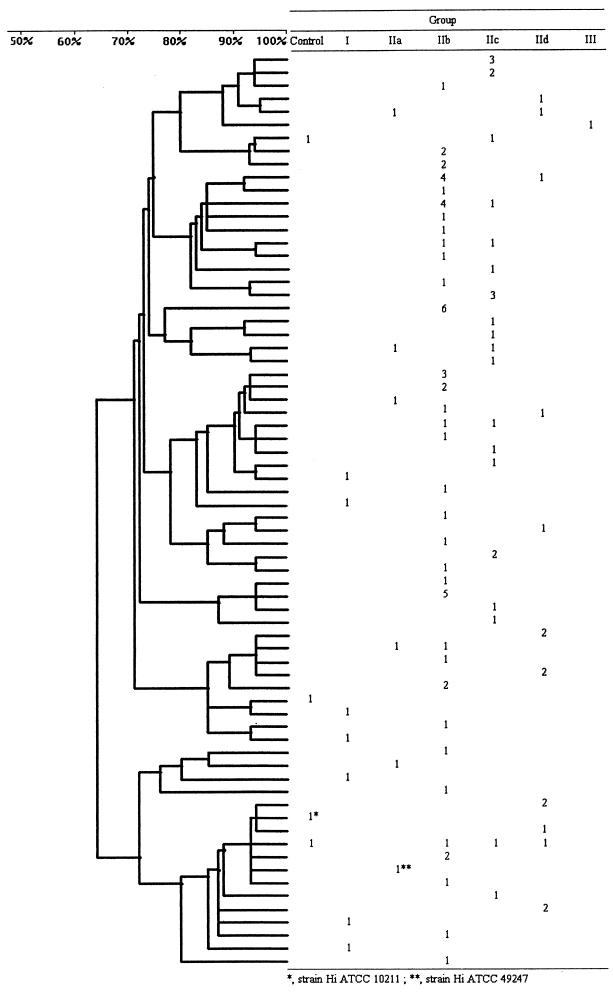
Genomic DNA from the 108 clinical strains, H. influenzae ATCC 49247, strain ME870, and four control strains including H. influenzae ATCC 10211 were examined by PFGE after treatment with endonuclease SmaI. The PFGE profiles obtained after SmaI digestion showed 10 to 12 fragments, ranging in size from approximately 20 to 400 kb. Among the 114 strains, 70 different profiles were obtained. The dendrogram was built by the inclusion of only one of the profiles with more than one strain. This choice facilitates the presentation and the reading of the results. The attribution of each profile to a group or subgroup is shown in Fig. 3.
FIG. 3.
PFGE analysis dendrogram showing the genotypic relationship among strains of H. influenzae (Hi) with decreased susceptibility to β-lactam antibiotics and distribution among the groups defined according to the patterns of amino acid substitutions deduced from the sequences of the ftsI gene.
The control strains and the strains of group I and subgroup IIa each had a unique pattern. In subgroup IIb (n = 56) 34 different profiles were identified, in subgroup IIc (n = 25) 19 profiles were identified, and in subgroup IId (n = 15) 11 profiles were identified. Forty-four profiles included only a single strain. Sixty profiles contained only strains belonging to a single group or subgroup (n = 86); the other 10 profiles contained strains belonging to different groups or subgroups.
Twenty-six clusters consisting of two to six strains were obtained (1 cluster of six strains, 3 clusters of five strains, 1 cluster of four strains, 3 clusters of three strains, and 18 clusters of two strains). Among the clusters of two strains, seven were composed of strains with the same amino acid substitution pattern, belonging to subgroups IIb (three clusters), IIc (one cluster), and IId (three clusters). Among six of these clusters three included isolates from children and three included isolates from adults.
Analysis of the other clusters indicates very variable situations. One cluster of three strains, one of four strains, and two of five strains are made up of strains with different substitution patterns. In contrast, for two clusters of three strains, one of five strains, and the one of six strains, the same substitution pattern occurred in each of the clusters. For the two clusters of three strains (subgroup IIc), there is dispersion in time and space except for two strains which were in fact isolated from the same child from the eye and the rhinopharynx.
The six-strain cluster is composed of strains from subgroup IIb isolated from blood cultures from adult subjects living in two distinct areas and of strains isolated from kindergarten children also from two separate areas. Finally, one five-strain cluster (IIb) is made up of strains isolated from the same area in subjects suffering from cystic fibrosis.
