Abstract
HlyD, a member of the membrane fusion protein family, is essential for the secretion of the RTX hemolytic toxin HlyA from Escherichia coli. Random point mutations affecting HlyA secretion were obtained, distributed in most periplasmic regions of the HlyD molecule. Analysis of the secretion phenotypes of different mutants allowed the identification of regions in HlyD involved in different steps of HlyA translocation. Four mutants, V349-I, T85-I, V334-I and L165-Q, were conditionally defective, a phenotype shown to be linked to the presence of inhibitory concentrations of Ca2+ in extracellular medium. Hly mutant T85-I was defective at an early stage in secretion, while mutants V334-I and L165-Q appeared to accumulate HlyA in the cell envelope, indicating a block at an intermediate step. Mutants V349-I, V334-I, and L165-Q were only partially defective in secretion, allowing significant levels of HlyA to be transported, but in the case of V349-I and L165-Q the HlyA molecules secreted showed greatly reduced hemolytic activity. Hemolysin molecules secreted from V349-I and V334-I are defective in normal folding and can be reactivated in vitro to the same levels as HlyA secreted from the wild-type translocator. Both V349-I and V334-I mutations mapped to the C-terminal lipoyl repeat motif, involved in the switching from the helical hairpin to the extended form of HlyD during assembly of the functional transport channel. These results suggest that HlyD is an integral component of the transport pathway, whose integrity is essential for the final folding of secreted HlyA into its active form.
Hemolysin A (HlyA) secretion from Escherichia coli proceeds via the Type I secretion pathway, which targets the hemolysin directly to the medium, bypassing the periplasm (7, 16, 21). HlyA carries a discrete, C-terminal targeting sequence which is not removed during secretion (9, 19, 27, 39, 46). Secretion is independent of SecA and SecY (18) but specifically requires dedicated transport proteins HlyB and HlyD, encoded by the hly determinant (hlyCABD) present in uropathogenic strains (7, 36, 38). HlyC is completely dispensable for secretion, but in combination with cellular acyl carrier protein, it promotes specific acylation of lysine residues in HlyA in a step essential for toxicity and hemolytic activity of HlyA (24, 47). The outer membrane protein, TolC, conserved in all E. coli strains, constitutes a third protein essential to completing the secretion pathway (51). Previous studies have shown that HlyB and HlyD form a cross-linkable complex in vivo in the absence of either HlyA or TolC (49, 54). TolC is then apparently recruited into the translocator in the presence of HlyA (3, 49). In fact, HlyD and HlyB show mutual stabilization in vivo, with HlyD apparently undergoing a conformational change in the presence of HlyB (40).
HlyB is an ABC transporter, integral to the cytoplasmic membrane (52), with a cytoplasmic nucleotide binding domain, the ABC-ATPase (43) which has been shown in vitro to specifically interact (competitively with ATP) with the C-terminal secretion signal of HlyA (6). Structural studies of TolC have revealed a trimeric structure composed of a β-strand open pore in the outer membrane with an extended helical domain of approximately 100 Å, capable of spanning most of the periplasm (30). The helical domain narrows to almost occlude the periplasmic opening, and Andersen et al. (2) and Eswaran et al. (13) have provided evidence that this orifice can open to approximately 30 Å in diameter, to allow passage, for example, of α-helical regions. Such a model is consistent with the translation of a largely unfolded HlyA molecule. Recent studies have provided further evidence that type I secretion involves translocation of unfolded proteins. These studies have shown that the secretion of a small 19-kDa protein, HasA, in Serratia marcescens is dependent upon the chaperone SecB and cannot be transported if allowed to fold in the periplasm (42, 53).
HlyD is a member of a large family of polypeptides, the MFP (for membrane fusion protein) family, proposed to span the periplasm, in some way linking the inner and outer membranes (40, 45, 52). MFPs, although involved in the export of a variety of compounds, from drug molecules to large polypeptides such as HlyA, are united by their similar overall structural organization, combined with some conserved regions, involving primary sequence and particularly secondary structure in the C-terminal periplasmic domain. Proteins belonging to the MFP family, such as HlyD, are characterized by a single transmembrane domain (TMD), followed by a large helical domain and a C-terminal domain, predicted to be composed largely of β strands (12). Recent structural data, obtained for MexA, the MFP component of the multidrug efflux system of Pseudomonas aeruginosa and an HlyD functional analogue, have confirmed this general organization, with (in particular) an extended periplasmic helical domain. This supports the idea that such proteins can indeed bridge the cytoplasm, specifically connecting, in the case of Mex A, the MexB (inner membrane) and OprM (outer membrane) partners of the translocator (1, 20).
HlyD is anchored in the inner membrane with an approximately 59-residue N terminal exposed to the cytoplasm (45, 52). Previous studies by Schülein et al. (44) identified two regions in HlyD, residues 127 to 170 and the C-terminal 33 residues, required for in vivo secretion. However, there is no clear indication at which stage in transport these mutants are blocked. Pimenta et al. (40) showed that deletion of the N-terminal 40 residues of HlyD blocked secretion of HlyA. More detailed studies (3) demonstrated that this region, including a predicted amphiphilic helix (residues 1 to 25) and a downstream charged region (residues 26 to 38) was necessary for interaction with HlyA and subsequent incorporation of TolC into the functional translocator, but it was not required for the oligomerization of HlyD or the formation of the HlyB/HlyD complex. A model was proposed in which HlyA binding to the cytosolic domain of HlyD promotes a conformational change, propagated to the periplasmic domain of HlyD, leading to the recruitment of a functional TolC.
In the HlyA translocation pathway, different possible roles for HlyD might be envisaged. In one view, HlyB would translocate HlyA across the inner membrane with HlyD constituting an essential part of the pathway across the periplasm, completed by the outer membrane protein, TolC, for final release into the medium. Alternatively, HlyD might simply act to bring the two surface membranes, and therefore the membrane domain of HlyB and the periplasmic extension of TolC, into close apposition, to allow translocation of HlyA to the medium without direct participation of HlyD in the transport channel.
In this study, we have tried to address the question of the role of HlyD and in particular of its periplasmic domain in the translocation of HlyA to the medium. We have identified several point mutations in the periplasmic domain of the HlyD, encoded by the hly2001 determinant (35). The effect of all the mutations on stability and assembly of HlyD was determined, as well as the effect of specific mutations in relation to different stages in the secretion process. The results support the idea that HlyD forms an integral component of the translocation pathway. Importantly, these results indicate, for the first time, that this MFP directly or indirectly affects the folding of HlyA following or during its transit through the translocator.
MATERIALS AND METHODS
Bacterial strains, plasmids, media, and growth conditions.
