Abstract
l-Lysine catabolism in Pseudomonas putida KT2440 was generally thought to occur via the aminovalerate pathway. In this study we demonstrate the operation of the alternative aminoadipate pathway with the intermediates d-lysine, l-pipecolate, and aminoadipate. The simultaneous operation of both pathways for the use of l-lysine as the sole carbon and nitrogen source was confirmed genetically. Mutants with mutations in either pathway failed to use l-lysine as the sole carbon and nitrogen source, although they still used l-lysine as the nitrogen source, albeit at reduced growth rates. New genes were identified in both pathways, including the davB and davA genes that encode the enzymes involved in the oxidation of l-lysine to δ-aminovaleramide and the hydrolysis of the latter to δ-aminovalerate, respectively. The amaA, dkpA, and amaB genes, in contrast, encode proteins involved in the transformation of Δ1-piperidine-2-carboxylate into aminoadipate. Based on l-[U-13C, U-15N]lysine experiments, we quantified the relative use of pathways in the wild type and its isogenic mutants. The fate of 13C label of l-lysine indicates that in addition to the existing connection between the d- and l-lysine pathways at the early steps of the catabolism of l-lysine mediated by a lysine racemase, there is yet another interconnection at the lower end of the pathways in which aminoadipate is channeled to yield glutarate. This study establishes an unequivocal relationship between gene and pathway enzymes in the metabolism of l-lysine, which is of crucial importance for the successful colonization of the rhizosphere of plants by this microorganism.
Pseudomonas putida KT2440, a derivative of P. putida mt-2 cured of the TOL plasmid, can grown on proline, lysine, glutamate, and other amino acids as the sole carbon and nitrogen source. The ability to assimilate these compounds confers on the strain a selective advantage to grow in the rhizosphere of a number of plants where these amino acids are part of the exudates (2, 11, 28, 29).
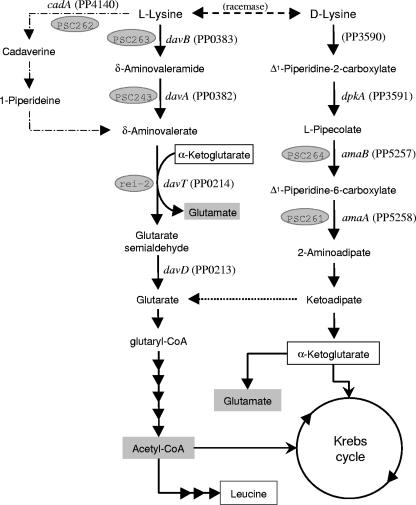
The catabolism of l-lysine by P. putida mainly involves the following steps: l-lysine → δ-aminovaleramide → δ-aminovalerate (AMV) → glutarate semialdehyde → glutarate, which is then channeled to the Krebs cycle. This pathway is known as the AMV pathway (Fig. 1) and was well characterized at the biochemical level in the late 1970s (6, 7, 13). In this route, the first step involves the oxidative decarboxylation of the amino acid to yield δ-aminovaleramide, which is hydrolyzed to produce ammonium and δ-aminovalerate. Thereafter, δ-aminovalerate is converted into glutarate via glutarate semialdehyde (6, 7) in reactions catalyzed by the products of the davD and the davT genes, the only genes of the pathway identified so far (11, 38). The davD gene forms an operon with davT, the gene order being davDT (38). The rei-2 mutant is a KT2440 derivative that is unable to use l-lysine as a carbon source and which was isolated after mutagenesis of the wild-type strain with mini-Tn5-′lux. Mini-Tn5-′lux was inserted within the davT gene, giving rise to a davDT:′lux transcriptional fusion. The relevance of this pathway during the colonization of the root system of corn by P. putida is evidenced by the fact that rei-2 cells emitted light in response to root exudates. In agreement with the pathway described above, both davD and davT mutants were unable to use l-lysine or δ-aminovalerate as a carbon source. The davD promoter was expressed at a certain level in the absence of l-lysine, but its expression increased about fourfold in response to the addition of exogenous l-lysine to the culture medium. However, the real inducer of this operon seems to be AMV because in a mutant unable to metabolize l-lysine to δ-aminovalerate, this compound, and not l-lysine, acted as an effector (38).
FIG. 1.
Proposed catabolic pathways for the degradation of l- and d-lysine by bacteria of the genus Pseudomonas. Reactions from d-lysine to 2-aminoadipate represent the AMA pathway, those from l-lysine to glutaric acid represent the AMV pathway, and the utmost left branch of reactions from l-lysine to δ-aminovalerate via cadaverine/1-piperidine represent the cadaverine pathway. When they are known, the corresponding gene and the number of its translated product are given. In shaded ovals are the mutant strain numbers corresponding to strains deposited in the publicly available Pseudomonas putida Stock Center.
In Pseudomonas aeruginosa, the early steps of the AMV pathway are not present (36) and l-lysine catabolism was proposed to proceed via the cadaverine pathway, which involves the decarboxylation of l-lysine to cadaverine and its subsequent metabolism to δ-aminovalerate (13, 36) (Fig. 1). No evidence for the operation of the cadaverine pathway is available in P. putida.
Several P. putida strains can use d-lysine in addition to l-lysine as a carbon and nitrogen source. The pathway involves the following steps: d-lysine → Δ1-piperidine-2-carboxylate → l-pipecolate → Δ1-piperidine-6-carboxylate → 2-aminoadipate (AMA) → α-ketoadipate → α-ketoglutarate (Fig. 1). This route is known as the AMA pathway, and part of these genes have been suggested to be plasmid encoded (5). Early studies by Chang and collaborators (6, 7) and a more recent one by Muramatsu et al. (30) suggest that the d- and l-lysine pathways are independent in P. putida.
To identify the genes encoding the enzymes involved in the catabolism of l-lysine by P. putida, we set up a strategy for the isolation of mutants deficient in the catabolism of this amino acid. The nature of the metabolic step was identified by determining accumulation of potential pathway intermediates and by using isotopically labeled l-lysine. Our results reveal that l-lysine catabolism occurs via two pathways, one in which the key metabolite is AMV and another one in which the key metabolites are pipecolate and AMA. The operation of the second pathway involves an early lysine racemase that converts l-lysine into d-lysine. The fate of 13C from l-lysine has allowed us to show that AMA pathway intermediates can be diverted to glutarate in the AMV pathway. Most of the genes involved in the key steps in both pathways have been identified.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
Pseudomonas putida KT2440, a derivative of the P. putida soil isolate mt-2, was used in this study as a prototrophic strain (14, 31). P. putida rei-2 is a KT2440 derivative that carries the ′luxCDABE genes fused in frame to the davT gene (11). Escherichia coli strain DH5α was used for cloning experiments, and E. coli CC118λpir was used as a host for replication of pUT-Km (Apr Kmr) (9) and pUT-Gm (Apr Gmr) (Soeren Molin, unpublished results), the suicide plasmids used for mini-Tn5 mutagenesis. These strains were grown at 37°C in lysogeny broth (LB) medium (39). Pseudomonas putida strains were grown at 30°C either in LB medium or in M9 minimal medium (40) supplemented with Fe-citrate, MgSO4 and trace metals, as described previously (1). As a carbon source we used benzoate (15 mM), glucose (0.5% [wt/vol]), or sodium citrate (10 mM). When indicated M9 minimal medium without NH4Cl was used; this culture medium is referred as M8. When indicated l-lysine (10 mM), d-lysine (10 mM), δ-aminovalerate (5 mM), glutarate (10 mM), or pipecolate (10 mM) was added to the culture medium. When appropriate, antibiotics were added at the following concentrations: chloramphenicol (Cm), 30 μg/ml; gentamicin (Gm), 30 μg/ml; kanamycin (Km), 50 μg/ml; and tetracycline (Tc), 15 μg/ml.