The PFGE results show the heterogeneity of the strains, with 70 different pulsotypes, but also strains closely related by pulsotype and substitution pattern, found in both adults and children.
DISCUSSION
In H. influenzae, changes in PBPs commonly result in a significant level of non-β-lactamase-mediated β-lactam resistance. The results of Clairoux et al. (4), Malouin et al. (19), Mendelman et al. (22, 25), and Parr and Bryan (29), demonstrated that the main factors involved in β-lactam resistance are the changes in two specific PBPs, PBP 3A and PBP 3B, involved in septal peptidoglycan synthesis. Amino acid substitutions in PBP 3 are involved in β-lactam resistance in BLNAR H. influenzae as described by Ubukata et al. (36).
The different patterns of amino acid substitution obtained with the strains studied here enable their attribution to groups I and II proposed by Ubukata et al. (36). However, the diversity of the patterns obtained and the occurrence of new patterns led us to propose different subgroups to account for the diversity.
It should be pointed out that in all the Japanese strains except one there was a substitution of Asn-350 for Asp-350 and that in the great majority (18 of 25) there was a substitution of Asn-357 for Ser-357.
With the French strains, the situation is very different because, in addition to the absence of strains belonging to group III, which will be discussed below, 63 of the 108 strains isolated in France had no substitution at position 350 and a single strain, in group IIb, had a double substitution at positions 350 and 357. The triple substitution at positions 377, 385, and 389 located near the conserved Ser-Ser-Asn (SSN) motif (observed in strain ME870 in group III) was not encountered in the French strains. The Japanese strains classified into group III were resistant to cephem antibiotics: cefotaxime MICs for these strains were 128- to 256-fold greater than those for susceptible strains. None of the French strains were found to belong to group III, and no strains resistant to cephems were observed.
The diversity of the substitutions noted in the French strains and the occurrence of mutations not reported in Japanese strains constitute an original situation. The French strains express a moderate decrease in susceptibility, in particular to cephem antibiotics. The inappropriate use of oral antibiotics for the treatment of community-acquired bronchopulmonary and ENT infections seems to be responsible for the selection of BLNAR strains. The difference could be related to dissimilar prescribing habits in the different countries. Now that the resistance mechanisms of the BLNAR strains are known, all possible measures should be taken to prevent their selection.
From a genomic point of view, if the double substitution at positions 350 and 357 must necessarily accompany the triple substitution at positions 377, 385, and 389 in addition to the substitution at position 526, then none of the French strains described here is a potential candidate for future inclusion in group III.
Noncapsulated strains of H. influenzae present a very high degree of heterogeneity. For 178 epidemiologically unrelated strains of H. influenzae, Saito et al. (31) have shown by PFGE that there was a variety of genome patterns and they obtained 165 genotypes (pulsotypes). Different studies, carried out on BLNAR strains, have shown the genotypic and phenotypic heterogeneity and the absence of clonal propagation of these strains (11, 24).
The analysis of the clonality of the strains in the present study can take different parameters into account: patterns of amino acid substitutions, analysis of sequence data, clusters from PFGE profiles, geographical location, age of patients, and date of specimen.
For Streptococcus pneumoniae the clonal diffusion of penicillin-resistant strains has been clearly demonstrated and the PBP genes present a mosaic structure (9, 21). For H. influenzae, unlike what was found for S. pneumoniae, the gene involved with β-lactam resistance does not present a mosaic structure. The strains studied here belong to 18 patterns of amino acid substitutions, but 5 patterns account for 70 strains (64.8%). By PFGE, 70 profiles were identified. Strains were taken as clonal if they presented the same amino acid substitution pattern and were in the same PFGE cluster.
Although there is evidence for a lack of clonality, the occurrence of certain PFGE clusters merits discussion. In particular, one cluster was present in a single geographic location and corresponds to strains isolated from patients suffering from cystic fibrosis. This indicates exchanges of strains between patients suffering from a particular disease but also the persistence of the strains over time (3, 26).
In contrast, the close phylogenetic relationship between the strains of the subgroup IId is not revealed by PFGE and the strains which were strongly genetically related with respect to ftsI belong to 11 PFGE profiles.