E. coli strain SE5000 (rpsL ara139 Δ[lacIPOZYA]U169 recA57 thi) was used, except when otherwise specified. Cultures were grown at 30°C or 37°C in LB medium, normally supplemented with 10 mM CaCl2 when hemolytic strains were used. Medium EH (8) buffered with 60 mM HEPES, with a low-phosphate content, was used for the analysis of the effect of Ca2+ on hemolysin secretion. Detection of hemolytic haloes on plates utilized either LB or M63 minimal medium (plus 1 mM Ca2+) supplemented with 0.5% glucose and 0.5-μg/ml thiamine. Antibiotics were supplied at the following final concentrations: kanamycin (25 μg/ml), chloramphenicol (25 μg/ml), ampicillin (100 μg/ml), and tetracycline (5 μg/ml). In vivo mutagenesis was performed using E. coli strain MUT1 (F− mutD5 metE lacZ trpA) (10). pLG815 carries the hlyBD genes cloned from the wild-type pathogenic strain LE2001 (33) and is identical to pLG814 (27) except for the orientation of the hlyBD insert. pLG813 is a pACYC derivative encoding the hlyCA genes and confers resistance to chloramphenicol (27). pLG570 is a pOU71 derivative (an R1 single-copy replicon at 30°C), containing the whole hly 2001 determinant (hlyCABD) and conferring resistance to ampicillin (34). pPSG152 is a pBluescript-derived plasmid, containing the AccI-XbaI fragment of pLG815 encoding the wild-type hlyD gene under the control of its own promoter (40). pLG154 is a pLG815 derivative, deleted for the AccI/HpaI fragment containing most of the hlyB gene.
Mutagenesis of hlyD.
In vitro hydroxylamine mutagenesis of pLG815 was performed as described by Humphreys et al. (23). The AccI/BamHI fragment (containing hlyBD) of the mutagenized plasmid was isolated from an agarose gel using the Geneclean kit (Bio 101, Inc.) and religated to a nonmutagenized pLG815 vector digested with the same enzymes. The ligation assay mixture was transformed into E. coli SE5000 cells made competent by CaCl2 treatment (41). The transformation mixture was used as a preinoculum for a 50-ml culture from which a population of mutagenized plasmids was isolated by the alkaline lysis method (37). Screening for the loss of ability to promote HlyA secretion was carried out in the presence of pLG813 (hlyCA), to allow complementation of the HlyA secretion system and identification of mutants. Colonies unable to produce hemolytic haloes in blood agar plates were isolated, and their plasmid DNA (pLG813 plus pLG815) was purified. These plasmids were reintroduced in SE5000 by calcium chloride transformation, and selection was made for colonies resistant to kanamycin and sensitive to chloramphenicol, thus allowing only the recovery of the pLG815 plasmids containing the hlyD mutations.
In vivo mutagenesis was performed by transforming pLG815 (hlyBD) plasmid into the mutator strain MUT1 (10). Subsequently, cells were screened for loss of secretion as above. Screening for mutations introduced specifically into hlyD, rather than in hlyB, by both the in vivo and in vitro methods and was performed by a complementation test on blood agar plates with cells also carrying pLG570 with a Tn5 insertion in hlyD (33).
Sequencing of hlyD mutations.
Sequence analysis was performed using the Sequenase II kit, version 2 (Amersham), as recommended by the supplier. Seven oligonucleotides were designed using Oligo v.4 Primer Analysis software (Wojciech Rychlik) for sequencing the mutations in hlyD and were as follows (sequences read from 5′ to 3′, lowercase letters stand for the extensions added to the original hlyD sequence; oligonucleotides 1 to 5 allow sequencing of the coding strand, and oligonucleotides 6 to 7 allow sequencing of the noncoding strand): (i) H7, cttaagCAGAAAGAACAGAAGAA; (ii) D190, ATTATGGGGTTTCTGGT; (iii) C5′, GCTAGCAAAGCCGTCTGGATGATTTCA; (iv) D950, AGTTAGAGAAAAATGAA; (v) D1310, ATAAGCACATTCCATTA; (vi) C3′, GGATCCTTAACGCTCATGTAAACTTTCTGTTAC; and (vii) N3′, GGATCCTGAAATCATCCACAGGGCTTT.
Computer analysis.
Primary sequence alignments were performed using the University of Wisconsin Genetics Computer Group package available on the MicroVAX. Similarity plots were obtained using the program SimPlot, version 1 (M. A. Blight) to plot the data obtained from a MicroVAX. MexA Protein Data Bank file IT5E was used for extracting MexA secondary structure. The HlyD secondary structure was predicted using the hidden Markov model SAM-T02 program at http://www.cse.ucsc.edu/research/compbio/HMM-apps/T02-query.html. Coiled-coil predictions were obtained using the Lupas method (32) at http://www.ch.embnet.org/software/COILS_form.html. The HlyD TMD was predicted from the TMHMM server at http://www.cbs.dtu.dk/services/TMHMM-2.0/.
Hemolytic assays.
Growth curves and secretion of active hemolysin to the supernatant of LB cultures were determined as previously described (19). Supernatant samples were tested for hemolytic activity at 30-min intervals during 6.5 h. Assay volumes were adjusted during the experiment to compensate for the loss of activity, and activity was recorded per milliliter of supernatant. For detection of hemolytic haloes on plates, 3% sheep blood was employed as previously, with 1 mM CaCl2 in the case of M63-blood agar.
Cellular fractionation.
E. coli SE5000 carrying appropriate plasmids was grown for 4 h in LB medium at 30°C until the culture density reached an optical density at 600 nm (OD600) of 1.5. Bacterial cells (100 ml) were harvested by low-speed centrifugation (5,000 × g; 5 min), resuspended in 0.05 volume of phosphate-buffered saline, and broken by two passages through a French pressure cell at 41,000 kPa; inner and outer membrane proteins were separated by Sarkosyl solubilization, as previously described (40). Membrane proteins and crude bacterial extracts were obtained from the cell pellet by solubilization in sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer, and proteins of the cell-free culture supernatant were precipitated by the addition of 10% (vol/vol) trichloroacetic acid (TCA).
Trypsin accessibility assays. (i) Cell-associated HlyA.
Exponentially growing cells (OD600, ≈0.1) were resuspended in 250 μl of ice-cold 0.25 M sucrose-20 mM Tris-HCl (pH 8.0) and incubated for 30 min at 4°C in the presence of trypsin (100 μg/ml) and 10 mM MgCl2. Proteolysis was stopped by addition of 2 mM phenylmethylsulfonyl fluoride and 200 μg of trypsin inhibitor/ml, followed by incubation at room temperature for 15 min. Cells were pelleted, resuspended in sample buffer, and analyzed by SDS-PAGE. Proteins were transferred onto a nitrocellulose membrane by electroblotting and exposed to anti-HlyA polyclonal antibodies.
(ii) Secreted HlyA.
Culture supernatants were collected by at the secretion peak (4.5 h of growth/37°C in LB plus 10 mM CaCl2). To compensate for differences in HlyA secretion levels, the supernatant from the culture expressing the wild-type HlyD was diluted (1:4) with fresh LB. Different amounts of trypsin were added to these supernatants and incubated for 30 min on ice; proteolysis was halted by addition of 2 mM phenylmethylsulfonyl fluoride and a twofold excess of trypsin inhibitor. Following precipitation by 10% TCA for 30 min on ice, proteins were collected by centrifugation, resuspended in sample buffer, and separated by SDS-PAGE. Proteins were then transferred onto a nitrocellulose membrane by electroblotting and exposed to anti-HlyA antibodies.
Denaturation-renaturation experiments.