Mini-Tn5 mutagenesis of P. putida strains.
Mini-Tn5 transposon mutagenesis was carried out by performing triparental matings between P. putida KT2440 (Cmr), E. coli CC118λpir(pUT-Km), and the helper strain E. coli HB101(pRK600), as described by de Lorenzo et al. (9). Among 3,000 Cmr Kmr transconjugants able to grow on M9 minimal medium with glucose as a carbon source, we searched for mutants unable to use l-lysine as the sole carbon and nitrogen source. Three mutants PSC261 (AmaA), PSC265 (DkpA), and PSC264 (AmaB) were identified and kept for further assays. P. putida rei-2 (lux+ Kmr) was mated with E. coli CC118λpir(pUT-Gm) and the helper strain E. coli HB101(pRK600), as described above. Transconjugants of P. putida rei-2 were selected on M9 minimal medium with citrate as the carbon source and with kanamycin and gentamicin. Approximately 10,000 independent transconjugants were tested for light emission in this minimal medium in the presence of 5 mM l-lysine. Five mutants were found to emit no light or significantly less light than the parental strain. To exclude those that may have resulted from the insertion of a mini-Tn5 cassette in the lux genes, total DNA from the mutants was hybridized against the entire lux operon labeled with digoxigenin. Four clones were discarded because of the inactivation of the lux genes, and the other one, called rei-2-1, was retained for further characterization (see below).
Site-specific homologous inactivation of davA, davB, and cadA.
To construct mutant strains bearing an inactivated chromosomal version of the davA, davB, and cadA genes, we generated the corresponding knockout using the appropriate derivatives of pCHESIΩKm. Plasmid pCHESIΩKm is based on pUC18 and bears the origin of transfer oriT of RP4 and the Ω-Km interposon of plasmid pHP45ΩKm cloned as a HindIII fragment (22). To generate the desired mutation, an internal fragment of about 600 bp of the target gene was amplified by PCR with primers provided with EcoRI and BamHI sites and was subsequently cloned between the EcoRI and BamHI sites of pCHESIΩKm in the same transcriptional direction as the Plac promoter. The recombinant plasmid was mobilized from E. coli CC118λpir into P. putida KT2440 by triparental mating with the E. coli HB101(pRK600) helper strain (18). P. putida transconjugants bearing a cointegrate of the plasmid in the host chromosome were selected on M9 minimal medium with benzoate (10 mM) as the sole carbon source and kanamycin. A few Kmr clones were chosen to confirm the generation of the corresponding knockout. A single random clone with the inactivated gene version was chosen. Mutant strains with knockouts in davA, davB, and cadA were called P. putida PSC243, PSC263, and PSC262, respectively.
Preparation of RNA and RT-PCR.
Pseudomonas putida strain KT2440 and its mutant derivatives were grown overnight in M9 minimal medium with benzoate. Cells were then diluted 100-fold in fresh medium, and different aliquots were incubated in duplicate in the absence or in the presence of 5 mM lysine, 5 mM δ-aminovalerate, and 10 mM pipecolate until the culture reached a turbidity of ca. 1.0 at 660 nm. Cells (1.5 ml) were harvested by centrifugation (5,000 × g for 10 min) and processed for RNA isolation according to the method described by Marqués et al. (24). Extracts were treated with RNase-free DNase I (50 U) in the presence of 3 U of an RNase inhibitor cocktail (Roche Laboratories).
Reverse transcription-PCR (RT-PCR) was done with 1 μg of RNA in a final volume of 20 μl using the Titan OneTube RT-PCR system according to the manufacturer's instructions (Roche Laboratories). The annealing temperature used for RT-PCR was 60°C, and the cycling conditions were as follows: 94°C for 30 s, 60°C for 30 s, and 68°C for 1 min. Positive and negative controls were included in all assays. The primers used to test contiguity in the mRNA of the davB and davA genes, as well as the amaA and amaB genes, will be made available on request.
14CO2 evolution from l-[14C]lysine.
Pseudomonas putida KT2440 and its mutant derivative PSC263 (davB mutant) were grown on M9 minimal medium with benzoate and 5 mM l-lysine until they reached the mid-logarithmic phase (0.7 to 0.8 turbidity units at 660 nm), then 7.8 μCi of l-[U-14C]lysine was added per assay and 14CO2 evolved for 5 min fixed on NaOH and 14C was determined in a Packard radiocounter.
13C, 15N-labeling assays.
For 13C, 15N-labeling experiments, aerobic cultures were grown in 50-ml baffled shake flasks with 10 ml M8 minimal medium supplemented with 5 mM l-[U-13C, U-15N]lysine and 0.5% (wt/vol) glucose at 30°C on a shaker at 225 rpm. Cell aliquots (5 ml) were harvested during the mid-exponential growth phase by centrifugation at 1,200 × g and 4°C for 20 min. The pellet was washed twice with 1 ml 0.9% (wt/vol) NaCl, hydrolyzed in 1.5 ml 6 M HCl for 24 h at 110°C in sealed 2-ml Eppendorf tubes, and desiccated overnight in a heating block at 85°C under a constant air stream. The hydrolysate was dissolved in 50 μl 99.8% dimethyl formamide and transferred immediately into a new Eppendorf tube. For derivatization, 30 μl N-methyl-N-(tert-butyldimethylsilyl)-trifluoroacetamide was added, which readily silylates hydroxyl groups, thiols, primary amines, amides, and carboxyl groups (8), and the mixture was incubated at 85°C with shaking at 550 rpm for 60 min. One microliter of the derivatized sample was injected into a Series 8000 gas chromatograph, combined with a MD 800 mass spectrometer (Fisons Instruments), and analyzed as described earlier (8, 12). The mass spectra of the derivatized leucine, alanine, and glutamic acid were corrected for the natural abundance of all stable isotopes and unlabeled biomass from the inoculum by using the MATLAB-based program Fiat Flux version 1.04, as described previously (12). The same software was used to finally calculate the mass isotopomer distribution vectors of leucine, alanine, and glutamic acid, which gave the relative abundances of each isotope of the different fragments.
Gas chromatography-mass spectrometry determination of l-lysine metabolites.
Aerobic cultures were grown in 500-ml baffled shake flasks with 50 ml M8 minimal medium supplemented with 5 mM l-lysine and 0.5% (wt/vol) glucose at 30°C on a rotary shaker at 225 rpm. When the turbidity at 600 nm was around 1, biomass was harvested by centrifugation at 4,000 × g and 4°C for 8 min. The pellet was suspended in methanol and incubated at 70°C for 30 min. After chilling on ice, it was centrifuged at 20,000 × g for 10 min. The extract was dried in a vacuum evaporation system (HetoVac VR-1 with a CT110 cold trap and Alcatel vacuum pump), and samples were derived as described above.
RESULTS
l-Lysine catabolism involves multiple pathways.
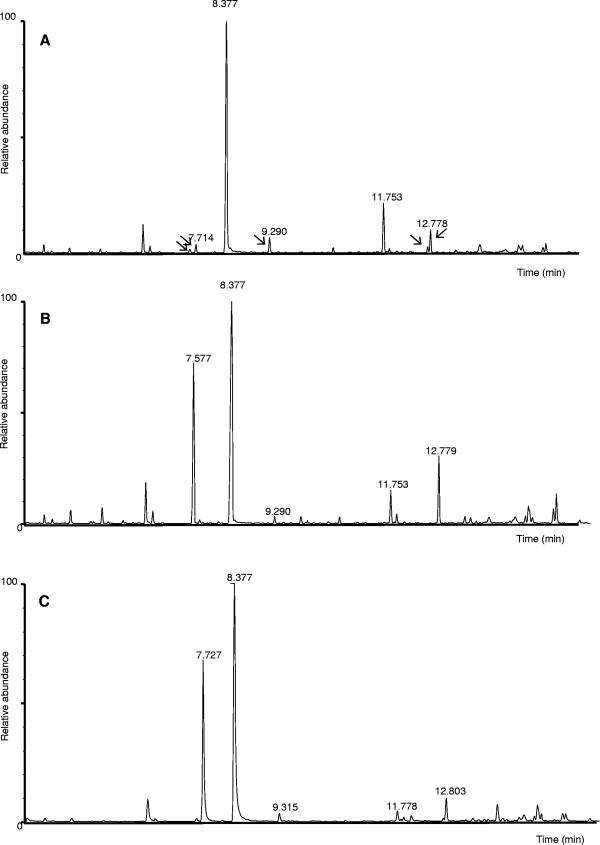
To identify potential intermediates involved in l-lysine catabolism, P. putida KT2440 cultures were grown to the mid-exponential growth phase on M8 minimal medium with glucose as a carbon source and l-lysine as the nitrogen source. Intracellular metabolites were then extracted from cell biomass and analyzed by gas chromatography-mass spectrometry (Fig. 2). Three products, δ-aminovalerate, pipecolate, and aminoadipate, were unequivocally identified based on identical retention times and identical mass fragmentation spectra compared to those of pure chemicals (Fig. 3 and 4), although glutarate was not detected. The intracellular concentrations of the three intermediates were in the range of 1 nmol per g of cell protein (Table 1), as quantified from standard curves with the pure compounds. These results indicated that l-lysine was metabolized by more than one pathway: at least one leading to δ-aminovalerate (note that cadaverine cannot be identified by the method used) and another probably involving the conversion of l-lysine into d-lysine through the action of a lysine racemase, in agreement with the early report by Ichihara et al. (19) on the existence of this activity in bacteria of the genus Pseudomonas, and the subsequent channeling to pipecolate and aminoadipate (see Fig. 1). Biochemical evidence for the existence of this activity was achieved with a polar mutant with a mutation in the dkpA gene that exerts a polar effect on d-lysine monooxygenase and which fails to metabolize d-lysine. In this mutant d-lysine accumulated in the culture supernatants from l-lysine with an estimated rate of 3 μmol/mg protein · min.
FIG. 2.
Gas chromatography analysis of the products from l-lysine accumulated intracellularly by P. putida. (A) Wild type; (B) amaB; (C) rei-2. Peaks at 7.71, 7.57, and 12.77 min correspond to pipecolate, AMV, and aminoadipate, respectively (see Fig. 3 and 4).
FIG. 3.
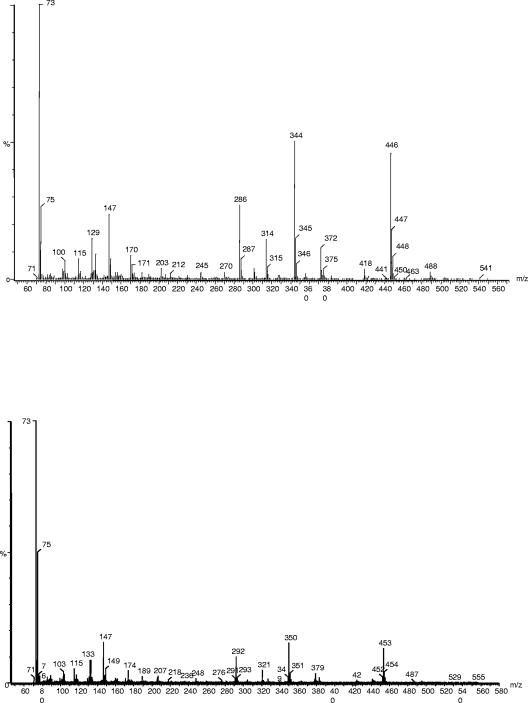
Mass spectra of products of aminovalerate and pipecolate in the metabolism of l-lysine by P. putida. P. putida KT2440 cells were grown on M9 minimal medium with glucose and unlabeled l-lysine or l-[U-15N, U-13C]lysine. The mass spectra of products corresponding to peaks at 7.57 and 7.71 are shown. (A) The fragmentation spectrum corresponds to AMV formed in cultures with unlabeled l-lysine. (B) The fragmentation spectrum corresponds to AMV derived from l-[15N, 13C]lysine. The two spectra at the bottom correspond to pipecolate formed from unlabeled l-lysine (C) or labeled l-lysine (D).
FIG. 4.
Mass spectra of aminoadipate in the metabolism of l-lysine by P. putida. Conditions are the same as those described for Fig. 3, except that the spectra corresponds to AMA formed from unlabeled l-lysine (top) or labeled l-lysine (bottom).
TABLE 1.
Intracellular concentrations of the different catabolites in the mutantsa
| Strain | Concn of catabolite (nmol/mg of total cell protein)
|
|||
|---|---|---|---|---|
| Mutant gene | AMV | Pipecolate | Aminoadipate | |
| KT2440 | None | 1.3 ± 0.5 | 1.4 ± 0.9 | 0.8 ± 0.2 |
| rei2 | davT | 25 ± 0.2 | 0.8 ± 0.05 | 0.7 ± 0.3 |
| PSC243 | davA | 1.4 ± 0.1 | 1.1 ± 0.2 | 0.7 ± 0.1 |
| PSC263 | davB | 0.7 ± 0.1 | 1.3 ± 0.1 | 0.8 ± 0.1 |
| PSC262 | cadA | 1 ± 0.1 | 0.7 ± 0.03 | 0.5 ± 0.2 |
| PSC264 | amaB | 1.1 ± 0.2 | 30 ± 7 | NDb |
| PSC261 | amaA | 1.2 ± 0.4 | 60 ± 20 | ND |
Extraction and analysis of these metabolites are described in Materials and Methods.
ND, not detected.
The complexity observed in the metabolism of l-lysine was somewhat unexpected because our earlier results indicated that the davD and davT mutants could not use l-lysine as a carbon source and also because the recent annotation of the P. putida KT2440 genome failed to identify open reading frames (ORFs) potentially involved in d-lysine catabolism (33).
Genetic approaches to the identification of the davA and davB genes, whose gene products are involved in the early steps of l-lysine metabolism in the AMV pathway.
To approach the identification of the functional genes involved in l-lysine metabolism, we designed different strategies. One of them took advantage of the rei-2 mutant, which emits light in response to the addition of l-lysine and δ-aminovalerate. We decided to mutagenize this strain and search for “dark” mutant derivatives in response to l-lysine. We assumed that the inactivation of a gene whose product led to the biosynthesis of δ-aminovalerate would produce no light emission. To this end, P. putida rei-2 was mated with E. coli S17-1λpir(pUT-Gm) and about 10,000 Gmr Kmr transconjugants were selected. Five clones emitted no light in response to l-lysine in the culture medium. In four of them the mini-Tn5-Gm inactivated the lux genes and was therefore discarded. The other mutant was called rei-2-1, and arbitrary PCR was used to identify the inactivated gene, which corresponded to the ORF that encodes protein PP0382. The sequence of PP0382 was compared with other amino acid sequences deposited in GenBank, revealing that it exhibited homology to hydrolases.
The analysis of the P. putida KT2440 genome revealed that PP0382 forms a cluster with three other genes transcribed in the same direction. The stop codon of PP0382 was found 18 bp upstream from the ATG of PP0383. The translated product of this ORF exhibited homology to monooxygenases and had been annotated as a tryptophan 2-monooxygenase (25, 33). Still, no genetic or biochemical evidence for the assignment was available. The other two ORFs were separated by more than 100 bp and encoded the PpgF protein and a regulator belonging to the AsnC family.
The transcriptional organization of these four genes was studied in the P. putida wild-type strain by RT-PCR. Pseudomonas putida KT2440 was grown with glucose as a carbon source and l-lysine as a nitrogen source. RNA was extracted, and RT-PCR was performed using the appropriate primers. This revealed that the ORFs encoding PP0383 and PP0382 formed an operon (not shown), whereas the other two genes were monocistronic. This suggests that both PP0383 and PP0382 could be involved in the metabolism of l-lysine.
Since rei-2-1 was obtained on a davT mutant background which prevented the metabolism of l-lysine as a carbon source, the potential role of PP0382 and PP0383 in l-lysine metabolism required the construction of mutants in each of these ORFs but in the wild-type genetic background. To construct such mutants, we generated the corresponding knockouts by site-specific inactivation of PP0382 and PP0383, yielding P. putida PSC243 (deficient in PP0382) and P. putida PSC263 (deficient in PP0383). While the wild type grew in M8 minimal medium with l-lysine as the sole carbon and nitrogen source, with a doubling time of 75 ± 3 min, the PSC243 and PSC263 mutants failed to grow in this culture medium, thus confirming their role in l-lysine metabolism. To assess the biochemical function of PP0383 in lysine metabolism, we determined the rate of 14CO2 produced from l-[14C]lysine by the wild-type strain and the mutant PSC263 strain. We found that the rate of 14CO2 evolution by the mutant strain was about 5% of that determined in the wild type, and this was interpreted as demonstrating that PP0383 is lysine monooxygenase. Since this step leads to δ-aminovalerate and the genes in this pathway have been called dav, this gene was denoted as davB. Note that the mutant deficient in PP0383 grew on tryptophan as the sole nitrogen source. The gene encoding PP0382 was called davA to maintain the nomenclature of the genes in the δ-aminovaleric acid pathway and encodes δ-aminovaleramide hydrolase. As expected, these two mutants grew on δ-aminovalerate as the sole carbon and nitrogen source with a generation time similar to that of the wild-type strain. The above series of results support that channeling of l-lysine through the early steps of the AMV branch is critical for the balanced growth of P. putida with l-lysine when supplied as the sole carbon and nitrogen source.
While the davA and davB mutants could not grow on l-lysine as the sole carbon source in M9 medium, they grew on M8 with glucose as a carbon source and with l-lysine as a nitrogen source. The doubling time, however, was significantly slower than that of the parental strain: i.e., 75 ± 5 min for the wild-type strain versus 115 ± 10 min for the davA mutant (P. putida PSC243) and 220 ± 20 min for davB mutant. Thus P. putida apparently has alternative pathways to use l-lysine as a nitrogen source, which agrees with the accumulation of AMA pathway intermediates in the wild type during growth on l-lysine (Fig. 2).
To obtain further evidence of an alternative pathway of l-lysine catabolism, we determined the intracellular concentration of AMV, pipecolate, and AMA in the rei-2, davB, and davA mutants (see Table 1). Generally, increased metabolite concentrations are expected upon the blocking of the flux by deletion of a downstream reaction. While the levels of AMV did not change significantly in the davA and davB mutants, rei-2 AMV levels were almost 20-fold higher than in the wild-type strain (Table 1). The levels of pipecolate and aminoadipate in the rei-2, davA, and davB mutants were similar to those in the wild type. This set of results supports the existing genetic data and provides evidence for the operation of a branch to catabolize l-lysine via pipecolate and aminoadipate. The accumulation of δ-aminovalerate in the davB mutant also supports that this compound can be synthesized via the cadaverine pathway (34).
Mutants unable to metabolize cadaverine grow well on l-lysine.
To test the potential of the cadaverine pathway in the catabolism of l-lysine, we searched for sequences homologous to the l-lysine decarboxylase involved in cadaverine biosynthesis using the cadA and ldc sequences of E. coli (21). Our search identified a single ORF that corresponded to PP4140, whose translated product had 60% similarity to these two proteins. The genetic organization revealed that the gene encoding PP4140 was present on the chromosome of KT2440 as a monocistronic unit. The ORF encoding PP4140 was inactivated using a pCHESI-Ω-Km derivative as described in Materials and Methods. The mutant grew well on l-lysine as the sole carbon and nitrogen source, albeit at a slightly slower rate than the parental strain. This suggests a minor role of this branch in l-lysine metabolism. Nevertheless, its contribution to AMV biosynthesis was still observable, at least in a davA mutant background; because the mutant's AMV levels were similar to those measured in the wild-type strain (Table 1). In the P. putida PSC262 mutant, with an inactivated cadA gene, l-lysine was channeled through the AMV and AMA pathways as deduced from the intracellular accumulation of aminovaleric acid, pipecolate, and aminoadipate (Table 1).
Mutants in the AMA branch.
Based on the above results, it was reasoned that the AMA pathway would be necessary for the efficient use of l-lysine as the sole carbon and nitrogen source. Consequently, we set up a massive screen in which KT2440 was mated with E. coli S17-1λpir(pUT-Km) and Kmr transconjugants were selected on M9 minimal medium with glucose as a carbon source. We then searched for mutants unable to grow on l-lysine and pipecolate as the sole carbon and nitrogen sources. Three mutants called PSC261, PSC265, and PSC264 were found to exhibit insertions in different ORFs. The mutants used l-lysine as a nitrogen source, although they grew more slowly than the wild type (i.e., around a 90-min generation time versus 75 min for the wild-type strain). Subsequently, we confirmed the blockage of the PSC261 and PSC264 mutants in the AMA pathway because when fed with l-lysine they accumulated pipecolate (20- to 40-fold higher levels than the wild type) and failed to produce aminoadipate (Table 1).
In the mutant PSC261, the mini-Tn5 was inserted in an ORF encoding PP5257, which exhibited around 30% identity to oxidases involved in the metabolism of d-amino acids in Burkholderia fungorum and Rhodopseudomonas sphaeroides. This mutant accumulates high levels of pipecolate (around 40-fold), and its phenotype is consistent with the inactivation of the enzyme that converts l-pipecolate into Δ1-piperidine-6-carboxylate. In the mutant PSC264, the inactivated ORF encodes PP5258, whose sequence revealed high identity (54 to 78%) with a number of Δ1-piperidine-6-carboxylate dehydrogenases that catalyze the synthesis of 2-aminoadipate from Δ1-piperidine-6-carboxylate. These results are consistent with the metabolic accumulation of pipecolate and the negligible levels of AMA in this mutant.
The PSC265 mutant failed to accumulate pipecolate (not shown), and the inactivated gene corresponded to dpkA, an NADPH-dependent reductase involved in the conversion of Δ1-piperidine-2-carboxylate into pipecolate. This mutant failed to grow with l-lysine as the sole carbon and nitrogen source, which is in agreement with a recent report by Muramatsu et al. (30) in which a mutant deficient in the synthesis of DpkA of a different P. putida strain also failed to grow on d-lysine or l-lysine as the sole carbon source. The PSC265 mutant grew with l-lysine as a nitrogen source and glucose as a carbon source, although its doubling time (110 ± 5 min) was higher than that of the wild-type strain in the same medium (75 ± 5 min). This series of results supports that utilization of l-lysine as the sole carbon and nitrogen source requires the simultaneous operation of the AMV and AMA pathways.
The two main branches for l-lysine catabolism are linked at the level of α-ketoadipate to glutarate.
To address the question of the interconnection between the AMV and AMA branches, we decided to monitor the incorporation of the l-[13C]lysine label into the carbon skeleton of three amino acids: l-glutamate, l-leucine, and l-alanine. In P. putida KT2440, synthesis of glutamate is mediated by the glutamate synthase and the glutamate dehydrogenase (4) and both enzymes use α-ketoglutarate as a substrate. In P. putida α-ketoglutarate can be synthesized either from aminoadipate, in the AMA pathway (34) (Fig. 1), or through the Krebs cycle. The synthesis of α-ketoglutarate via the AMA pathway guarantees that all carbon atoms in this compound are derived from l-lysine; hence all carbon atoms in l-glutamate should be labeled. When α-ketoglutarate is generated in the Krebs cycle, not all carbon atoms of the ketoacid are labeled, since the cells are using [12C]glucose as a carbon source. Table 2 shows that up to 15.9% of the total glutamate in wild-type cells was completely 13C labeled, thus obtaining the complete and unbroken carbon backbone from [U-13C]lysine (Table 2). Furthermore, we detected a very low fraction of fully labeled in the PSC264 mutant (m6 in Table 2), whereas in the rei-2 mutant the relative level of glutamate with all carbon labeled was similar to that of the wild-type strain. This is in agreement with l-lysine being metabolized via the AMA pathway in rei-2. These results unequivocally show that l-lysine is metabolized via two alternative pathways that lead to central metabolism.
TABLE 2.
15N, 13C labeling in glutamic acid, leucine, and alanine in P. putida growing with l-[U-13C,U-15N2]lysinea
| Labeled amino acid and strain | Mass increase
|
|||||||
|---|---|---|---|---|---|---|---|---|
| m0 | m1 | m2 | m3 | m4 | m5 | m6 | m7 | |
| Glutamic acid | ||||||||
| KT2440 | 0 | 32 | 18 | 17 | 11.5 | 5.2 | 15.9 | |
| PSC264 (amaB) | 0 | 45 | 22 | 25 | 8.5 | 2.3 | 1.0 | |
| rei-2 (davT) | 0 | 33 | 19 | 17 | 10.5 | 3.9 | 12 | |
| Leucine | ||||||||
| KT2440 | 0 | 42 | 19 | 28 | 7 | 3 | 0 | 0 |
| PSC264 (amaB) | 0 | 49 | 19 | 25 | 5 | 0.7 | 0 | 0 |
| rei-2 (davT) | 0 | 44 | 20 | 25 | 6 | 2 | 0 | 0 |
| Alanine | ||||||||
| KT2440 | 0 | 80 | 8.5 | 5 | 5 | 0 | 0 | |
| PSC264 (amaB) | 0 | 87 | 8.3 | 3.5 | 0.6 | 0 | 0 | |
| rei-2 (davT) | 0 | 87 | 8 | 4 | 5 | 0 | 0 | |
Growth conditions and analyses are described in Materials and Methods. No value in m0 is due to +1 unit mass because of the incorporation of 15N; other mass increases correspond to each of the 13C molecules incorporated in the carbon skeletons of these amino acids. Standard deviations were in all cases below 5% of the given value.
The synthesis of l-leucine involves an early condensation of pyruvate and acetyl coenzyme A (acetyl-CoA). Therefore, the 13C label of l-leucine should originate from labeled acetyl-CoA formed from labeled lysine via the AMV pathway (Fig. 1). The patterns of mass increase in l-leucine in the wild-type strain and in the rei-2 mutant were similar (Table 2). This was surprising because the blockage of the AMV pathway should greatly reduce the inflow of 13C from l-lysine and yield a significantly lower fractional abundance of labeled acetyl-CoA. Since there is no direct outflow of the acetyl-CoA from the tricarboxylic acid cycle and since there is no increase in the m6 of glutamate in the rei-2 mutant—which would indicate that the AMA pathway operates down to α-ketoglutarate—our results unequivocally support that l-lysine contributes significantly to the synthesis of acetyl-CoA in the rei-2 mutant. This can be achieved upon the decarboxylation of ketoadipate to glutarate, thus establishing a link between both pathways (see Fig. 1). These results are in accordance with the early studies by Perfetti et al. (35) showing that a different P. putida strain, called P2, when incubated with α-amino [6-14C]adipate formed radioactive [14C]glutarate. In the PSC264 (amaB) mutant, we found a pattern of mass increase similar to that of the wild-type strain, which confirms that the channeling of l-lysine through the AMV pathway leads to acetyl-CoA.
It could also have been possible that in the amaB mutants the balance between catabolism and anapleurosis (i.e., citrate synthase versus phosphoenolpyruvate [PEP] carboxylase) had been disrupted. In other words, acetyl-CoA could have been produced from PEP and pyruvate. In this case, a significant fraction of l-alanine should have been labeled with 13C in the mutant. Nonetheless, almost 80% of l-alanine corresponded to the m1 fraction, indicating the incorporation of 15N but not 13C from l-[15N, 13C]lysine, which supports the finding that acetyl-CoA in the PSC264 mutant is made from the α-ketoglutarate generated in the AMA pathway rather than via PEP.
The above results indicate that l-lysine can be metabolized through alternative pathways that converge at the level of Krebs cycle intermediates and that l-lysine can be used as the sole nitrogen source when any of the branches are operative, but that inactivation of one of them results in l-lysine not being used as a carbon source. To shed light on this issue, we decided to complement the mutants by rescuing wild-type genes from a P. putida library. We chose a mutant in each branch: PSC264 in the AMA pathway and rei-2 in the AMV route. We identified cosmids by hybridization and then transferred them to the corresponding mutant. Cosmids bearing the davD and amaB genes restored all parameters to the corresponding mutants. The answer to the above question could be that slow metabolism of l-lysine through either the AMV or AMA pathway results in an unbalanced C/N ratio in the cells and when a C source, such as glucose, is available such imbalance is overcome. It may also be possible that one pathway for l-lysine catabolism is not enough to generate all the necessary ATP for growth. Another alternative is that accumulation of AMA pathway intermediates exerts a negative feedback control on the AMV pathway and vice versa. We cannot rule out the possibility of some accumulated metabolites being either toxic or capable of altering the redox balances of the cells.
DISCUSSION
The lysine catabolic genes in the genome of P. putida KT2440 are distributed on the chromosome at both sides of the ori of chromosomal replication. This unlinked distribution allows the cells to activate different segments of the l-lysine degradation pathways in response to different nutritional situations. For instance, P. putida can grow on AMV or pipecolate as the sole carbon source when these substrates are available in the culture medium (35, 38); however, the genes encoding the enzymes for the metabolism of l-lysine to AMV (1) or l-lysine into pipecolate are not induced under these conditions (our unpublished results). The physical organization of the genes is, therefore, in compliance with our earlier proposal stating that the l-lysine pathway was made up of a series of patches (38). Our present results showing that l-lysine is metabolized through two main interconnected branches contrast with early results in other P. putida strains in which the l-lysine and d-lysine pathways were proposed to be independent.
The interconnection of pathways in KT2440 is supported by the accumulation of d-lysine pathway metabolites in mutants blocked in this pathway upon growth on l-lysine. Further support is obtained from genetic analyses showing that mutants with mutation in either of the branches failed to grow with l-lysine as the sole carbon and nitrogen source. l-Lysine racemase is a periplasmic enzyme, whereas the rest of the catabolic pathways are cytoplasmic. The action of this enzyme prior to the transport of l-lysine into the cells could be critical to determine the metabolic fate of this amino acid. Once in the cytoplasm, l-lysine and d-lysine follow their pathways, but the intracellular detection of AMV, pipecolate, and aminoadipate indicates that the metabolism of these chemicals represents the bottleneck step in each of the branches in P. putida. Our results tracing the fate of 13C in l-lysine revealed that the two pathways can be linked at a lower level via a ketoadipate-decarboxylase that converts ketoadipate into glutarate, which is then channeled to acetyl-CoA. Evidence for this link is derived from 13C labeling in l-leucine and also from the early studies of Perfetti et al. (35) that showed the synthesis of glutarate from aminoadipate. It then follows that l-lysine metabolism involves interconnected branches and fluxes that can be modulated to supply enough carbon and nitrogen for growth.
In a recent report, Muramatsu et al. (30) described that a P. putida dpkA mutant grew very slowly with l-lysine as the sole carbon and nitrogen source. This is in accordance with our results showing that mutations in the d-lysine pathway prevent the metabolism of l-lysine, and, therefore, our observation of multichanneling may be more widespread than was initially thought. Given that in this study a number of genes for lysine metabolism were found (davB, davA, amaA, dkpA, amaB, and cadA) and that absence of the AMV pathway was reported for P. aeruginosa, we decided to test whether the genes identified in this study, and others identified in our laboratory before for l-lysine uptake, are present in strains of the genus Pseudomonas whose genomes have been sequenced (3, 33, 40; www.sanger.org). We carried out BLAST comparisons of the identified sequences in P. putida versus the genomes of the P. aeruginosa, P. fluorescens Pf01, and Pseudomonas syringae pv. syringae (Table 3). In all tested Pseudomonas strains, the different uptake systems identified by Revelles et al. (38) before regarding transport of l-lysine were present. These inducible systems are likely to be the ones responsible for the high rate of lysine uptake described by Miller and Rodwell (27) in P. putida. We found that in the genome of P. aeruginosa, davA and davB were absent (in agreement with the lack of l-lysine catabolism via the early steps of the AMV pathway); however, the cadA gene and those of the pipecolate pathway were present (Table 2). This is in agreement with the proposal by Rahman and Clarke (36) that this species can use the cadaverine pathway for l-lysine metabolism. But whether the strain uses the pipecolate pathway or not will depend on the strain exhibiting a racemase for the conversion of l-lysine into d-lysine for its subsequent metabolism. The racemase gene has not yet been identified in the genus Pseudomonas, although its activity has been measured in cell extracts of P. putida cells growing on l-lysine (19). In contrast, the cadA gene was not found in P. syringae, although the genes for the AMV and AMA pathways were present. Finally, in P. fluorescens Pf0-1, both the cadA gene and those of the AMV and AMA pathways were found. It remains to be explored whether l-lysine catabolism involves the interconnection of the l- and d-lysine pathways in P. syringae and P. fluorescens strains. It is of interest to note that certain Pseudomonas sp. strains we examined are able to produce cadaverine from l-lysine; however, its potential role in response to external acid pH has not been analyzed, in contrast with the situation in E. coli (32).
TABLE 3.
Conservation of lysine catabolic genes among Pseudomonas species
| Gene name | Protein function | % of similarity to P. putida KT2440a
|
||
|---|---|---|---|---|
| P. aeruginosa PAO1 | P. syringae sv.syringae | P. fluorescens PfO-1 | ||
| davD (PP0213) | Glutaric semialdehyde dehydrogenase | 91 (PA0265) | 91 (PSPTO0300) | 91 (PflO2004773) |
| davT (PP214) | δ-Aminovalerate aminotransferase | 89 (PA0266) | 92 (PSPTO0301) | 92 (PflO2004774) |
| davB (PP0383) | Lysine monooxygenase | None | 91 (PSPTO0518) | 91 (PflO2005316) |
| davA (PP0382) | δ-Aminovaleramide amidohydrolase | None | 75 (PSPTO0517) | 73 (PflO2005317) |
| amaA (PP5257) | l-Pipecolate oxidase | 86 (PA1027) | 90 (PSPTO1891) | 84 (PflO2000241) |
| amaB (PP5258) | Δ1-Piperideine-6-carboxylate dehydrogenase | 79 (PA1028) | 73 (PSPTO1892) | 75 (PluO2000242) |
| cadA (PP4140) | Lysine decarboxylase | 88 (PA1818) | None | 90 (PluO2001874) |
| Periplasmic protein (PP0282) | Amino acid ABC transporter | 86 (PA5153) | 82 (PSPTO5358) | 62 (PfluO2003365) |
| Periplasmic protein (PP4486) | Basic amino acid ABC transporter | 94 (PA0892) | 92 (PSPTO1830) | 92 (PfluO2003361) |
The number in parentheses refers to the corresponding polypeptide number deduced from the annotated genome sequences of P. aeruginosa, P. syringae, and P. fluorescens.
The complexity of l-lysine metabolism in P. putida is not unique since in eukaryotes such as fungi, plants, and animals this amino acid can also be metabolized through different pathways, or its degradation can be tissue specific in the case of higher eukaryotes (16, 17, 26). In Penicillium chrysogenum l-lysine catabolism can proceed via a racemase leading to d-lysine and subsequent catabolism to yield 2-aminoadipate via l-pipecolate. Biosynthesis of aminoadipate can take also place via an l-lysine:γ-ketoglutarate-6-aminotransferase, which converts l-lysine into Δ1-piperidine-6-carboxylate that is catabolized to 2-aminoadipate (10). This pathway is also found in a soil bacterium such as Flavobacterium lutescens (15) and would add a third connection point between the two pathways described here. However, no evidence for this activity is available for P. putida or P. aeruginosa (13), and the results obtained here with mutants in the early steps of each pathway (davB and dpkA) indicate that if it exists, its contribution to lysine metabolism in KT2440 is minor.
In plants, l-lysine catabolism involves the action of a bifunctional enzyme with lysine:α-ketoglutarate reductase activity and saccharopine dehydrogenase (LKR/SDH) that catalyzes the first two steps in a catabolic pathway leading to 2-aminoadipate semialdehyde that is subsequently transformed to 2-aminoadipate (20). Two documented functions of the 2-aminoadipate pathway in plants are to balance lysine levels and also to regulate carbon/nitrogen partition in response to abiotic stresses (16, 17). The LKR/SDH enzymes also operate in animals (41). In mammals, lysine catabolism is stimulated in brain tissues and is apparently used to generate glutamate, which regulates signal transmissions via glutamate receptors (34, 37). Defects in the LKR/SDH gene in humans are associated with a severe genetic disorder called familial hyperlysinemia, which is associated with mentally retarded patients (23). The availability of bacterial enzymes capable of preventing l-lysine accumulation can be of interest for medical treatments.
Acknowledgments
We would like to thank the three anonymous reviewers whose comments have enlightened this article. We thank Carmen Lorente and M. Mar Fandila for secretarial assistance.
This study was supported by grants from the CICYT (BIO 2003-00515), the European Commission (QLK3-CT-2002-01923), and PAI from Junta de Andalucía to group CIV191.
REFERENCES
- 1.Abril, M.-A., C. Michán, K. N. Timmis, and J. L. Ramos. 1989. Regulator and enzyme specificities of the TOL plasmid-encoded upper pathway for degradation of aromatic hydrocarbons and expansion of the substrate range of the pathway. J. Bacteriol. 171:6782-6790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Basic, A., S. F. Moody, and A. E. Clarke. 1986. Structural analysis of secreted root slime from maize (Zea mays L.). Plant Physiol. 80:771-777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buell, C. R., V. Joardar, M. Lindeberg, J. Selengut, I. T. Paulsen, M. L. Gwinn, R. J. Dodson, R. T. Deboy, A. S. Durkin, J. F. Kolonay, R. Madupu, S. Daugherty, L. Brinkac, M. J. Beanan, D. H. Haft, W. C. Nelson, T. Davidsen, N. Zafar, L. Zhou, J. Lu, Q. Yuan, H. Khouri, N. Fedorova, B. Tran, D. Russell, K. Berry, T. Utterback, S. E. Van Aken, T. V. Feldblyum, M. D'Ascenzo, W. L. Deng, A. R. Ramos, J. R. Alfano, S. Cartinhour, A. K. Chatterjee, T. P. Delaney, S. G. Lazarowitz, G. B. Martin, D. J. Schneider, X. Tang, C. L. Bender, O. White, C. M. Fraser, and A. Collmer. 2003. The complete genome sequence of the Arabidopsis and tomato pathogen Pseudomonas syringae pv. tomato DC3000. Proc. Natl. Acad. Sci. USA 100:10181-10186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Caballero, A., A. Esteve-Núñez, G. J. Zylstra, and J. L. Ramos. 2005. Assimilation of nitrogen from nitrite and trinitrotoluene in Pseudomonas putida JLR11. J. Bacteriol. 187:396-399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cao, X., J. Kolonay, Jr., K. A. Saxton, and R. A. Hartline. 1993. The OCT plasmid encodes D-lysine membrane transport and catabolic enzymes in Pseudomonas putida. Plasmid 30:83-89. [DOI] [PubMed] [Google Scholar]
- 6.Chang, Y. F., and E. Adams. 1977. Glutarate semialdehyde dehydrogenase of Pseudomonas. J. Biol. Chem. 252:7979-7986. [PubMed] [Google Scholar]
- 7.Chang, Y. F., and E. Adams. 1977. Factors influencing growth on L-lysine by Pseudomonas. J. Biol. Chem. 252:7987-7991. [PubMed] [Google Scholar]
- 8.Dauner, M., and U. Sauer. 2000. GC-MS analysis of amino acids rapidly provides rich information for isotopomer balancing. Biotechnol. Prog. 16:642-649. [DOI] [PubMed] [Google Scholar]
- 9.de Lorenzo, V., M. Herrero, U. Jakubzik, and K. N. Timmis. 1990. Mini-Tn5 transposon derivatives for insertion mutagenesis, promoter probing, and chromosomal insertion of cloned DNA in gram-negative eubacteria. J. Bacteriol. 172:6568-6572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Esmahan, C., E. Alvarez, E. Montenegro, and J. F. Martín. 1994. Catabolism of lysine in Penicillium chrysogenum leads to formation of 2-aminoadipic acid, a precursor of penicillin biosynthesis. Appl. Environ. Microbiol. 60:1705-1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Espinosa-Urgel, M., and J.-L. Ramos. 2001. Expression of a Pseudomonas putida aminotransferase involved in lysine catabolism is induced in the rhizosphere. Appl. Environ. Microbiol. 67:5219-5224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fischer, E., and U. Sauer. 2003. Metabolic flux profiling of Escherichia coli mutants in central carbon metabolism using GC-MS. Eur. J. Biochem. 270:880-891. [DOI] [PubMed] [Google Scholar]
- 13.Forthegill, J. L., and J. R. Guest. 1977. Catabolism of L-lysine by Pseudomonas aeruginosa. J. Gen. Microbiol. 99:139-155. [DOI] [PubMed] [Google Scholar]
- 14.Franklin, F. C. H., M. Bagdasarian, M. M. Bagdasarian, and K. N. Timmis. 1981. Molecular and functional analysis of the TOL plasmid pWW0 from Pseudomonas putida and cloning of genes for the entire regulated aromatic ring meta-cleavage pathway. Proc. Natl. Acad. Sci. USA 78:7458-7462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fuiji, T., T. Narita, H. Agematu, N. Agata, and K. Isshiki. 2000. Characterization of L-lysine-6-aminotransferase and its structural gene in Flavobacterium lutescens IF 03084. J. Biochem. 128:391-397. [DOI] [PubMed] [Google Scholar]
- 16.Galili, G. 2002. New insights into the regulation and functional significance of lysine metabolism in plants. Annu. Rev. Plant Physiol. Mol. Biol. 53:27-43. [DOI] [PubMed] [Google Scholar]
- 17.Galili, G., G. Tang, X. Zhu, and B. Gakiere. 2001. Lysine catabolism: a stress and development super-regulated metabolic pathway. Curr. Opin. Plant Biol. 4:261-266. [DOI] [PubMed] [Google Scholar]
- 18.Herrero, M., V. de Lorenzo, and K. N. Timmis. 1990. Transposon vectors containing non-antibiotic resistance selection markers for cloning and stable chromosomal insertion of foreign genes in gram-negative bacteria. J. Bacteriol. 172:6557-6567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ichihara, A., S. Furiya, and M. Suda. 1960. Metabolism of L-lysine by bacterial enzymes. J. Biochem. 48:277-283. [DOI] [PubMed] [Google Scholar]
- 20.Karchi, H., O. Saul, and G. Galili. 1994. Lysine synthesis and catabolism are co-ordinately regulated during tobacco seed development. Proc. Natl. Acad. Sci. USA 91:2577-2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kikuchi, Y., M. Koyima, T. Tanaka, Y. Takatsuka, and Y. Kamio. 1997. Characterization of a second lysine decarboxylase isolate from Escherichia coli. J. Bacteriol. 179:4486-4492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Llamas, M. A., J. J. Rodríguez-Herva, R. E. W. Hancock, W. Bitter, J. Tommasen, and J. L. Ramos. 2003. Role of the Pseudomonas putida tol-oprL gene products in uptake of solutes through the cytoplasmic membrane. J. Bacteriol. 185:4707-4716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Markovitz, P. J., D. T. Chuang, and R. P. Cox. 1984. Familial hyperlysinemias: purification and characterization of the bifunctional aminoadipic semialdehyde synthase with lysine-ketoglutarate reductase and saccharopine dehydrogenase activities. J. Biol. Chem. 259:11643-11646. [PubMed] [Google Scholar]
- 24.Marqués, S., J. L. Ramos, and K. N. Timmis. 1993. Analysis of the mRNA structure of the Pseudomonas putida TOL meta fission pathway operon around the transcription initiation point, the xylTE and the xylFJ region. Biochim. Biophys. Acta 1216:227-237. [DOI] [PubMed] [Google Scholar]
- 25.Martins dos Santos, V. A. P., S. Heim, E. R. B. Moore, M. Stranz, and K. N. Timmis. 2004. Insights into the genomic basis of niche specificity of Pseudomonas putida KT2440. Environ. Microbiol. 6:1264-1286. [DOI] [PubMed] [Google Scholar]
- 26.Meghal, S. K., H. S. Cheung, R. M. O'Neal, and R. E. Koeppe. 1966. Metabolism of D, L-lysine-2- and -6-14C in rats and dogs. J. Biol. Chem. 241:2622-2625. [PubMed] [Google Scholar]
- 27.Miller, D. L., and V. W. Rodwell. 1971. Metabolism of basic amino acids in Pseudomonas putida. Properties of the inducible lysine transport system. J. Biol. Chem. 246:14742-14747. [PubMed] [Google Scholar]
- 28.Molina, L., C. Ramos, E. Duque, M. C. Ronchel, J. M. García, L. Wyke, and J. L. Ramos. 2000. Survival of Pseudomonas putida KT2440 in soil and in the rhizosphere of plants under greenhouse and environmental conditions. Soil Biol. Biochem. 32:315-321. [Google Scholar]
- 29.Mozafar, A. 1992. Effect of Pseudomonas fluorescens on the root exudates of tomato mutants differently sensitive to Fe chlorosis. Plant Soil 144:167-176. [Google Scholar]
- 30.Muramatsu, H., H. Mihara, R. Kakutani, M. Yasuda, M. Veda, T. Kurihara, and N. Esaki. 2005. The putative malate/lactate dehydrogenase from Pseudomonas putida is a NADPH-dependent Δ1-piperideine-2-carboxylate/Δ1-pyrroline-2-carboxylate reductase involved in the catabolism of D-lysine and D-proline. J. Biol. Chem. 280:5329-5335. [DOI] [PubMed] [Google Scholar]
- 31.Nakazaka, T. 2002. Travels of a Pseudomonas, from Japan around the world. Environ. Microbiol. 4:782-786. [DOI] [PubMed] [Google Scholar]
- 32.Neely, M. N., and E. R. Olson. 1996. Kinetics of expression of the Escherichia coli cad operon as a function of pH and lysine. J. Bacteriol. 178:5522-5528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nelson, K. E., C. Weinel, I. T. Paulsen, R. J. Dodson, H. Hilbert, V. A. P. Martins dos Santos, D. E. Fouts, S. R. Gill, M. Pop, M. Holmes, L. Brinkac, M. Beanan, R. T. DeBoy, S. Daugherty, J. Kolonay, R. Madupu, W. Nelson, O. White, J. Peterson, H. Khouri, I. Hance, P. Chris Lee, E. Holtzapple, D. Scanlan, K. Tran, A. Moazzez, T. Utterback, M. Rizzo, K. Lee, D. Kosack, D. Moestl, H. Wedler, J. Lauber, D. Stjepandic, J. Hoheisel, M. Straetz, S. Heim, C. Kiewitz, J. Eisen, K. N. Timmis, A. Düsterhöft, B. Tümmler, and C. M. Fraser. 2002. Complete genome sequence and comparative analysis of the metabolically versatile Pseudomonas putida KT2440. Environ. Microbiol. 4:799-808. [DOI] [PubMed] [Google Scholar]
- 34.Papes, F., E. L. Kemper, G. Cord-Neto, F. Langone, and P. Arruda. 1999. Lysine degradation through saccharopine pathway in mammals: involvement of both bifunctional and monofunctional lysine-degrading enzymes in mouse. Biochem. J. 344:555-563. [PMC free article] [PubMed] [Google Scholar]
- 35.Perfetti, R., R. J. Campbell, J. Titus, and R. A. Hartline. 1972. Catabolism of pipecolate to glutamate in Pseudomonas putida. J. Biol. Chem. 247:4089-4095. [PubMed] [Google Scholar]
- 36.Rahman, M., and P. H. Clarke. 1980. Gene and enzymes of lysine catabolism by Pseudomonas aeruginosa. J. Gen. Microbiol. 116:357-369. [DOI] [PubMed] [Google Scholar]
- 37.Rao, V. V., X. Pan, and C. Y. Chang. 1992. Development changes in L-lysine-ketoglutarate reductase in rat brain and liver. Comp. Biochem. Physiol. 103B:221-224. [DOI] [PubMed] [Google Scholar]
- 38.Revelles, O., M. Espinosa-Urgel, S. Molin, and J. L. Ramos. 2004. The davDT operon of Pseudomonas putida, involved in lysine catabolism, is induced in response to the pathway intermediate δ-aminovaleric acid. J. Bacteriol. 186:3439-3446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 40.Stover, C. K., X. Q. Pham, A. L. Erwin, S. D. Mizoguchi, P. Warrener, M. J. Hickey, F. S. Brinkman, W. O. Hufnagle, D. J. Kowalik, M. Lagrou, R. L. Garber, L. Goltry, E. Tolentino, S. Westbrock-Wadman, Y. Yuan, L. L. Brody, S. N. Coulter, K. R. Folger, A. Kas, K. Larbig, R. Lim, K. Smith, D. Spencer, G. K. Wong, Z. Wu, I. T. Paulsen, J. Reizer, M. H. Saier, R. E. Hancock, S. Lory, and M. V. Olson. 2000. Complete genome sequence of Pseudomonas aeruginosa PA01, an opportunistic pathogen. Nature 406:959-964. [DOI] [PubMed] [Google Scholar]
- 41.Zhu, X., G. Tang, and G. Galili. 2002. The activity of the Arabidopsis bifunctional lysine-ketoglutarate reductase/saccharopine dehydrogenase enzyme of lysine catabolism is regulated by functional interaction between its two enzyme domains. J. Biol. Chem. 272:49655-49661. [DOI] [PubMed] [Google Scholar]