Encapsulated type b strains can occasionally have low susceptibility to β-lactams, but most BLNAR strains are noncapsulated (4, 13, 20, 27, 30). These strains were most often isolated from patients with chronic respiratory infections (2, 3, 24, 26). This is unlike the situation reported for Japan, where BLNAR strains were mainly observed among isolates from children and where there was an increasing proportion of group III strains (28, 33, 36).
In our study the presence of strains isolated from both children and adults in a single cluster supports the notion of circulation and exchange within and among the different populations.
The detection of decreased susceptibility to β-lactam antibiotics remains controversial. No single resistance breakpoint has been unanimously chosen, and different values have been proposed (1, 13, 30, 37). The study of Barry et al. (1) again stresses the difficulty in standardizing procedures and reaching a universal definition of what a BLNAR strain is. This unresolved issue has become more complex with the isolation of β-lactamase-producing strains which are resistant to amoxicillin-clavulanic acid (7, 8, 12). This type of strain occurred in the study of Ubukata et al. (36) and is also presented in our study.
β-Lactamase-producing strains resistant to amoxicillin-clavulanic acid, with both resistance mechanisms, are a further complication for detection, and some of them show only a slight decrease in susceptibility and are not always effectively resistant to amoxicillin-clavulanic acid.
The clinical relevance of resistance through the modification of PBPs has yet to be unambiguously demonstrated. The role in therapeutic failure of such resistant strains has not been clearly demonstrated, and therapeutic failure when resistant strains are present is infrequent (23).
Understanding the intricacies of the resistance mechanisms and using genetically investigated strains should lead to the development of reliable tests for the detection of such strains. However, the involvement of other resistance mechanisms in current or future strains cannot be excluded.
Acknowledgments
We are indebted to our colleagues for providing us with the strains isolated in their laboratories and for their contribution to the activity of the Centre National de Référence des Haemophilus influenzae. We thank K. Ubukata, who kindly provided us with strain ME870. We also thank O. Fayet and M. F. Prère for their help in the choice of primers, Mélanie Loriaux for her help, and Michèle Dhers for secretarial assistance.
This work was supported in part by SmithKline Beecham (France) and by a grant from Université Paul Sabatier Toulouse (AT UPS).
REFERENCES
- 1.Barry, A. L., P. C. Fuchs, and S. D. Brown. 2001. Identification of β-lactamase-negative, ampicillin-resistant strains of Haemophilus influenzae with four methods and eight media. Antimicrob. Agents Chemother. 45:1585-1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bell, S. M., and D. Plowman. 1980. Mechanisms of ampicillin resistance in Haemophilus influenzae from respiratory tract. Lancet i:279-280. [DOI] [PubMed] [Google Scholar]
- 3.Campos, J., F. Román, M. Georgiou, C. Garcia, R. Gómez-Lus, R. Cantón, H. Escobar, and F. Baquero. 1996. Long-term persistence of ciprofloxacin-resistant Haemophilus influenzae in patients with cystic fibrosis. J. Infect. Dis. 174:1345-1347. [DOI] [PubMed] [Google Scholar]
- 4.Clairoux, N., M. Picard, A. Brochu, N. Rousseau, P. Gourde, D. Beauchamp, T. R. Parr, M. G. Bergeron, and F. Malouin. 1992. Molecular basis of the non-β-lactamase-mediated resistance to β-lactam antibiotics in strains of Haemophilus influenzae isolated in Canada. Antimicrob. Agents Chemother. 36:1504-1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dabernat, H., and C. Delmas. 1998. Activité du Centre National de Référence des Haemophilus influenzae, années 1996-1997: le déclin du type b. Med. Mal. Infect. 28:940-946. [Google Scholar]
- 6.de Groot, R., G. Dzoljic-Danilovic, B. Van Klingeren, W. H. F. Goessens, and H. J. Neyens. 1991. Antibiotic resistance in Haemophilus influenzae: mechanisms, clinical importance and consequences for therapy. Eur. J. Pediatr. 150:534-546. [DOI] [PubMed] [Google Scholar]
- 7.Doern, G. V., A. B. Brueggemann, G. Pierce, H. P. Holley, and A. Rauch. 1997. Antibiotic resistance among clinical isolates of Haemophilus influenzae in the United States in 1994 and 1995 and detection of β-lactamase-positive strains resistant to amoxicillin-clavulanate: results of a national multicenter surveillance study. Antimicrob. Agents Chemother. 41:292-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Doern, G. V., R. N. Jones, M. A. Pfaller, K. Kugler, and the Sentry Participants Group. 1999. Haemophilus influenzae and Moraxella catarrhalis from patients with community-acquired respiratory tract infections: antimicrobial susceptibility patterns from the SENTRY antimicrobial surveillance program (United States and Canada, 1997). Antimicrob. Agents Chemother. 43:385-389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dowson, C. G., T. J. Coffey, and B. G. Spratt. 1994. Origin and molecular epidemiology of penicillin-binding-protein-mediated resistance to β-lactam antibiotics. Trends Microbiol. 2:361-366. [DOI] [PubMed] [Google Scholar]
- 10.Foxwell, A. R., J. M. Kyd, and A. W. Cripps. 1998. Nontypeable Haemophilus influenzae: pathogenesis and prevention. Microbiol. Mol. Biol. Rev. 62:294-308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gazagne, L., C. Delmas, E. Bingen, and H. Dabernat. 1998. Molecular epidemiology of ampicillin-resistant non-β-lactamase-producing Haemophilus influenzae. J. Clin. Microbiol. 36:3629-3635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jacobs, M. R., and S. Bajaksouzian. 1997. Evaluation of Haemophilus influenzae isolates with elevated MICs to amoxicillin/clavulanic acid. Diagn. Microbiol. Infect. Dis. 28:105-112. [DOI] [PubMed] [Google Scholar]
- 13.James, P. A., D. A. Lewis, J. Z. Jordens, J. Cribb, S. J. Dawson, and S. A. Murray. 1996. The incidence and epidemiology of β-lactam resistance in Haemophilus influenzae. J. Antimicrob. Chemother. 37:737-746. [DOI] [PubMed] [Google Scholar]
- 14.Jordens, J. Z., and M. P. E. Slack. 1995. Haemophilus influenzae: then and now. Eur. J. Clin. Microbiol. Infect. Dis. 14:935-948. [DOI] [PubMed] [Google Scholar]
- 15.Jorgensen, J. H. 1992. Update on mechanisms and prevalence of antimicrobial resistance in Haemophilus influenzae. Clin. Infect. Dis. 14:1119-1123. [DOI] [PubMed] [Google Scholar]
- 16.Jorgensen, J. H., J. S. Redding, L. A. Mahe, and A. W. Howell. 1987. Improved medium for antimicrobial susceptibility tests of Haemophilus influenzae. J. Clin. Microbiol. 25:2105-2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kilian, M. 1976. A taxonomic study of the genus Haemophilus, with the proposal of a new species. J. Gen. Microbiol. 93:9-62. [DOI] [PubMed] [Google Scholar]
- 18.Madore, D. V. 1996. Impact of immunization on Haemophilus influenzae type b disease. Infect. Agents Dis. 5:8-20. [PubMed] [Google Scholar]
- 19.Malouin, F., A. B. Schryvers, and L. E. Bryan. 1987. Cloning and expression of genes responsible for altered penicillin-binding proteins 3a and 3b in Haemophilus influenzae. Antimicrob. Agents Chemother. 31:286-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Markowitz, S. M. 1980. Isolation of an ampicillin-resistant, non-β-lactamase-producing strain of Haemophilus influenzae. Antimicrob. Agents Chemother. 17:80-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McGee, L., L. McDougal, J. Zhou, B. G. Spratt, F. C. Tenover, R. George, R. Hakenbeck, W. Hryniewicz, J. C. Lefèvre, A. Tomasz, and K. P. Klugman. 2001. Nomenclature of major antimicrobial-resistant clones of Streptococcus pneumoniae defined by the pneumococcal molecular epidemiology network. J. Clin. Microbiol. 39:2565-2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mendelman, P. M., D. O. Chaffin, and G. Kalaitzoglou. 1990. Penicillin-binding proteins and ampicillin resistance in Haemophilus influenzae. J. Antimicrob. Chemother. 25:525-534. [DOI] [PubMed] [Google Scholar]
- 23.Mendelman, P. M., D. O. Chaffin, L. Krilov, G. Kalaitzoglou, D. A. Serfass, and O. Onay. 1990. Cefuroxime treatment failure of nontypeable Haemophilus influenzae meningitis associated with alteration of penicillin-binding proteins. J. Infect. Dis. 162:1118-1123. [DOI] [PubMed] [Google Scholar]
- 24.Mendelman, P. M., D. O. Chaffin, J. M. Musser, R. De Groot, D. A. Serfass, and R. K. Selander. 1987. Genetic and phenotypic diversity among ampicillin-resistant, non-β-lactamase-producing, nontypeable Haemophilus influenzae isolates. Infect. Immun. 55:2585-2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mendelman, P. M., D. O. Chaffin, T. L. Stull, C. E. Rubens, K. D. Mack, and A. L. Smith. 1984. Characterization of non-β-lactamase-mediated ampicillin resistance in Haemophilus influenzae Antimicrob. Agents Chemother. 26:235-244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Möller, L. V. M., A. G. Regelink, H. Grasselier, L. van Alphen, and J. Dankert. 1998. Antimicrobial susceptibility of Haemophilus influenzae in the respiratory tracts of patients with cystic fibrosis. Antimicrob. Agents Chemother. 42:319-324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Offit, P. A., J. M. Campos, and S. A. Plotkin. 1982. Ampicillin-resistant, β-lactamase-negative Haemophilus influenzae type b. Pediatrics 69:230-231. [PubMed] [Google Scholar]
- 28.Ohkusu, K., A. Nakamura, and K. Sawada. 2000. Antibiotic resistance among recent clinical isolates of Haemophilus influenzae in Japanese children. Diagn. Microbiol. Infect. Dis. 36:249-254. [DOI] [PubMed] [Google Scholar]
- 29.Parr, T. R., Jr., and L. E. Bryan. 1984. Mechanism of resistance of an ampicillin-resistant, β-lactamase-negative clinical isolate of Haemophilus influenzae type b to β-lactam antibiotics. Antimicrob. Agents Chemother. 25:747-753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Powell, M. 1988. Antimicrobial resistance in Haemophilus influenzae. J. Med. Microbiol. 27:81-87. [DOI] [PubMed] [Google Scholar]
- 31.Saito, M., A. Umeda, and S.-I. Yoshida. 1999. Subtyping of Haemophilus influenzae strains by pulsed-field gel electrophoresis. J. Clin. Microbiol. 37:2142-2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scriver, S. R., S. L. Walmsley, C. L. Kau, D. J. Hoban, J. Brunton, A. McGeer, T. C. Moore, E. Witwicki, the Canadian Haemophilus Study Group, and D. E. Low. 1994. Determination of antimicrobial susceptibilities of Canadian isolates of Haemophilus influenzae and characterization of their β-lactamases. Antimicrob. Agents Chemother. 38:1678-1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seki, H., Y. Asahara, K. Ohta, K. Ohta, Y. Saikawa, R. Sumita, A. Yachie, S. I. Fujita, and S. Koizumi. 1999. Increasing prevalence of ampicillin-resistant, non-beta-lactamase-producing strains of Haemophilus influenzae in children in Japan. Chemotherapy 45:15-21. [DOI] [PubMed] [Google Scholar]
- 34.Serfass, D. A., P. M. Mendelman, D. O. Chaffin, and C. A. Needham. 1986. Ampicillin resistance and penicillin-binding proteins of Haemophilus influenzae. J. Gen. Microbiol. 132:2855-2861. [DOI] [PubMed] [Google Scholar]
- 35.Tenover, F. C., M. B. Huang, J. K. Rasheed, and D. H. Persin. 1994. Development of PCR assays to detect ampicillin resistance genes in cerebrospinal fluid samples containing Haemophilus influenzae. J. Clin. Microbiol. 32:2729-2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ubukata, K., Y. Shibasaki, K. Yamamoto, N. Chiba, K. Hasegawa, Y. Takeuchi, K. Sunakawa, M. Inoue, and M. Konno. 2001. Association of amino acid substitutions in penicillin-binding protein 3 with β-lactam resistance in β-lactamase-negative ampicillin-resistant Haemophilus influenzae. Antimicrob. Agents Chemother. 45:1693-1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zerva, L., D. J. Biedenbach, and R. N. Jones. 1996. Reevaluation of interpretive criteria for Haemophilus influenzae by using meropenem (10-microgram), imipenem (10-microgram), and ampicillin (2- and 10-microgram) disks. J. Clin. Microbiol. 34:1970-1974. [DOI] [PMC free article] [PubMed] [Google Scholar]