Duplicate cultures of SE5000 secreting HlyA from wild-type or mutant HlyD translocators were grown to an OD600 of ≈6 and centrifuged as before. Culture supernatants were collected and recentrifuged to remove any remaining cells, and sodium azide was added to a final concentration of 5 mM. The HlyA activity of the supernatant was measured, and secreted hemolysin was then precipitated following the protocol adapted from Stanley et al. (48). In brief, HlyA protein was precipitated by the slow addition of solid ammonium sulfate (40% [wt/vol]) to the supernatant with mixing at 4°C for 1 h. The protein was recovered by centrifugation at 16,000 × g for 10 min and resuspended in a 1/10 volume 25 mM HEPES (pH 8.0) buffer containing 5 mM EDTA, 0.5 mM β-mercaptoethanol, and 6 M guanidinium HCl for denaturation. Aliquots of HlyA in GnCl were diluted into the assay mixture to measure the activity of renatured protein.
Activity of HlyA determined by the titration method.
Samples of HlyA in LB medium containing 10 mM CaCl2 were subjected to 12 twofold serial dilutions in the same medium. To measure renatured HlyA activity, aliquots of HlyA in 6 M guanidine chloride (GnCl) were diluted (1/60) directly into LB medium (with 10 mM CaCl2) and immediately serially diluted as above. To 0.3 ml of each dilution was added 1 ml of a 0.65% solution of sheep erythrocytes prewashed in 10 mM Tris (pH 7.5), 155 mM NaCl, 20 mM CaCl2, 2 mM MgCl2, and 5 mM KCl. Samples were incubated for 1 h at 37°C and centrifuged at 14,000 × g for 1 min; the A543 of the supernatant was measured as before. In this experiment, one hemolytic unit (HU50) is defined as the amount of HlyA necessary to produce 50% lysis of the erythrocytes.
SDS-PAGE and immunoblotting.
Proteins were separated in 8% or 11% acrylamide gels (37:1 acrylamide-bisacrylamide ratio) in the presence of sodium dodecyl sulfate (SDS-PAGE) as previously described (31), and Western blots for immunodetection of HlyD protein were carried out with purified polyclonal antiserum raised against the 85-kDa glutathione S-transferase-HlyD fusion, as previously described (40).
Western blots were shown to be quantitative over at least a 10-fold range.
RESULTS
Isolation and sequencing of point mutations in different domains of HlyD.
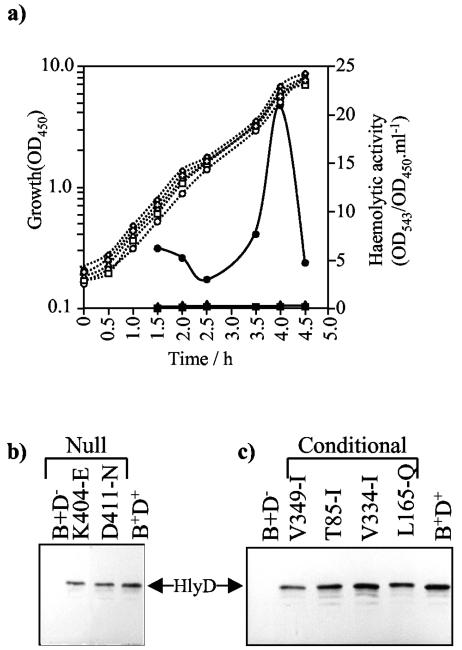
Plasmid pLG815 (expressing both hlyB and hlyD) was mutagenized in vitro with hydroxylamine (see Materials and Methods). Following transformation of pLG815 into strain SE5000, we screened for mutations specifically in hlyD. Transformants in which secretion was restored on LB-blood agar in the presence of wild-type hlyB hlyD but not by wild-type hlyB hlyD::Tn5 were considered defective in hlyD (see Materials and Methods). HlyD mutants obtained by this method (V349-I and L165-Q) were finally classified as conditional according to halo size detected on LB or minimal medium-blood agar plates, as summarized in Table 1. In addition, hlyD was mutagenized in vivo by passage of the plasmid pLG815 hlyB hlyD through the E. coli mutator strain MUT1, as described in Materials and Methods. Mutants specifically defective in hlyD were again identified and classified by complementation with pLG570 (Tn5::hlyD hlyCAB) as before. This procedure yielded the null mutants, completely lacking halos (K404-E and D411-N) and conditional mutants (T85-I and V334-I) shown in Table 1. Conditional mutants V349-I, T85-I, V334-I, and L165-Q gave clearly detectable haloes on minimal medium-blood agar, but virtually undetectable haloes on Luria broth-blood agar. For this reason, these mutants will be henceforth referred to as conditionally defective with respect to secretion of active hemolysin. The null mutants gave no detectable haloes under any conditions.
TABLE 1.
Amino acid changes corresponding to the hlyD mutants sequenced and secretion phenotype (production or not of hemolytic haloes on blood agar plates)
| HlyD mutation |
HlyA secretion phenotype | ||
|---|---|---|---|
| HlyD mutant | Amino acid change | Codon change | |
| HlyD7 | K404→E | 1210AAG→GAG | Null |
| HlyD8 | V349→I | 1045GTT→AAT | Conditionala |
| HlyD35 | T85→I | A254CT→ATT | Conditional |
| HlyD45 | V334→I | 1000GTT→ATT | Conditional |
| HlyD54 | L165→Q | C496TA→CAA | Conditional |
| HlyD55 | D411→N | 1231GAT→AAT | Null |
Mutants producing extremely small hemolytic haloes on LB blood agar plates but displaying clearly detectable haloes on minimal medium.
In total, six random mutations were identified and each one was characterized by DNA sequencing, together with the wild-type hlyD encoded by the determinant, Hly2001, used in these studies. Specific mutations were identified in all cases, and their positions and amino acid changes are presented in Table 1 and Fig. 1, together with the predicted secondary structure of HlyD. The complete sequence of HlyD2001 has been deposited in the EMBL data bank (accession number Y13891) and is virtually identical to that described previously for E. coli J96 (14). As shown in Fig. 1 and Table 1, six unique missense mutants (K404-E, V349-I, T85-I, V334-I, L165-Q, and D411-N) were identified. All the mutations isolated mapped in the periplasmic domain of HlyD.
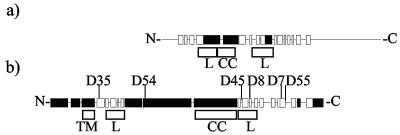
FIG. 1.
Representations of MexA and HlyD secondary structures. Black boxes, alpha helices; white boxes, beta strands. (a) MexA (383 amino acids) secondary structure taken from Protein Data Bank entry 1T5E. Only residues 29 to 259 are shown in the structure. (b) HlyD (478 amino acids) secondary structure prediction from the SAM-T02 server based upon 536 MFP sequences (http://www.cse.ucsc.edu/research/compbio/HMM-apps/T02-query.html). The positions of the mutations identified in HlyD are as indicated, together with the positions of the lipoyl motifs (boxed L), TMD (boxed TM), and coiled-coil domain (boxed CC). The precise amino acid changes in the different mutants are shown in Table 1. The first 40 residues forming an amphiphilic helix are essential for secretion of HlyA (40) being involved in the initial interaction with HlyA and triggering of TolC recruitment to the translocator (3, 49).
As shown in Fig. 1, the two to three large helical regions in the periplasmic domain are precisely flanked by two half lipoyl boxes, conserved in both the HlyD-like and the MexC-like subfamilies of MFPs and periplasmic efflux proteins, respectively. Lipoyl domains are proposed to play an essential role in the assembly of HlyD into the translocator (25). As indicated in Fig. 1b, two of the conditional mutations studied here, V334-I and V349-I, map precisely to the C-terminal lipoyl domain.
HlyD mutant proteins are stably associated with the membrane in vivo.
To determine any effect of the mutations in vivo on the level of expression, stability and localization of HlyD to the membrane, total cell proteins and cytoplasmic membrane fractions were prepared from cells grown in LB medium (see Materials and Methods). Proteins were separated by SDS-PAGE and probed with anti-HlyD antibody on immunoblots. The results for the detection of HlyD missense mutants in isolated envelopes are shown in Fig. 2. In all of the null (K404-E and D411-N) (Fig. 2b) and conditional (V349-I, T85-I, V334-I, and L165-Q) (Fig. 2c) mutants, HlyD proteins were detected in repeated experiments at levels not significantly different from that of wild-type HlyD expressed from the isogenic plasmid, indicating that the secretion defects caused by these mutations are not due to the absence of the HlyD protein in the bacterial envelope. Subsequent studies concentrated upon the analysis of HlyA secreted by these conditional mutant translocators.
FIG. 2.
(a) HlyA activity secreted by E. coli SE5000 (pLG813, hlyCA), growing in LB-10 mM CaCl2 at 30°C, expressing wild-type BD+ or mutant hlyD encoded by pLG815. ○/•, wild-type translocator (D+); /✚, V349-I; ⋄/⧫, T85-I; ▵/▴, V334-I; □/▪, L165-Q. Open symbols and dotted lines indicate growth (A450, which was identical for all cultures); filled symbols and lines indicate hemolytic activity detected in culture supernatants (OD543/OD450 · ml−1). (b) Immunodetection of HlyD in the isolated envelope fraction of cultures of SE5000 expressing different hlyD mutations. Cell envelopes were isolated as described in Materials and Methods. Protein samples (1.5 OD450 equivalent cell loadings) were analyzed by SDS-PAGE (10% acrylamide), followed by immunodetection with anti-HlyD antibodies. Different HlyD null mutants (numbers above the tracks) were expressed from pLG815 BD−. Strains carrying plasmid vectors alone or the wild-type BD+ translocator were used as controls. All cultures also expressed hlyCA (pLG813). (c) The same results as shown in panel b, but with the conditional hlyD mutations.
The conditional mutants V349-I, T85-I, V334-I, and L165-Q produce little hemolytic activity in liquid cultures.
The conditionally defective mutants V349-I, T85-I, V334-I, and L165-Q only produced haloes on minimal medium-blood agar but no detectable haloes with LB medium. The secretion efficiency of all these HlyD mutants was analyzed with respect to hemolytic activity in culture supernatants with liquid LB culture (plus 10 mM CaCl2, normally required for maximal hemolytic activities in LB) at 30°C. The results shown in Fig. 2a confirmed that, as on LB plates, mutants T85-I, V334-I, L165-Q, and V349-I all displayed extremely low levels of hemolytic activity in LB culture supernatants. No detectable activity was observed for the null mutants.
Analysis of HlyA levels in the cell envelope fraction of the HlyD mutants.
Depending upon possible different roles of HlyD in HlyA secretion, functional defects might be expected to block access of HlyA to the translocator or to lead to accumulation of hemolysin either within the cell envelope or on the cell surface. The latter would contrast with the phenotype observed in the complete absence of either HlyB or HlyD, where only a small percentage of the expected amount of HlyA was detected in the cytoplasmic fraction, indicating that both HlyB and HlyD are required for an early initiation step and that nonsecreted (or nonengaged) HlyA is highly unstable (19). We therefore decided to search for the presence of accumulated HlyA in different subcellular compartments, which might indicate whether the mutants were blocked at an early or a late step in the translocation process.
E. coli SE5000 expressing hlyCA (pLG813) together with wild-type or mutant derivatives of hlyD, including wild-type hlyB encoded by pLG815 as above, were grown in LB medium at 30°C for 3.5 h to an OD600 of 6.0. With wild-type HlyD, this corresponds to the time just before the peak of hemolysin secretion (22) under these conditions (Fig. 2a). Culture supernatants were obtained by centrifugation, cell envelopes were isolated, and proteins were separated by SDS-PAGE and subsequently analyzed by Western blot with anti-HlyA antibody.
As shown in Fig. 3b, significant amounts of HlyA were always detected in the isolated envelope fraction of cells specifically expressing a wild-type translocator. This probably represents HlyA molecules exposed on the cell surface following secretion, since this is largely removed by treatment of cells with trypsin (Fig. 3c). Cells expressing the HlyD conditional mutants showed three phenotypes with respect to secreted and membrane-associated HlyA. T85-I secreted low levels of HlyA to the medium (Fig. 3a); similarly, very little HlyA was detected in association with the cell envelope (Fig. 3b). In contrast, in the case of both mutants V334-I and L165-Q, although secreting low levels of HlyA to the medium (Fig. 3a), significant levels of HlyA could be reproducibly detected that were associated with the envelope fraction (Fig. 3b).
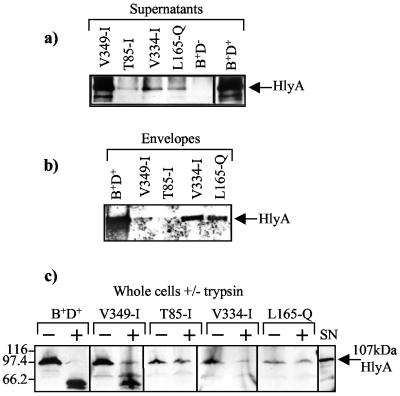
FIG. 3.
Effect of conditional HlyD mutations (V349-I, T85-I, V334-I, and L165-Q) on HlyA levels in the culture supernatant and in the cell envelope. (a) HlyA secreted by E. coli SE5000 (pLG813, hlyCA) growing exponentially in LB-10 mM CaCl2 at 30°C, expressing different hlyD mutations encoded by pLG815 (V349-I, T85-I, V334-I, and L165-Q). Supernatant samples (equivalent to 1.5 OD450 of cells) were TCA precipitated and submitted to SDS-PAGE, and HlyA was detected by immunoblotting. (b) The same results as shown in panel a, with the immunodetection of HlyA in isolated cell envelope preparations. (c) Trypsin treatment of whole cells of E. coli SE5000 (pLG813, hlyCA) expressing different mutations in hlyD harbored by pLG815. +, presence of trypsin in the digestion buffer; −, absence of trypsin in the digestion buffer; SN, supernatant sample from a culture expressing wild-type hlyCABD, used as a control for HlyA. Cells were harvested at OD450 = 1, and 0.5 OD450 equivalents were loaded, following trypsin treatment.
The above results indicated that T85-I, with a mutation close to the transmembrane region, may be defective at an early stage in the secretion pathway. On the other hand, mutations V334-I and L165-Q, mapping to either end of the large helical domain (Fig. 1b), led to the accumulation of a possible translocation intermediate and appeared to affect a later step in transport. With the null, missense mutants K404-E and D411-N producing no haloes under all conditions, little or no HlyA was detected in either the supernatants or the envelopes (data not shown). These results indicate that these mutants were blocked at the earliest stage of secretion. In the case of the conditional mutant V349-I, little HlyA could be found in association with the envelope. Other unexpected properties of this mutant will be discussed below.
Accessibility of cell-associated HlyA to trypsin digestion in the presence of a wild-type or mutant HlyD translocators.
SDS-PAGE analysis of total protein from cells expressing a wild-type translocator consistently showed substantial amounts of cell-associated hemolysin (Fig. 3b). To characterize further the specific defects caused by the mutations introduced in the HlyD translocator, a trypsin accessibility experiment was designed in the attempt to distinguish surface-bound from cytoplasmic HlyA or even to distinguish HlyA from molecules still trapped in the translocator.
Figure 3c shows the analysis of the accessibility of HlyA molecules when whole cells expressing the wild-type or a mutant HlyD translocator were treated with trypsin. The results demonstrate that, with the wild-type HlyD translocator, the majority of cell-associated HlyA was digested by trypsin to a lower-molecular-weight form, indicating the presence of HlyA on the cell surface postsecretion. Similarly, with V349-I, which also secretes substantial levels of HlyA under these conditions (see below), the majority of cell-associated HlyA was also accessible to trypsin.
In contrast to the wild type and V349-I mutant, the low levels of cell-associated HlyA in the T85-I mutant were largely inaccessible to trypsin. This mutant secreteds very poorly, with little HlyA recovered with the isolated envelope (Fig. 3b), and this result indicates that in this case the cell-associated HlyA may be cytoplasmic. With mutant V334-I, cell-associated HlyA was to some extent accessible to exogenous trypsin, although no degradation products were detected. This result, combined with the data shown in Fig. 3b, indicates that with V334-I, some HlyA is present in the cell surface and some is present inside the translocator. Interestingly, with mutant L165-Q, which like V334-I shows relative enrichment of HlyA in the isolated envelope (Fig. 3b), cell-associated HlyA was inaccessible to trypsin. Moreover, in an additional control experiment with L165-Q, insignificant amounts of HlyA were detected in the cytoplasmic fraction by immunoblotting (data not shown). Taken together, these results indicate that some HlyA accumulates within the translocator with the L165-Q mutant, with little on the cell surface, and is consistent with the conclusion that this mutation blocks HlyA translocation at a intermediate stage. The data indicating that mutants V334-I and L165-Q accumulated HlyA within the translocator were supported by results obtained when secretion of a LacZ-HlyA fusion (CIZ-HlyA) (26) was analyzed. In those experiments, high levels of the fusion protein, inaccessible to trypsin, were also recovered in the envelopes of L165-Q and V334-I cells (data not shown).
Levels of HlyA in the culture supernatants reveals that the V349-I mutation affects the activity of secreted hemolysin.
Analysis of the levels of HlyA protein secreted to the culture supernatant of the conditional HlyD mutants demonstrated that mutants T85-I, V334-I, and L165-Q, as expected, secreted low levels of HlyA compared to the wild-type translocator (Fig. 3a). The null mutants (K404-E and D411-N) gave no detectable HlyA signal by Western blotting. Unexpectedly, in contrast, the V349-I mutant secreted significant levels of HlyA protein to the culture supernatant (Fig. 3a). Western blot analysis indicated that V349-I, despite the low levels of hemolytic activity in the medium, still secreted at least 25% of the amount of HlyA protein detected with an isogenic strain expressing wild-type HlyD.
Calculations indicated that the hemolysin released to the medium from the V349-I translocator had a specific activity approximately 20-fold lower than that of the wild type. This suggested that the mutation might have an additional effect on the folding of HlyA, necessary for the release of an active molecule. This was further investigated in more detail.
HlyA secreted from the V349-I translocator is not unstable.
The greatly reduced activity of HlyA protein secreted from cells carrying the V349-I mutation could be accounted for by a folding defect in the secreted HlyA molecules or increased instability of HlyA secreted by the mutant V349-I. To distinguish between these two possibilities, we measured the half-life of hemolytic activity in culture supernatants from wild-type HlyD- and V349-I-expressing cells in LB at 37°C. Both wild-type- and V349-I-secreted HlyA had an identical half-life of approximately 30 min (data not shown), showing that HlyA secreted via the V349-I translocator displayed normal stability with respect to activity. This therefore suggested that the reduced specific activity of the HlyA molecules secreted by the V349-I translocator is probably due to a folding defect. To test the hypothesis that the majority of molecules secreted from the V349-I translocator were misfolded, two additional experiments were performed, as described below.
HlyA secreted from the HlyD8 translocator is hypersensitive to trypsin.
Trypsin treatments of HlyA obtained from supernatants of cultures expressing either the mutant V349-I or wild-type HlyD translocators were carried out. The digestion patterns of hemolysin secreted through the mutant and wild-type translocator, as a function of trypsin concentration, were compared by SDS-PAGE and followed by immunoblotting with anti-HlyA antibodies (Fig. 4). HlyA secreted from the HlyD8 translocator was very sensitive to trypsin (Fig. 4a), being completely degraded when >2.5 μg/ml of the protease was present. In contrast, HlyA secreted to the medium from the wild-type translocator was significantly more resistant to trypsin treatment, with 70- to 80-kDa resistant fragments remaining at high concentrations of protease (>100 μg/ml) (Fig. 4b). This latter is characteristic of normal HlyA, as observed in many different types of experiments, including time course digestions as shown in Fig. 4c, where HlyA, treated with 10-μg/ml trypsin, accumulated progressively as a 70-kDa species and smaller fragments.
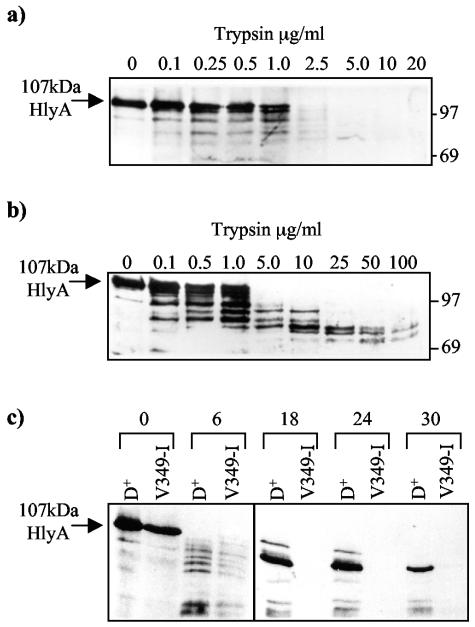
FIG. 4.
Trypsin treatment of HlyA obtained from supernatants of cultures (pLG813, hlyCA) expressing either the V349-I mutant translocator (a) or the wild-type HlyD harbored by pLG815 (b). The wild-type supernatant was first diluted in LB to give an HlyA level similar to that of the supernatant from the V349-I mutant. The final concentration of Ca2+ in both cases was 10 mM. Numbers on top of each track indicate the amount of trypsin (in micrograms per milliliter) incubated with the samples for 30 min on ice. Following the addition of trypsin inhibitor and TCA precipitation of HlyA protein present in each sample, samples were analyzed by SDS-PAGE (8% acrylamide); molecular weight markers are indicated at the right. (c) Time course experiment. HlyA in the supernatant from a culture of with HlyD or mutant V349-I was treated with 10-μg/ml trypsin at 25°C for different times (in minutes) indicated at top and was analyzed by SDS-PAGE.
Time course experiments for HlyA secreted from the V349-I mutant translocator (Fig. 4c) confirmed the greater sensitivity to the protease, with the 70- to 80-kDa fragments apparently completely sensitive to trypsin. Moreover, using proteinase K, we also observed an enhanced sensitivity of HlyA secreted from the V349-I translocator compared with wild-type HlyD (data not shown). These results are therefore consistent with the misfolding of the majority of HlyA molecules secreted by the V349-I mutant.
Activity of HlyA secreted from either the V349-I or L165-Q-translocator wais increased by in vitro denaturation and refolding.
Previous studies (17, 48) have shown that HlyA can be renatured efficiently in vitro, following denaturation in urea or GnCl, with restoration of high levels of hemolytic activity. We considered that if HlyA aberrantly secreted from the V349-I translocator was misfolded, then denaturation followed by in vitro refolding could result in a regain in hemolytic activity, bringing the level of activity expressed per unit of HlyA protein to that displayed by HlyA secreted from a wild-type translocator. To test this hypothesis, HlyA was precipitated from cell-free culture supernatants by ammonium sulfate, denatured in 6 M GnCl, and renatured by rapid dilution into an assay mixture (containing 10 mM CaCl2) before HlyA activity was measured.
The hemolytic activity of renatured HlyA secreted from a wild-type or V349-I cell is shown in Table 2. In the case of HlyA secreted from the wild-type translocator, the recovery of hemolytic activity was slightly more than 100%. This excess could be expected if, for example, the initial supernatant sample contained a small fraction of inactive molecules due to aggregation. Importantly, HlyA secreted from the mutant V349-I strain showed a marked 11-fold gain in activity compared to the initial low value, resulting in an activity when expressed per unit of HlyA protein, similar to that of the wild-type control. The HlyA secreted from the L165-Q mutant translocator was also analyzed, and results were even more striking, as shown in Table 2. This protein is secreted to the medium at lower levels than with V349-I, with HlyA having in this case a calculated specific activity of only 0.4% of HlyA secreted from a wild-type HlyD. Dramatically, after the denaturation-renaturation experiment, the previously inactive HlyA secreted from L165-Q displayed an apparent specific activity much closer (74%) to that of the wild-type control. These results provided strong evidence that HlyA secreted from either the V349-I or L165-Q translocator was largely misfolded in some way, ruling out all possibility that the mutation in the V349-I translocator affected in some way the action of cytoplasmic HlyC, which is required to activate toxin molecules prior to secretion (24, 38, 47). In contrast, this result clearly demonstrates that secretion by a translocator containing a mutated HlyD perturbs the normal folding of the HlyA molecule, suggesting that HlyD constitutes an inherent component of the transport pathway.
TABLE 2.
Hemolytic activity before and after denaturation-renaturation of HlyA secreted from wild-type or mutant translocators V349-I (D8) or L165-Q (D54)a
| Source of HlyA molecules | HlyA activity (per unit of HlyA protein) |
Increase in HlyA activity/unit of protein after renaturation | |
|---|---|---|---|
| Before denaturation-renaturation | After denaturation-renaturation | ||
| Wild-type HlyD | 35.2 | 36.7 | 1.04 |
| Mutant V349-I | 3.06 | 32.4 | 10.6 |
| Mutant L165-Q | 0.14 | 26.8 | 191 |
Hemolytic activities were measured by the titration method and expressed as HU per unit of HlyA protein in the culture supernatant, with HlyA protein quantified by Western blot analysis (see Materials and Methods). Values in column 4 are derived by dividing the values in column 3 by those in column 2.
Effect of extracellular calcium on the action of the V349-I and HlyL165-Q translocator.
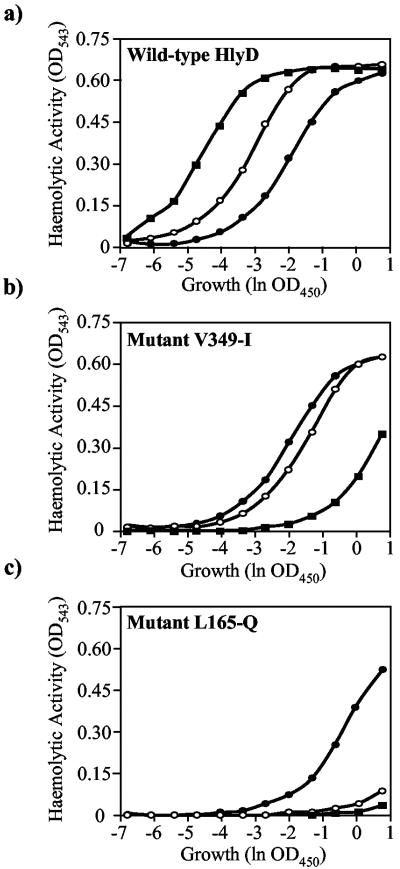
Production of high levels of hemolytic activity in the medium of E. coli cultures is normally associated with high concentrations of extracellular calcium. Such a Ca2+ requirement is consistent with the presence of repetitive Ca2+ binding motifs in HlyA upstream of the secretion signal, which in folded molecules of this RTX protein family forms an extremely stable parallel β-roll structure in association with several Ca2+ atoms (4, 5). Importantly, in this context we noted that the calcium concentration was markedly different between LB medium (10 mM) and the M63 minimal medium (1 mM), used here to distinguish between conditional and null mutants with respect to HlyA secretion. It was of interest, therefore, to determine whether directly varying the extracellular Ca2+ concentration would differentially affect the function of the conditional mutants V349-I and L165-Q.
Cultures were grown in EH medium (a low-phosphate medium to avoid precipitation of Ca2+) containing different levels of added Ca2+, and supernatant samples were obtained at the peak time for secretion, as before. Secreted hemolytic activity and levels of HlyA protein at different Ca2+ concentrations were determined. With the wild-type translocator, both the amount of hemolysin secreted and its specific activity increased four- to fivefold with increasing Ca2+ concentrations. In complete contrast, the hemolytic activity in the supernatant with the mutants V349-I and L165-Q was significantly reduced as the Ca2+ concentration increased in the medium. This is illustrated in Fig. 5, where the amount of hemolytically active HlyA secreted from the wild-type HlyD, V349-I, or L165-Q cells was determined for different Ca2+ concentrations.
FIG. 5.
Effect of extracellular Ca2+ on the activity of HlyA secreted by wild-type and mutant HlyD translocators. E. coli expressing hlyCA (pLG813), hlyB, and different hlyD alleles (pLG815) was grown in EH medium (containing different Ca2+ concentrations) to an OD450 of ∼6. Culture supernatants were obtained, and hemolytic activity was determined (hemoglobin release measured at OD453) by serial dilution assay in the presence of different CaCl2 concentrations (Materials and Methods). (a) Wild-type HlyD; (b) mutant V349-I; (c) mutant L165-Q. Filled circles, 0.1 mM CaCl2; open circles, 1 mM CaCl2; filled squares, 10 mM CaCl2.
HU50 (see Materials and Methods), which reflects both the amount of HlyA and its specific activity, was found to decrease with Ca2+ levels for HlyA secreted from the wild-type translocator. Conversely, the HU50 of HlyA molecules secreted from the V349-I or L165-Q translocator increased markedly as the Ca2+ concentration in the medium increased, reflecting the inhibitory action of Ca2+ on the initial folding of the toxin to calcium. These results could nicely explain the observed conditional phenotype of these mutants in the initial screen on LB and M63 media. Most importantly, these observations indicated that the availability of Ca2+ in the extracellular medium and/or in the bacterial cell surface is an important factor required for the correct folding of the secreted HlyA molecule as it emerges from the translocator channel.
DISCUSSION
The predicted secondary structure for HlyD indicates a possible two-domain organization of the periplasmic domain, one that is largely helical and followed by a β-strand domain at the C terminus (45, 52). From this, it has been postulated that HlyD spans the periplasm mainly through its strongly helical domain, including a coiled-coil region, to interact directly with TolC and/or the outer membrane. In line with the proposal of Nikaido (39a) for the related MFP AcrA involved in multidrug transport, we assume that the HlyD-TolC interaction provides a tight seal, completely separating the interior of the translocator from the periplasm. This model is consistent with the absence of any periplasmic intermediate in hemolysin secretion (15, 19, 28, 29). The recently described crystal structures of MexA (1, 20) have allowed modeling of the docking between the oligomeric MexA (MFP) with the TolC structure, providing convincing support for the idea of a continuous transport structure or tunnel across the periplasm and through the outer membrane for such translocators, which should also be applicable in principle to the type 1 protein translocators.
The four conditional mutations shown here to affect translocation and or final folding of HlyA all mapped to the periplasmic domain of HlyD, likely to constitute part of the translocation channel with TolC. Despite the functional relationship between MexA and HlyD, primary sequence similarity is limited; HlyD is substantially larger, with two additional large proximal helices (Fig. 1). Unfortunately, in view of the major differences between HlyD and MexA, we were unable in any meaningful way to assign these mutations to a possible three-dimensional organization of HlyD based on MexA.
The null mutants K404-E and D411-N together with the conditional T85-I mutant (Fig. 1) secrete little or no HlyA protein, at least in LB medium. Similarly, little HlyA was detected with these mutants in the intact cells or after isolation of the envelope. The simplest interpretation of the properties of these mutations, affecting the periplasmic domain, is that secretion is blocked at an early stage, abolishing entry into the pathway. These mutations could, for example, affect the recruitment of TolC. HlyA in such mutants does not accumulate to high levels in the cytoplasm (data not shown); this is not surprising, since previous studies (19) demonstrated that in the complete absence of hlyD or hlyB, HlyA does not accumulate in the cell and is presumably unstable if confined to the cytoplasm.
When analyzed in detail in LB medium, two of the conditional mutants (V334-I and L165-Q) produced substantially reduced levels of secreted HlyA protein. Mutation L165-Q colocalizes with a region (residues 127 to 170) shown previously to be required for secretion. Moreover, HlyD molecules deleted for this region apparently failed to oligomerize, as indicated by genetic competition studies with the wild-type HlyD (44). Unlike the null mutants, L165-Q and (to a lesser extent) V334-I retained significant levels of HlyA protein (compared to the amount secreted) in the isolated envelopes, inaccessible to exogenous trypsin. These results indicated that transport of HlyA through the L165-Q translocator is perturbed, allowing some HlyA to accumulate in the translocator. This conclusion was further supported by the analysis of secretion of the hybrid LacZ-HlyA protein, CIZ-HlyA, from these HlyD translocators. This hybrid protein (26) is normally poorly secreted to the medium but accumulates in the cell envelope specifically in the presence of HlyBD. With mutant V334-I and L165-Q translocators, secretion of the CIZ-HlyA hybrid was completely abolished, but the fusion protein still accumulated in the cell envelope, resistant to trypsin (data not shown). These results support the idea that HlyD constitutes an inherent component of the transport pathway and is not simply an adaptor, bringing HlyB and TolC into contact.
Evidence has been provided that after interacting with the allocrite, HlyA, a major reorganization of the periplasmic domain of HlyD monomers allows the recruitment of TolC into the translocator complex (3, 49). Models proposed to explain this organization of HlyD are as follows. The lipoyl motifs, in the absence of any transport substrate, form a hybrid, globular structure, resulting from the interlocking of two large helices. In molecules like HlyD, it is proposed that the association-dissociation of the two motifs controls the interchange of HlyD between two forms (25). These are a collapsed form, as a helical hairpin restrained by the association of the lipoyl motifs, or an extended form, in which the lipoyl motifs separate, allowing HlyD to produce a long continuous channel with TolC across the periplasm. Disruption of the lipoyl motifs is presumed to be triggered by docking of the HlyA signal region with the translocator HlyD-HlyB complex (49). The lipoyl motifs, capable perhaps of forming either intramolecular or intermolecular hybrid complexes (25, 30), could contribute to either the spring-like properties of MFP (periplasmic efflux protein) molecules or their lateral packing into oligomers and should in either case play a key role in HlyD function.
It is intriguing, therefore, that two mutations, V349-I and V334-I, map to the C-terminal lipoyl peptide (residues 330 to 360). These mutations, although both are conservative Val-Ile changes, map to relatively conserved regions of the two different β-strands in this lipoyl motif (25). As a consequence, such mutations, although not affecting oligomerization of HlyD per se (54), possibly affect the formation and/or the stable packing of HlyD molecules in some way, resulting in aberrant movement of HlyA through the translocator. As a result, in the most perturbed case of mutant V334-I and (to a lesser extent) L165-Q, this actually causes accumulation of some molecules of HlyA in the channel.
The results presented here show that the low level of toxin activity of HlyA secreted by mutant V349-I or L165-Q translocator in LB medium is due to misfolding of secreted HlyA molecules. This conclusion is supported by two lines of evidence. (i) HlyA molecules secreted from the V349-I mutant are hypersensitive to proteolytic enzymes, indicating differences in the folding pathway and leading to the exposure of different protease digestion sites. (ii) Most convincingly, HlyA secreted from either mutant translocator V349-I or L165-Q can be refolded normally to give full or nearly full hemolytic activity, following denaturation and renaturation in vitro. In both cases, however, these properties might be best interpreted in terms of a translocation mechanism that normally tightly couples the rapid translocation of HlyA with its final folding on the cell surface as molecules emerge from TolC. We propose that this requires a functional periplasmic domain of HlyD, including the major helical regions. In the V349-I and L165-Q mutants, slowing down of translocation might, for example, perturb this coupling and result in misfolding. Interestingly, Vakharia et al. (50) previously described mutations in TolC that also resulted in secretion of HlyA showing a reduced hemolytic activity; the majority of those mutations located to the periplasmic domain of TolC.
Given the narrow tunnel formed by TolC (30), we assume that HlyA and other type I polypeptides are translocated in an unfolded form. This is supported by recent studies of HasA, a small 21-kDa protein that folds in the cytoplasm in the absence of the chaperone SecB and cannot be translocated (11, 53). Consequently, folding of such type 1 proteins, including HlyA, must take place on the cell surface. Thus, it is likely that several factors (most importantly, the nature of the bacterial cell surface environment, the concentration of Ca2+ available to bind to the RTX repeat motifs, and the speed at which HlyA molecules emerge on the surface) will play an important role in coordinating the final translocation and release of HlyA to the medium. The results presented here for the wild-type HlyD (Fig. 5) show that both secretion of HlyA and its hemolytic activity (that is, the specific activity) are enhanced by high Ca2+ levels. On the other hand, the folding of HlyA secreted from the mutant translocators V349-I and L165-Q is actually hypersensitive to high (10 mM) Ca2+ levels. This finding also provides an explanation for the conditional phenotype of mutants V349-I, T85-I, V334-I, and L165-Q, almost completely nonhemolytic on LB (10 mM Ca2+) but producing hemolytic haloes on minimal medium (1 mM Ca2+). In the case of the incorrectly folded HlyA molecules analyzed here, we can only speculate that high Ca2+ levels induce a conformation change which further exaggerates the aberrant folding already present, with consequent reduction in hemolytic activity.
Major questions still arise concerning the overall mechanism used to ensure correct folding of HlyA molecules emerging from the cell surface in the apparent absence of conventional chaperones. It is tempting to speculate that, as an RTX protein, HlyA could bind extracellular calcium, as the molecule exits the translocator to promote a final folding step. Results presented here add an extra piece to this puzzle, indicating that the integrity of the channel formed by HlyD molecules through the periplasm is important not only for the transport of HlyA itself, but also to ensure a correct folding step for the translocated toxin. Since all known RTX toxins are supposed to be translocated in an unfolded form, coupling of the final secretion step to the binding of Ca2+ may represent a common mechanism adopted by proteins of this family to ensure their correct folding in external medium.
Acknowledgments
This work was partially supported by the Brazilian Government, through the Conselho Nacional de Desenvolvimento e Pesquisa (CNPq), contract number 260.127/90.6. We thank the CNRS and Université de Paris XI for support. K.R. is pleased to acknowledge the support of a postdoctoral Chateaubriand Fellowship from CIES.
We thank J. Kuhn for the purification of anti-HlyD antibodies.
REFERENCES
- 1.Akama, H., T. Matsuura, S. Kashiwagi, H. Yoneyama, S.-i. Narita, T. Tsukihara, A. Nakagawa, and T. Nakae. 2004. Crystal structure of the membrane fusion protein, MexA, of the multidrug transporter in Pseudomonas aeruginosa. J. Biol. Chem. 279:25939-25942. [DOI] [PubMed] [Google Scholar]
- 2.Andersen, C., E. Koronakis, C. Hughes, and V. Koronakis. 2002. An aspartate ring at the TolC tunnel entrance determines ion selectivity and presents a target for blocking by large cations. Mol. Microbiol. 44:1131-1139. [DOI] [PubMed] [Google Scholar]
- 3.Balakrishnan, L., C. Hughes, and V. Koronakis. 2001. Substrate-triggered recruitment of the TolC channel-tunnel during type I export of hemolysin by Escherichia coli. J. Mol. Biol. 313:501-510. [DOI] [PubMed] [Google Scholar]
- 4.Baumann, U. 1994. Crystal structure of the 50 kDa metalloprotease from Serratia marcescens. J. Mol. Biol. 242:244-251. [DOI] [PubMed] [Google Scholar]
- 5.Baumann, U., S. Wu, K. M. Flaherty, and D. B. McKay. 1993. Three-dimensional structure of the alkaline protease of P. aeruginosa: a two-domain protein with a calcium binding parallel beta roll motif. EMBO J. 12:3357-3364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benabdelhak, H., S. Kiontke, C. Horn, R. Ernst, M. A. Blight, I. B. Holland, and L. Schmitt. 2003. A specific interaction between the NBD of the ABC-transporter HlyB and a C-terminal fragment of its transport substrate haemolysin A. J. Mol. Biol. 327:1169-1179. [DOI] [PubMed] [Google Scholar]
- 7.Blight, M. A., C. Chervaux, and I. B. Holland. 1994. Protein secretion in Escherichia coli. Curr. Opin. Biotechnol. 5:468-474. [DOI] [PubMed] [Google Scholar]
- 8.Brockman, R. W., and L. A. Heppel. 1968. On the localization of alkaline phosphatase and cyclic phosphodiesterase in Escherichia coli. Biochemistry 7:2554-2562. [DOI] [PubMed] [Google Scholar]
- 9.Chervaux, C., and I. B. Holland. 1996. Random and directed mutagenesis to elucidate the functional importance of helix II and F-989 in the C-terminal secretion signal of Escherichia coli hemolysin. J. Bacteriol. 178:1232-1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Degnen, G. E., and E. C. Cox. 1974. Conditional mutator gene in Escherichia coli: isolation, mapping, and effector studies. J. Bacteriol. 117:477-487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Delepelaire, P., and C. Wandersman. 1998. The SecB chaperone is involved in the secretion of the Serratia marcescens HasA protein through an ABC transporter. EMBO J. 17:936-944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dinh, T., I. T. Paulsen, and M. H. Saier, Jr. 1994. A family of extracytoplasmic proteins that allow transport of large molecules across the outer membranes of gram-negative bacteria. J. Bacteriol. 176:3825-3831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eswaran, J., C. Hughes, and V. Koronakis. 2003. Locking TolC entrance helices to prevent protein translocation by the bacterial type I export apparatus. J. Mol. Biol. 327:309-315. [DOI] [PubMed] [Google Scholar]
- 14.Felmlee, T., S. Pellet, E. Y. Lee, and R. A. Welch. 1985. Nucleotide sequence of an Escherichia coli chromosomal hemolysin. J. Bacteriol. 163:94-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Felmlee, T., and R. A. Welch. 1988. Alterations of amino acid repeats in the Escherichia coli hemolysin affects cytolytic activity and secretion. Proc. Natl. Acad. Sci. USA 85:5269-5273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Genin, S., and C. A. Boucher. 1994. A superfamily of proteins involved in different secretion pathways in gram-negative bacteria: modular structure and specificity of the N-terminal domain. Mol. Gen. Genet. 243:112-118. [DOI] [PubMed] [Google Scholar]
- 17.Gonzalez-Carrero, M. I., J. C. Zabala, F. de la Cruz, and J. M. Ortiz. 1985. Purification of alpha-hemolysin from an overproducing E. coli strain. Mol. Gen. Genet. 199:106-110. [DOI] [PubMed] [Google Scholar]
- 18.Gray, L., K. Baker, B. Kenny, N. Mackman, R. