Abstract
Pyrococcus furiosus and Pyrococcus woesei grow optimally at temperatures near 100°C and were isolated from the same shallow marine volcanic vent system. Hybridization of genomic DNA from P. woesei to a DNA microarray containing all 2,065 open reading frames (ORFs) annotated in the P. furiosus genome, in combination with PCR analysis, indicated that homologs of 105 ORFs present in P. furiosus are absent from the uncharacterized genome of P. woesei. Pulsed-field electrophoresis indicated that the sizes of the two genomes are comparable, and the results were consistent with the hypothesis that P. woesei lacks the 105 ORFs found in P. furiosus. The missing ORFs are present in P. furiosus mainly in clusters. These clusters include one cluster (Mal I, PF1737 to PF1751) involved in maltose metabolism and another cluster (PF0691 to PF0695) whose products are thought to remove toxic reactive nitrogen species. Accordingly, it was found that P. woesei, in contrast to P. furiosus, is unable to utilize maltose as a carbon source for growth, and the growth of P. woesei on starch was inhibited by addition of a nitric oxide generator. In P. furiosus the ORF clusters not present in P. woesei are bracketed by or are in the vicinity of insertion sequences or long clusters of tandem repeats (LCTRs). While the role of LCTRs in lateral gene transfer is not known, the Mal I cluster in P. furiosus is a composite transposon that undergoes replicative transposition. The same locus in P. woesei lacks any evidence of insertion activity, indicating that P. woesei is a sister or even the parent of P. furiosus. P. woesei may have acquired by lateral gene transfer more than 100 ORFs from other organisms living in the same thermophilic environment to produce the type strain of P. furiosus.
The shallow marine volcanic vents of Vulcano Island, Italy, have proven to be a rich source of thermophilic archaea. More than a dozen organisms have been isolated from this location (2, 22, 26, 27, 34, 50, 57, 58, 62, 63, 68), including two species of Pyrococcus, Pyrococcus furiosus (26) and Pyrococcus woesei (68). P. furiosus was the first of these organisms to be discovered in the Vulcano Island ecosystem and is now one of the best studied of the hyperthermophilic archaea. It grows optimally at temperatures near 100°C and utilizes peptides and carbohydrates as carbon and energy sources, generating organic acids and hydrogen or, if elemental sulfur is present, hydrogen sulfide as end products. The physiology of P. woesei appears to be very similar to that of P. furiosus. These organisms have the same growth temperature range and use the same carbon sources and terminal electron acceptors (26, 68). The genome of P. furiosus has been sequenced. It is 1.9 Mb long and contains more than 2,000 open reading frames (ORFs) (51). Although the genome of P. woesei has not been sequenced, 19 protein sequences and two RNA sequences are available in public databases, and all of these sequences exhibit at least 99% identity to their homologs in P. furiosus (5, 13-15, 16-19, 21, 35, 39, 41, 52, 64, 70); this includes the 16S rRNA sequences, which are 100% identical (39).
The question arises as to how these two Pyrococcus species originated and what the evolutionary relationship between them is. This is an intriguing issue given the fact that these organisms are found in the same geothermal environment. Indeed, because of the identity of their 16S rRNA sequences, the striking similarities in their physiological properties, and the disruption of a putative Na+/H+ antiporter gene (napA, PF0275) by an insertion sequence (IS), it was recently concluded that P. woesei should be classified as a subspecies of P. furiosus (39). To evaluate the overall genetic differences between the two organisms, we utilized DNA microarrays based on the complete genome of P. furiosus (55, 56). The fundamental question to be addressed is, does P. woesei contain homologs of all the genes found in P. furiosus? Moreover, if the answer is no, what are the consequences of any differences in terms of evolution, physiology, and metabolism? So far, genome-based DNA microarray comparisons have been restricted to mesophilic bacteria, where the goals were to determine the presence or absence of genes associated with pathogenic and nonpathogenic strains (10, 28, 33, 42, 49, 53, 67). We show here that the results of DNA microarray comparisons allow testable predications to be made about physiology and metabolism. In addition, the whole-genome approach also provides an opportunity to gain insight into interactions between members of the Vulcano Island environment. This may provide a means to assess global genetic exchanges that potentially occurred at a time when primitive archaea lived on a hot earth and acquired or disseminated genetic innovation, such as stress resistance or utilization of a carbon source, for survival.
MATERIALS AND METHODS
Array design and DNA preparation.
Microarray slides were designed and processed as previously described (55, 56). P. furiosus DSM 3638 and P. woesei DSMZ 3773 were grown in 1-liter culture bottles using maltose or peptides as the carbon source (65). Cells were harvested at the end of exponential growth, and genomic DNA (gDNA) was isolated by a phenol-chloroform protocol (54).
Preparation of labeled DNA and hybridization conditions.
Labeled DNA was prepared using a Prime-It Fluor kit (Stratagene, La Jolla, CA) according to the manufacturer's instructions, except that a dUTP-aminoallyl tag (Sigma, St. Louis, MO) was used. Tagged DNA products and Alexa-labeled DNA were purified using a QIAquick PCR purification kit (QIAGEN, Valencia, CA). Aminoallyl-labeled DNA was coupled with Alexa dyes 488, 546, 594, and 647 (Molecular Probes, Eugene, OR) according to the manufacturer's instructions. Labeled genomic DNA samples were hybridized to a microarray slide containing PCR products for all 2,065 ORFs in the P. furiosus genome (55, 56) using a Genetac hybridization station (Genomic Solutions, Ann Arbor, MI) for 14 h at 65°C. The slides were then washed for 20 s each in 2× sodium chloride-sodium citrate buffer (SSC) containing 0.1% Tween 20, 0.2× SSC containing 0.1% Tween 20, and 0.2× SSC, rinsed in distilled water, and blown dry with compressed air. The fluorescence intensities of the four dyes, which represented one experiment in triplicate with one control, were measured using a Scan Array 5000 slide reader (Perkin-Elmer, Boston, MA) with the appropriate laser and filter settings.
Data analysis.
Spots were identified and quantitated using the Gleams software package (Nutec, Houston, TX). The relative fluorescence intensities were averaged from three sets of microarray data generated from slides that contained the P. furiosus genome printed in triplicate (a total of nine arrays). The values for spots that gave negative fluorescence intensities with P. woesei DNA (78 of the 2,065 spots examined) were converted to an arbitrary value of 200 U, and the values for spots that gave negative fluorescence intensities with P. furiosus DNA (16 of 2,065 spots) were converted to the minimum detection limit (2,000 U). Instead of eliminating negative P. furiosus numbers, the conversions were used to keep positive P. woesei hybridizations for the spots even though the P. furiosus control did not. The fluorescence intensities collected from the P. woesei data set were divided into the P. furiosus data set for fluorescence intensities. The resulting values were then multiplied by −1 and added to 100. Values less than 98 were interpreted to indicate that a P. furiosus gene did not have a homolog in the P. woesei genome (corresponding to where the P. woesei fluorescence intensity was 50% of the P. furiosus fluorescence intensity). An ORF analysis was conducted using the InterProScan tool (version 3.3; http://www.ebi.ac.uk/interpro/). ISs were analyzed using ISfinder (http://www-is.biotoul.fr/). Homologs of P. furiosus ORFs in the genomes of Pyrococcus abyssi (9, 24), Pyrococcus horikoshii (31, 40), and Thermococcus kodakaraensis were defined as ORFs which encoded proteins that exhibited at least 75% sequence similarity over at least 75% of the protein length when the data were analyzed by BLASTP.
PCR and sequencing.
Primers were synthesized and PCR products were sequenced by the University of Georgia Integrated Biotech Laboratories (http://www.ors.uga.edu/ibl/index.html/). PCR analyses were carried out in triplicate with different annealing temperatures and positive controls. The primers used to amplify the Mal I locus were forward primer 5′-AAT ACG CTC ATA GAA TCA AAG-3′ and reverse primer 5′-CCC TAT GAC TGC CTT TGG ATT-3′. All PCR reagents were obtained from Stratagene, and previously described standard molecular biology techniques were used (54).
Growth studies.
The two types of maltose used in the growth studies were 95% grade (M2250; Sigma) and 99% grade (M9171; Sigma). Roussin's black salt (RBS) {Na+[Fe4S3(NO)7]−} (8) was provided by Martin Hughes (King's College, London, United Kingdom). The dried powder was suspended in degassed water to a final concentration of approximately 0.9 μM prior to use.
PFGE.
The pulsed-field gel electrophoresis (PFGE) procedure was adapted from the procedure described by Borges et al. (6). Gel plugs were made by suspending cells of P. furiosus or P. woesei to a concentration of 5 × 109 cells/ml in 1% (wt/vol) agarose. The plugs were incubated with 0.1 M EDTA, 1% (wt/vol) cetyltrimethylammonium bromide, 1% (wt/vol) sodium dodecyl sulfate, 1% (vol/vol) Triton X-200, and proteinase-K (2.0 mg/ml) for 24 h at 42°C. The plugs were washed twice with 10 mM Tris buffer (pH 8.0) containing 1 mM EDTA (TE buffer) for 15 min at 4°C, incubated with 1 mM phenylmethylsulfonyl fluoride in 10 mM TE buffer at 23°C for 2 h, washed with 10 mM TE buffer at 23°C for 2 h, and equilibrated with 1× restriction enzyme buffer (New England Biolabs, Ipwich, MA) for 20 min at 4°C. Using fresh 1× restriction enzyme buffer, each plug was incubated with 25 U of NotI (New England Biolabs) for 16 h at 37°C. The plugs were inserted into a 1% (wt/vol) agarose gel and electrophoresed using the CHEF-DR II system (Bio-Rad, Hercules, CA) for 20 h at 200 V with an alternating field (90°/90 s for 1 h and 90° from 1 to 25 s for 19 h). DNA bands were stained with ethidium bromide.
RESULTS
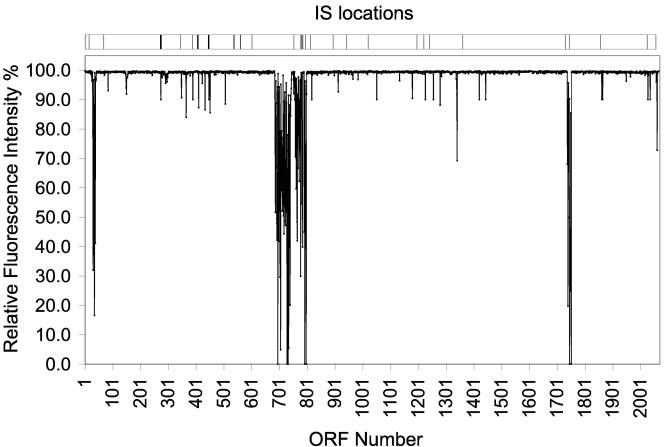
gDNA from P. woesei was hybridized to the DNA microarray containing spots representing the 2,065 ORFs annotated in the P. furiosus genome. The fluorescence intensities were compared directly with those obtained using gDNA from P. furiosus. The results showed that close homologs of 1,890 (92%) of the 2,065 P. furiosus ORFs were present in the P. woesei genome. This included all 21 sequences of P. woesei genes available in the NCBI database (http://www.ncbi.nlm.nih.gov/) that are known to be (virtually) identical as determined by direct sequence comparisons. The remaining 175 P. furiosus ORFs (representing 8% of the P. furiosus genome) were not detected in P. woesei DNA at a significant level by microarray analysis, implying that close homologs of these genes are not present in the P. woesei genome. It is possible that some genes are apparently absent because they are highly divergent. However, this seems unlikely given the almost complete identity of all genes (and proteins) examined so far in the two species. The arrangement of the proposed missing ORFs on the P. furiosus genome is shown in Fig. 1. It is readily apparent that one striking feature is that in P. furiosus the missing ORFs form clusters or ORF islands. This pattern is not unlike the previously proposed (25) suggestion that these ORFs form islands or gene cassettes because of functional interactions. If P. woesei is as closely related as suspected and synteny is conserved, then the regions of missing ORFs are restricted to specific areas of the genome.
FIG. 1.
Hybridization of genomic DNA from P. woesei to the P. furiosus DNA microarray. The data were normalized as described in Materials and Methods. The abscissa indicates P. furiosus ORFs (ORFs 1 to 2065). The bar at the top indicates the positions of P. furiosus insertion sequences.
The veracity of the microarray results was assessed by direct PCR analysis using primer pairs covering 137 of the 175 ORFs proposed by the microarray results (78% coverage). PCR products of the expected size were obtained for all 137 ORFs analyzed using gDNA from P. furiosus, but only 32 ORFs yielded PCR products when P. woesei gDNA was used. This analysis therefore confirmed the absence in the P. woesei genome of homologs of 105 P. furiosus ORFs (or 77% of the ORFs indicated by the DNA microarray analysis). Table 1 lists the P. furiosus ORFs missing from the P. woesei genome. Of the 105 ORFs, 93 were present in 20 gene clusters consisting of two or more genes, and these clusters are indicated in Table 1 (in which shading indicates potential operons). Analysis by InterPro of the amino acid sequences revealed that 37 of the 105 ORFs (35%) encode proteins with unknown functions that have no known homologs in other organisms. Conversely, 407 of the 444 ORFs that are annotated as hypothetical ORFs in P. furiosus appear to be present in the P. woesei genome according to the DNA microarray analysis. This suggests that these hypothetical ORFs in P. furiosus do indeed encode proteins. The results of a BLASTP analysis of the 105 P. furiosus ORFs not present in P. woesei and ORFs of three closely related species, P. abyssi (9, 24), P. horikoshii (31, 40), and T. kodakaraensis (29), are shown in Table 1. Only seven of the P. furiosus ORFs have homologs in the genomes of all three species, and only 11 ORFs have homologs in two of the three organisms. In P. furiosus, these ORFs are scattered in the genome, except for the gene cluster containing PF0764 to PF0770, a homolog of which is found in P. abyssi but not in the other two organisms.
TABLE 1.
P. furiosus ORFs that lack homologs in the P. woesei genome based on DNA microarray and PCR analysesa
| ORFb | A | B | C | InterPRO annotation | ORFb | A | B | C | InterPRO annotation | |
|---|---|---|---|---|---|---|---|---|---|---|
| PF0025 | * | * | * | Glutamine amidotransferase II | PF0735 | No InterPRO entry | ||||
| PF0026 | * | * | * | DNA polymerase, beta region | PF0737 | No InterPRO entry | ||||
| PF0029 | No InterPRO entry | PF0738 | S-Adenosylmethionine binding protein | |||||||
| PF0030 | No InterPRO entry | PF0739 | Bacterial regulator, AsnC/Lrp | |||||||
| PF0031 | PLP enzyme, beta subunit | PF0740 | * | Copper-transporting ATPase | ||||||
| PF0032 | No InterPRO entry | PF0742 | * | Ferritin and Dps | ||||||
| PF0034 | * | No InterPRO entry | PF0743 | Coenzyme A binding domain | ||||||
| PF0035 | * | ABC transporter | PF0744 | * | ABC transporter | |||||
| PF0037 | ARM repeat fold | PF0758 | * | * | HEPN, nucleotide-binding | |||||
| PF0038 | * | * | Beta-lactamase-like | PF0760 | * | * | Transposase, IS605 OrfB | |||
| PF0041 | * | * | * | Elongator protein 3/MiaB/NifB | PF0762 | * | * | No InterPRO entry | ||
| PF0151 | * | Glycoside hydrolase, family 5 | PF0763 | No InterPRO entry | ||||||
| PF0152 | * | * | Protein of unknown function | PF0764 | * | * | * | Archaeal ATPase | ||
| PF0154 | * | * | * | Glutamine amidotransferase II | PF0765 | * | 6-Phosphogluconate DH, C terminal | |||
| PF0365 | Protein of unknown function | PF0766 | * | Oxidoreductase, N terminal | ||||||
| PF0366 | * | * | * | No InterPRO entry | PF0767 | * | Deg T aminotransferase | |||
| PF0685 | Protein of unknown function | PF0768 | * | Transferase hexapeptide repeat | ||||||
| PF0687 | Transcription factor TFIIB | PF0769 | * | Glycosyl transferase, family 2 | ||||||
| PF0689 | Prismane-like | PF0770 | * | UTP-glucose-1-Pi transferase | ||||||
| PF0691 | * | Lambda repressor-like | PF0772 | * | DNA polymerase, beta-like | |||||
| PF0692 | * | Prismane | PF0773 | No InterPRO entry | ||||||
| PF0693 | SirA-like | PF0775 | No InterPRO entry | |||||||
| PF0694 | Flavodoxin/NO synthase | PF0776 | * | No InterPRO entry | ||||||
| PF0695 | * | Protein of unknown function | PF0777 | No InterPRO entry | ||||||
| PF0697 | Sugar transporter superfamily | PF0781 | * | * | Nucleotide binding protein, PINc | |||||
| PF0698 | No InterPRO entry | PF0783 | * | No InterPRO entry | ||||||
| PF0701 | * | * | No InterPRO entry | PF0790 | No InterPRO entry | |||||
| PF0702 | * | * | Peptidase M24 | PF0791 | Glycosyl transferase, group 1 | |||||
| PF0703 | No InterPRO entry | PF0792 | No InterPRO entry | |||||||
| PF0704 | Protein of unknown function | PF0794 | UDP-N-Ac gluc.-2-epimerasec | |||||||
| PF0705 | Cytochrome c biogenesis | PF0795 | Glycosyl transferase, group 1 | |||||||
| PF0706 | Protein of unknown function | PF0796 | Transferase hexapeptide repeat | |||||||
| PF0707 | 4-Oxalocrotonate tautomerase | PF0797 | No InterPRO entry | |||||||
| PF0708 | Extrusion protein MatE | PF0798 | Glycosyl transferase, family 2 | |||||||
| PF0712 | No InterPRO entry | PF1337 | * | * | TENA/THI-4 protein | |||||
| PF0713 | * | Winged helix DNA binding | PF1339 | Protein of unknown function | ||||||
| PF0715 | Nitroreductase | PF1340 | Protein of unknown function | |||||||
| PF0716 | 6-Phosphogluconate dehydrogenase | PF1737 | No InterPRO entry | |||||||
| PF0717 | S-Adenosylmethionine binding motif | PF1738 | Ribokinase | |||||||
| PF0718 | No InterPRO entry | PF1339 | Protein of unknown function | |||||||
| PF0719 | No InterPRO entry | PF1740 | Membrane transport component | |||||||
| PF0720 | No InterPRO entry | PF1741 | Membrane transport component | |||||||
| PF0721 | NADPH: flavin mononucleotide reductase | PF1742 | * | Glycosyl transferase, group 1 | ||||||
| PF0722 | Alkyl hydroperoxide reductase | PF1743 | * | Protein of unknown function | ||||||
| PF0723 | Iron permease FTR1 | PF1744 | * | * | * | ABC transporter | ||||
| PF0725 | Coenzyme A binding domain | PF1745 | * | * | l-Fucose isomerase | |||||
| PF0727 | No InterPRO entry | PF1746 | Glycoside transferase | |||||||
| PF0729 | 4Fe-4S ferredoxin | PF1747 | Protein of unknown function | |||||||
| PF0730 | * | No InterPRO entry | PF1748 | * | Membrane transport component | |||||
| PF0731 | Ferritin-like | PF1749 | * | Membrane transport component | ||||||
| PF0732 | ABC transporter | PF1750 | * | * | * | ABC transporter | ||||
| PF0733 | ABC transporter | PF1751 | * | * | Thiamine ABC transporter | |||||
| PF0734 | No InterPRO entry |
Shading indicates potential ORF clusters (in which adjacent ORFs can be on the positive or negative strand). An asterisk indicates the presence of a homolog. See text for details.
For each ORF, the presence of a homolog in P. horikoshii (A), P. abyssi (B), or T. kodakaraensis (C) is shown.
UDP-N-acetylglucosamine-4-epimerase.
To gain further insight into the differences between the genomes of P. furiosus and P. woesei and the proposed absence of 105 ORFs in the latter organism, a PFGE analysis was performed with DNA isolated from both species after digestion using the NotI restriction enzyme. For P. furiosus DNA, this enzyme should generate six DNA fragments that are approximately 43, 132, 224, 385, 416, and 709 kbp long. Assuming that the two genomes differ only by the 105 ORFs, treatment of P. woesei DNA should also yield six fragments, two of which (42 and 132 kbp) are the same as fragments in P. furiosus. Each of the other four fragments from P. woesei DNA are predicted to be smaller (206, 371, 401, and 667 kbp) than the corresponding fragments from P. furiosus DNA. PFGE analysis revealed the expected six bands from P. furiosus DNA, and six bands were also seen after digestion of P. woesei DNA, all of which corresponded to the P. furiosus DNA fragments (data not shown). Five of the bands appeared to be the same in both species since differences of less than 20 kb were not resolved. However, the sixth fragment (667 kb) from P. woesei DNA was distinguishable from the fragment from P. furiosus DNA (which was predicted to be 41 kb larger). Given that no fragments were obtained from P. woesei DNA that were larger than predicted, we concluded that the genome of this organism is approximately the same size as the P. furiosus genome and lacks all 105 ORFs (equivalent to 88.8 kbp) predicted by the microarray and PCR analyses.
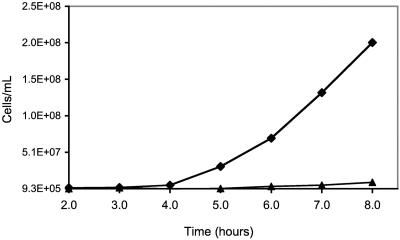
The power of the comparative DNA microarray approach is that it enables predictions regarding metabolism and physiology. Thus, of the ORFs listed in Table 1, of particular interest are the ORFs encoding proteins having known or predicted functions that are amenable to phenotypic analysis. One such gene cluster is the cluster containing PF1737 to PF1751, which includes the Mal I operon found in P. furiosus (1, 55) and in the related genus Thermococcus (50, 66). This operon encodes an ABC-type maltose/trehalose transporter (malEFG and malK, represented by PF1739 to PF1741 and PF1744, respectively), as well as a trehalose-degrading enzyme (PF1742) (1, 32, 36, 37, 44, 66). Interestingly, Thermococcus litoralis, which contains the Mal I operon, was also isolated from a shallow marine volcanic vent at Vulcano Island, Italy. In contrast, as shown by the BLASTP results (Table 1), P. horikoshii, P. abyssi, and T. kodakaraensis do not have a complete Mal I gene cluster. These three organisms were isolated from deep sea hydrothermal environments (4, 24, 31), perhaps implying that the availability of the Mal I gene cluster is limited to the vicinity of Vulcano Island. However, the apparent absence of the Mal I operon from P. woesei is inconsistent with a report that the organism is able to grow on maltose (68). Indeed, in our hands P. woesei exhibited very good growth (densities of >108 cells/ml) when the standard P. furiosus maltose-containing medium was used (65). This inconsistency was resolved by the finding that P. woesei did not exhibit significant growth on high-purity maltose (99%) rather than the technical grade usually used (95%, which contain 5% glucose and polysaccharides) (Fig. 2). Hence, in agreement with the DNA microarray analysis, maltose does not support growth of P. woesei.
FIG. 2.
Growth of Pyrococcus species on 99% pure maltose. Symbols: ⧫, P. furiosus; ▴, P. woesei.
A second gene cluster of interest in P. furiosus, which was absent in P. woesei, is the cluster containing PF0691 to PF0695. This cluster contains an ORF (PF0694) which encodes a protein that exhibits between 32 and 65% sequence similarity to the flavoprotein nitric oxide reductase (NOR) from the anaerobic bacteria Moorella thermoacetica (59), Desulfovibrio vulgaris (60), and Desulfovibrio gigas (59, 61). The protein encoded by PF0694 has the conserved residues required to coordinate the binuclear nonheme iron site found in NOR (11, 20, 30). Analysis of the genome sequences available for 23 archaea revealed that only 6 of them have a homolog of the gene encoding the bacterial NOR. These organisms include Archaeoglobus fulgidus and the methanogens Methanobacterium thermoautotrophicum, Methanococcus janaschii, Methanosarcina acetivorans, and Methanosarcina mazei. As indicated in Table 1, a close homolog of PF0694, which is annotated by InterPro as NO synthase, is not present in P. abyssi, P. horikoshii, or T. kodakaraensis.
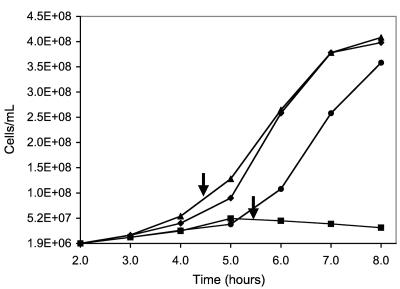
In light of the presence of a putative NOR system in P. furiosus and the apparent absence of this system in P. woesei, the question arose as to whether there are any differences between the two organisms in their responses to reactive nitrogen species (RNS). However, sensitivity of archaea to RNS has not been reported. To investigate the responses of the two Pyrococcus species, we used the NO generator known as RBS. This iron-sulfur-nitrosyl compound delivers seven molar equivalents of NO and is a potent antimicrobial agent (7, 8, 38, 47, 48). If P. woesei does not contain a homolog of PF0694, the organism should be more susceptible to this NO generator than P. furiosus. As shown in Fig. 3, growth studies using RBS showed that P. furiosus was not significantly affected by addition of 0.9 μM RBS, while under the same conditions the P. woesei cultures were not viable. These results strongly suggest that the cluster containing PF0691 to PF0695, particularly PF0694, plays a key role in detoxifying RNS (3).
FIG. 3.
Growth of P. woesei and P. furiosus in the presence of the NO generator RBS. The arrows indicate times at which RBS (0.9 μM) was added. Symbols: ▴, P. furiosus with RBS; ⧫, P. furiosus without RBS; ▪, P. woesei with RBS; •, P. woesei without RBS.
It is therefore clear that P. woesei and P. furiosus share a close genetic origin. Interestingly, analysis of the P. furiosus genome revealed that the ORF islands not detected in P. woesei are either bracketed by or are close to ISs. This is illustrated in Fig. 1. A notable exception is the putative gene cluster (PF0691 to PF0695) that encodes NOR (Table 1). However, this cluster has an IS on one side (PF0756), and on the other side, adjacent to PF0688, there is a ∼3.5-kb stretch of a long cluster of tandem repeats (LCTR). The tandem repeat that composes the LCTR is 29 nucleotides long. An LCTR is also located next to the cluster containing PF0025 to PF0032, which is another ORF cluster that is absent in P. woesei (Table 1). LCTRs are noncoding repeat sequences in tandem that are believed to behave like mobile elements and that have been proposed to participate in gene transfer (69). The positions of LCTRs next to some of the ORF clusters that are not present in P. woesei strongly support this proposition.
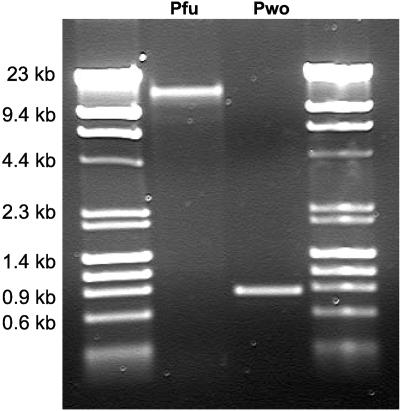
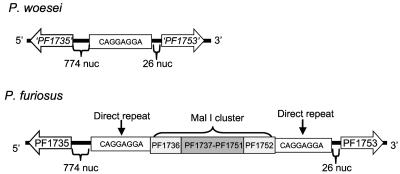
ISs are mobile elements that can transpose within the genome or into extrachromosomal elements (12, 46). The Mal I gene cluster found in P. furiosus (but not in P. woesei) is particularly noteworthy as it is packaged as a composite transposon. In other words, the Mal I is bracketed by two identical ISs, and the whole composite transposon (including ISs) is flanked by matching direct repeats (DRs), an indication of insertion as a complete composite transposon. In fact, the sequences of the Mal I gene cluster are virtually identical in P. furiosus and T. litoralis, and it has been proposed that a lateral gene transfer event was responsible (23, 37). To investigate whether the nature of IS and DR elements around the Mal I gene cluster could provide insight into the phylogenetic relationship between P. furiosus and P. woesei, the sequence of the relevant region in P. woesei was determined. PCR primers were designed to anneal outside the vicinity of the composite Mal I transposon in P. furiosus to determine what was present in the corresponding region in P. woesei. The same experiment with P. furiosus produced a PCR product that was approximately 17 kb long (the Mal I composite transposon is 17,854 bases long), while P. woesei produced a fragment that was about 780 bp long (Fig. 4). The sequence of the PCR product from P. woesei (accession number DQ202294) revealed that synteny of surrounding ORFs was conserved in P. woesei and P. furiosus. An eight-nucleotide sequence, CAGGAGGA, was found in the P. woesei locus where the Mal I cluster is located in P. furiosus. This sequence is not spurious, as the DRs that bracket the P. furious Mal I composite transposon have the same sequence (Fig. 5).
FIG. 4.
Products of the Mal I region of P. furiosus and P. woesei as determined by PCR analysis. The products were analyzed on an agarose gel (0.5%, wt/vol) and were stained with ethidium bromide. The λ/HindIII-φX174/HaeIII DNA ladder was obtained from Stratagene.
FIG. 5.
Sequences of the PCR products of the Mal I regions of P. furiosus and P. woesei. See text for details. The sequenced ORFs of the unsequenced P. woesei genome are labeled ‘PF1735’ and ‘PF1753.’ The nucleotide sequences of these two ORFs in P. woesei are identical to the nucleotide sequences of the corresponding P. furiosus ORFs.
DISCUSSION
Insertion sequences are suspected of playing key roles in the shaping of the P. furiosus genome that led to evolutionary divergence from P. abyssi and P. horikoshii (43, 45, 69). A total of 28 transposases are annotated in the P. furiosus genome, and our analysis of them showed that they comprise four groups. These groups were given the formal names groups ISPfu1 (8 isoforms, IS6 family), ISPfu2 (11 isoforms, IS6 family), ISPfu3 (5 isoforms, IS982 family), and ISPfu5 (4 isoforms, IS6 family) after analysis by ISFinder (http://www-is.biotoul.fr/). The ISs that bracket the Mal I gene cluster are isoforms belonging to group ISPfu1. This group is a member of the IS6 family, which transpose via replicative transposition. This is accomplished by formation of cointegrates between the donor and target sites that resolve, leaving a copy of the IS in the target and the donor site (46). Once a member of the IS6 family inserts into a locus, the copy remains at the new location, potentially replicating at other target sites in time. However, the PF1735/PF1753 locus of P. woesei does not contain an IS or a composite transposon, nor is there any indication that the locus has been involved in replicative transposition. Assuming that the archaeal ISs follow the observed IS6 replicative progression, we concluded that P. woesei is a sister and possibly the parent of P. furiosus. A P. furiosus strain would therefore be the type strain and presumably acquired ORFs (missing from P. woesei) from external sources at temperatures near 100°C in the same hydrothermal environment.
ISs likely play a pivotal role in shaping the genomes of the thermophilic community found at Vulcano Island and at similar locations on Earth. The use of DNA microarrays enables the first step to be taken toward understanding genomic phenomena such as the dissemination of gene cassettes within a community. This focuses attention on gene clusters found in archaeal communities rather than on individual genes or an individual organism. However, as demonstrated here, additional molecular and phenotypic characterizations are necessary to confirm the implications of array results.
Acknowledgments
We thank Martin Hughes for his generous gift of Roussin's black salt, Patricia Siguier for assistance with the IS analysis, Bryan Gibson, Jon Voigt, and Melani Atmodjo for assistance with the growth studies, Farris Poole for the annotation analyses, Anna Glasgow Karls and Jim Holden for helpful discussions, and Bao Phan for assistance with PFGE.
This research was supported by grants BES-0317911 and MCB 0129841 from the National Science Foundation.
REFERENCES
- 1.Albers, S. V., S. M. Koning, W. N. Konings, and A. J. Driessen. 2004. Insights into ABC transport in archaea. J. Bioenerg. Biomembr. 36:5-15. [DOI] [PubMed] [Google Scholar]
- 2.Amend, J. P., D. R. Meyer-Dombard, S. N. Sheth, N. Zolotova, and A. C. Amend. 2003. Palaeococcus helgesonii sp. nov., a facultatively anaerobic, hyperthermophilic archaeon from a geothermal well on Vulcano Island, Italy. Arch. Microbiol. 179:394-401. [DOI] [PubMed] [Google Scholar]
- 3.Amend, J. P., K. L. Rogers, E. L. Shock, S. Gurrieri, and S. Inguaggiato. 2003. Energetics of chemolithoautotrophy in the hydrothermal system of Vulcano Island, southern Italy. Geobiology 1:37-58. [Google Scholar]
- 4.Atomi, H., T. Fukui, T. Kanai, M. Morikawa, and T. Imanaka. 2004. Description of Thermococcus kodakaraensis sp. nov., a well studied hyperthermophilic archaeon previously reported as Pyrococcus sp. KOD1. Archaea 1:263-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baumann, P., and S. P. Jackson. 1996. An archaebacterial homologue of the essential eubacterial cell division protein FtsZ. Proc. Natl. Acad. Sci. USA 93:6726-6730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borges, K. M., S. R. Brummet, A. Bogert, M. Davis, K. M. Hujer, S. T. Domke, J. Szasz, J. Ravel, J. DiRuggiero, C. Fuller, J. W. Chase, and F. T. Robb. 1996. A survey of the genome of the hyperthermophilic archaeon, Pyrococcus furiosus. Genome Sci. Technol. 1:37-46. [Google Scholar]
- 7.Bourassa, J., B. Lee, S. Bernard, J. Schoonover, and P. C. Ford. 1999. Flash photolysis studies of Roussin's Black Salt anion: Fe4S3(NO)7−. Inorg. Chem. 38:2947-2952. [DOI] [PubMed] [Google Scholar]
- 8.Cammack, R., C. L. Joannou, X. Y. Cui, C. Torres Martinez, S. R. Maraj, and M. N. Hughes 1999. Nitrite and nitrosyl compounds in food preservation. Biochim. Biophys. Acta 1411:475-488. [DOI] [PubMed] [Google Scholar]
- 9.Cohen, G. N., V. Barbe, D. Flament, M. Galperin, R. Heilig, O. Lecompte, O. Poch, D. Prieur, J. Querellou, R. Ripp, J. C. Thierry, J. Van der Oost, J. Weissenbach, Y. Zivanovic, and P. Forterre. 2003. An integrated analysis of the genome of the hyperthermophilic archaeon Pyrococcus abyssi. Mol. Microbiol. 47:1495-1512. [DOI] [PubMed] [Google Scholar]
- 10.Costa de Oliveira, R., G. M. Yanai, N. H. Muto, D. B. Leite, A. A. de Souza, H. D. Coletta Filho, M. A. Machado, and L. R. Nunes. 2002. Competitive hybridization on spotted microarrays as a tool to conduct comparative genomic analyses of Xylella fastidiosa strains. FEMS Microbiol. Lett. 216:15-21. [DOI] [PubMed] [Google Scholar]
- 11.Coufal, D. E., P. Tavares, A. S. Pereira, B. H. Hyunh, and S. J. Lippard. 1999. Reactions of nitric oxide with the reduced non-heme diiron center of the soluble methane monooxygenase hydroxylase. Biochemistry 38:4504-4513. [DOI] [PubMed] [Google Scholar]
- 12.Craig, N. L. 1996. Transposition, 2nd ed., vol. 2. ASM Press, Washington, DC.
- 13.Creti, R., F. Citarella, O. Tiboni, A. Sanangelantoni, P. Palm, and P. Cammarano. 1991. Nucleotide sequence of a DNA region comprising the gene for elongation factor 1 alpha (EF-1 alpha) from the ultrathermophilic archaeote Pyrococcus woesei: phylogenetic implications. J. Mol. Evol. 33:332-342. [DOI] [PubMed] [Google Scholar]
- 14.Creti, R., P. Londei, and P. Cammarano. 1993. Complete nucleotide sequence of an archaeal (Pyrococcus woesei) gene encoding a homolog of eukaryotic transcription factor IIB (TFIIB). Nucleic Acids Res. 21:2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Creti, R., P. Sterpetti, M. Bocchetta, E. Ceccarelli, and P. Cammarano. 1995. Chromosomal organization and nucleotide sequence of the fus gene encoding elongation factor 2 (EF-2) of the hyperthermophilic archaeum Pyrococcus woesei. FEMS Microbiol. Lett. 126:85-90. [DOI] [PubMed] [Google Scholar]
- 16.Dabrowski, S., G. Sobiewska, J. Maciunska, J. Synowiecki, and J. Kur. 2000. Cloning, expression, and purification of the His6-tagged thermostable beta-galactosidase from Pyrococcus woesei in Escherichia coli and some properties of the isolated enzyme. Protein Expr. Purif. 19:107-112. [DOI] [PubMed] [Google Scholar]
- 17.Dabrowski, S., and B. Kiaer Ahring. 2003. Cloning, expression, and purification of the His6-tagged hyper-thermostable dUTPase from Pyrococcus woesei in Escherichia coli: application in PCR. Protein Expr. Purif. 31:72-78. [DOI] [PubMed] [Google Scholar]
- 18.Dabrowski, S., and J. Kur. 1998. Cloning and expression in Escherichia coli of the recombinant His-tagged DNA polymerases from Pyrococcus furiosus and Pyrococcus woesei. Protein Expr. Purif. 14:131-138. [DOI] [PubMed] [Google Scholar]
- 19.Dabrowski, S., J. Maciunska, and J. Synowiecki. 1998. Cloning and nucleotide sequence of the thermostable beta-galactosidase gene from Pyrococcus woesei in Escherichia coli and some properties of the isolated enzyme. Mol. Biotechnol. 10:217-222. [DOI] [PubMed] [Google Scholar]
- 20.da Costa, P. N., M. Teixeira, and L. M. Saraiva. 2003. Regulation of the flavorubredoxin nitric oxide reductase gene in Escherichia coli: nitrate repression, nitrite induction, and possible post-transcription control. FEMS Microbiol. Lett. 218:385-393. [DOI] [PubMed] [Google Scholar]
- 21.De Wachter, R., P. Willekens, and W. Zillig. 1989. Nucleotide sequence of the 5S ribosomal RNA of the archaebacterium Pyrococcus woesei. Nucleic Acids Res. 17:5848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dirmeier, R., M. Keller, D. Hafenbradl, F. J. Braun, R. Rachel, S. Burggraf, and K. O. Stetter. 1998. Thermococcus acidaminovorans sp. nov., a new hyperthermophilic alkalophilic archaeon growing on amino acids. Extremophiles 2:109-114. [DOI] [PubMed] [Google Scholar]
- 23.Diruggiero, J., D. Dunn, D. L. Maeder, R. Holley-Shanks, J. Chatard, R. Horlacher, F. T. Robb, W. Boos, and R. B. Weiss. 2000. Evidence of recent lateral gene transfer among hyperthermophilic archaea. Mol. Microbiol. 38:684-693. [DOI] [PubMed] [Google Scholar]
- 24.Erauso, G., A.-L. Reysenbach, A. Godfroy, J.-R. Meunier, B. Crump, F. Partensky, J. A. Baross, V. Marteinsson, G. Babrbier, N. R. Pace, and D. Prieur. 1993. Pyrococcus abyssi sp. nov., a new hyperthermophilic archaeon isolated from a deep-sea hydrothermal vent. Arch. Microbiol. 160:338-349. [Google Scholar]
- 25.Ettema, T., J. van der Oost, and M. Huynen. 2001. Modularity in the gain and loss of genes: applications for function prediction. Trends Genet. 17:485-487. [DOI] [PubMed] [Google Scholar]
- 26.Fiala, G., and K. O. Stetter. 1986. Pyrococcus furiosus sp. nov. represents a novel genus of marine heterotrophic archaebacteria growing optimally at 100°C. Arch. Microbiol. 145:56-61. [Google Scholar]
- 27.Fiala, G., K. O. Stetter, H. W. Jannasch, T. A. Langworthy, J. Madon 1986. Staphylothermus marinus sp. nov. represents a novel genus of extremely thermophilic submarine heterotrophic archaebacteria growing up to 98°C. Syst. Appl. Microbiol. 8:106-113. [Google Scholar]
- 28.Fukiya, S., H. Mizoguchi, T. Tobe, and H. Mori. 2004. Extensive genomic diversity in pathogenic Escherichia coli and Shigella strains revealed by comparative genomic hybridization microarray. J. Bacteriol. 186:3911-3921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fukui, T., H. Atomi, T. Kanai, R. Matsumi, S. Fujiwara, and T. Imanaka. 2005. Complete genome sequence of the hyperthermophilic archaeon Thermococcus kodakaraensis KOD1 and comparison with Pyrococcus genomes. Genome Res. 15:352-363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gomes, C. M., A. Giuffre, E. Forte, J. B. Vicente, L. M. Saraiva, M. Brunori, and M. Teixeira. 2002. A novel type of nitric-oxide reductase. Escherichia coli flavorubredoxin. J. Biol. Chem. 277:25273-25276. [DOI] [PubMed] [Google Scholar]
- 31.Gonzalez, J. M., Y. Masuchi, F. T. Robb, J. W. Ammerman, D. L. Maeder, M. Yanagibayashi, J. Tamaoka, and C. Kato. 1998. Pyrococcus horikoshii sp. nov., a hyperthermophilic archaeon isolated from a hydrothermal vent at the Okinawa Trough. Extremophiles 2:123-130. [DOI] [PubMed] [Google Scholar]
- 32.Greller, G., R. Riek, and W. Boos. 2001. Purification and characterization of the heterologously expressed trehalose/maltose ABC transporter complex of the hyperthermophilic archaeon Thermococcus litoralis. Eur. J. Biochem. 268:4011-4018. [DOI] [PubMed] [Google Scholar]
- 33.Grimm, V., S. Ezaki, M. Susa, C. Knabbe, R. D. Schmid, and T. T. Bachmann. 2004. Use of DNA microarrays for rapid genotyping of TEM beta-lactamases that confer resistance. J. Clin. Microbiol. 42:3766-3774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hafenbradl, D., M. Keller, R. Dirmeier, R. Rachel, P. Rossnagel, S. Burggraf, H. Huber, and K. O. Stetter. 1996. Ferroglobus placidus gen. nov., sp. nov., a novel hyperthermophilic archaeum that oxidizes Fe2+ at neutral pH under anoxic conditions. Arch. Microbiol. 166:308-314. [DOI] [PubMed] [Google Scholar]
- 35.Hess, D., K. Kruger, A. Knappik, P. Palm, and R. Hensel. 1995. Dimeric 3-phosphoglycerate kinases from hyperthermophilic Archaea. Cloning, sequencing and expression of the 3-phosphoglycerate kinase gene of Pyrococcus woesei in Escherichia coli and characterization of the protein. Structural and functional comparison with the 3-phosphoglycerate kinase of Methanothermus fervidus. Eur. J. Biochem. 233:227-237. [DOI] [PubMed] [Google Scholar]
- 36.Horlacher, R., K. B. Xavier, H. Santos, J. DiRuggiero, M. Kossmann, and W. Boos. 1998. Archaeal binding protein-dependent ABC transporter: molecular and biochemical analysis of the trehalose/maltose transport system of the hyperthermophilic archaeon Thermococcus litoralis. J. Bacteriol. 180:680-689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Imamura, H., B. S. Jeon, and T. Wakagi. 2004. Molecular evolution of the ATPase subunit of three archaeal sugar ABC transporters. Biochem. Biophys. Res. Commun. 319:230-234. [DOI] [PubMed] [Google Scholar]
- 38.Joannou, C. L., X. Y. Cui, N. Rogers, N. Vielotte, C. L. Torres Martinez, N. V. Vugman, M. N. Hughes, and R. Cammack. 1998. Characterization of the bactericidal effects of sodium nitroprusside and other pentacyanonitrosyl complexes on the food spoilage bacterium Clostridium sporogenes. Appl. Environ. Microbiol. 64:3195-3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kanoksilapatham, W., J. M. Gonzales, D. L. Maeder, J. DiRuggiero, and F. T. Robb. 2004. A proposal to rename the hyperthermophile Pyrococcus woesei as Pyrococcus furiosus subsp. woesei. Archaea 1:275-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kawarabayasi, Y., M. Sawada, H. Horikawa, Y. Haikawa, Y. Hino, S. Yamamoto, M. Sekine, S. Baba, H. Kosugi, A. Hosoyama, Y. Nagai, M. Sakai, K. Ogura, R. Otsuka, H. Nakazawa, M. Takamiya, Y. Ohfuku, T. Funahashi, T. Tanaka, Y. Kudoh, J. Yamazaki, N. Kushida, A. Oguchi, K. Aoki, and H. Kikuchi. 1998. Complete sequence and gene organization of the genome of a hyper-thermophilic archaebacterium, Pyrococcus horikoshii OT3. DNA Res. 5:55-76. [DOI] [PubMed] [Google Scholar]
- 41.Kohlhoff, M., A. Dahm, and R. Hensel. 1996. Tetrameric triosephosphate isomerase from hyperthermophilic archaea. FEBS Lett. 383:245-250. [DOI] [PubMed] [Google Scholar]
- 42.Koide, T., P. A. Zaini, L. M. Moreira, R. Z. Vencio, A. Y. Matsukuma, A. M. Durham, D. C. Teixeira, H. El-Dorry, P. B. Monteiro, A. C. da Silva, S. Verjovski-Almeida, A. M. da Silva, and S. L. Gomes. 2004. DNA microarray-based genome comparison of a pathogenic and a nonpathogenic strain of Xylella fastidiosa delineates genes important for bacterial virulence. J. Bacteriol. 186:5442-5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lecompte, O., R. Ripp, V. Puzos-Barbe, S. Duprat, R. Heilig, J. Dietrich, J. C. Thierry, and O. Poch. 2001. Genome evolution at the genus level: comparison of three complete genomes of hyperthermophilic archaea. Genome Res. 11:981-993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee, S. J., A. Engelmann, R. Horlacher, Q. Qu, G. Vierke, C. Hebbeln, M. Thomm, and W. Boos. 2003. TrmB, a sugar-specific transcriptional regulator of the trehalose/maltose ABC transporter from the hyperthermophilic archaeon Thermococcus litoralis. J. Biol. Chem. 278:983-990. [DOI] [PubMed] [Google Scholar]
- 45.Maeder, D. L., R. B. Weiss, D. M. Dunn, J. L. Cherry, J. M. Gonzalez, J. DiRuggiero, and F. T. Robb. 1999. Divergence of the hyperthermophilic archaea Pyrococcus furiosus and P. horikoshii inferred from complete genomic sequences. Genetics 152:1299-1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mahillon, J., and M. Chandler. 1998. Insertion sequences. Microbiol. Mol. Biol. Rev. 62:725-774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maraj, S. R., S. Khan, X. Y. Cui, R. Cammack, C. L. Joannon, and M. N. Hughes 1995. Interactions of nitric oxide and redox-related species with biological targets. Analyst 120:699-703. [Google Scholar]
- 48.Megson, I. L. 2000. Nitric oxide donor drugs. Drugs Future 25:701-715. [Google Scholar]
- 49.Murray, A. E., D. Lies, G. Li, K. Nealson, J. Zhou, and J. M. Tiedje. 2001. DNA/DNA hybridization to microarrays reveals gene-specific differences between closely related microbial genomes. Proc. Natl. Acad. Sci. USA 98:9853-9858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Neuner, A., H. W. Jannasch, S. Belkin, and K. O. Stetter 1990. Thermococcus litoralis sp. nov.: a new species of extremely thermophilic marine archaebacteria. Arch. Microbiol. 153:205-207. [Google Scholar]
- 51.Robb, F. T., M. D., J. R. Brown, J. DiRuggiero, M. D. Stump, R. K. Yeh, R. B. Weiss, and D. M. Dunn 2001. Genomic sequence of a hyperthermophile, Pyrococcus furiosus: implications for physiology and enzymology. Methods Enzymol. 330:134-157. [DOI] [PubMed] [Google Scholar]
- 52.Rowlands, T., P. Baumann, and S. P. Jackson. 1994. The TATA-binding protein: a general transcription factor in eukaryotes and archaebacteria. Science 264:1326-1329. [DOI] [PubMed] [Google Scholar]
- 53.Sachse, K., H. Hotzel, P. Slickers, T. Ellinger, and R. Ehricht. 2005. DNA microarray-based detection and identification of Chlamydia and Chlamydophila spp. Mol. Cell. Probes 19:41-50. [DOI] [PubMed] [Google Scholar]
- 54.Sambrook, J., E. F. Fritsch, and T. Maniatis 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 55.Schut, G. J., S. D. Brehm, S. Datta, and M. W. Adams. 2003. Whole-genome DNA microarray analysis of a hyperthermophile and an archaeon: Pyrococcus furiosus grown on carbohydrates or peptides. J. Bacteriol. 185:3935-3947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schut, G. J., J. Zhou, and M. W. Adams. 2001. DNA microarray analysis of the hyperthermophilic archaeon Pyrococcus furiosus: evidence for a new type of sulfur-reducing enzyme complex. J. Bacteriol. 183:7027-7036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Segerer, A., T. A. Langworthy, and K. O. Stetter 1988. Thermoplasma acidophilum and Thermoplasma volcanium sp. nov. from solfatara fields. Syst. Appl. Microbiol. 10:161-171. [Google Scholar]
- 58.Segerer, A., A. Neuner, J. K. Kristjansson, and K. O. Stetter 1986. Acidianus infernus gen. nov., sp. nov., and Acidianus brierleyi comb. nov.: facultatively aerobic, extremely acidophilic, thermophilic, sulfur-metabolizing archaebacteria. Int. J. Syst. Bacteriol. 36:559-564. [Google Scholar]
- 59.Silaghi-Dumitrescu, R., E. D. Coulter, A. Das, L. G. Ljungdahl, G. N. Jameson, B. H. Huynh, and D. M. Kurtz, Jr. 2003. A flavodiiron protein and high molecular weight rubredoxin from Moorella thermoacetica with nitric oxide reductase activity. Biochemistry 42:2806-2815. [DOI] [PubMed] [Google Scholar]
- 60.Silaghi-Dumitrescu, R., K. Y. Ng, R. Viswanathan, and D. M. Kurtz, Jr. 2005. A flavo-diiron protein from Desulfovibrio vulgaris with oxidase and nitric oxide reductase activities. Evidence for an in vivo nitric oxide scavenging function. Biochemistry 44:3572-3579. [DOI] [PubMed] [Google Scholar]
- 61.Silva, G., S. Oliveira, J. LeGall, A. V. Xavier, and C. Rodrigues-Pousada. 2001. Analysis of the Desulfovibrio gigas transcriptional unit containing rubredoxin (rd) and rubredoxin-oxygen oxidoreductase (roo) genes and upstream ORFs. Biochem. Biophys. Res. Commun. 280:491-502. [DOI] [PubMed] [Google Scholar]
- 62.Stetter, K. O. 1988. Archaeoglobus fulgidus gen. nov., sp. nov.: a new taxon of extremely thermophilic archaebacteria. Syst. Appl. Microbiol. 10:172-173. [Google Scholar]
- 63.Stetter, K. O., H. König, and E. Stackebrandt 1983. Pyrodictium, a new genus of submarine disc-shaped sulfur reducing archaebacteria growing optimally at 105°C. Syst. Appl. Microbiol. 4:535-551. [DOI] [PubMed] [Google Scholar]
- 64.Tiboni, O., P. Cammarano, and A. M. Sanangelantoni. 1993. Cloning and sequencing of the gene encoding glutamine synthetase I from the archaeum Pyrococcus woesei: anomalous phylogenies inferred from analysis of archaeal and bacterial glutamine synthetase I sequences. J. Bacteriol. 175:2961-2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Verhagen, M. F., A. L. Menon, G. J. Schut, and M. W. Adams. 2001. Pyrococcus furiosus: large-scale cultivation and enzyme purification. Methods Enzymol. 330:25-30. [DOI] [PubMed] [Google Scholar]
- 66.Xavier, K. B., R. Peist, M. Kossmann, W. Boos, and H. Santos. 1999. Maltose metabolism in the hyperthermophilic archaeon Thermococcus litoralis: purification and characterization of key enzymes. J. Bacteriol. 181:3358-3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang, L., U. Srinivasan, C. F. Marrs, D. Ghosh, J. R. Gilsdorf, and B. Foxman. 2004. Library on a slide for bacterial comparative genomics. BMC Microbiol. 4:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zillig, W., I. Holz, H.-P. Klenk, J. Trent, S. Wunderl, D. Janekovic, E. Imsel, and B. Haas. 1987. Pyrococcus woesei, sp. nov., an ultra-thermophilic marine archaebacterium, representing a novel order, Thermococcales. Syst. Appl. Microbiol. 9:62-70. [Google Scholar]
- 69.Zivanovic, Y., P. Lopez, H. Philippe, and P. Forterre. 2002. Pyrococcus genome comparison evidences chromosome shuffling-driven evolution. Nucleic Acids Res. 30:1902-1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zwickl, P., S. Fabry, C. Bogedain, A. Haas, and R. Hensel. 1990. Glyceraldehyde-3-phosphate dehydrogenase from the hyperthermophilic archaebacterium Pyrococcus woesei: characterization of the enzyme, cloning and sequencing of the gene, and expression in Escherichia. coli. J. Bacteriol. 172:4329-4338. [DOI] [PMC free article] [PubMed] [Google Scholar]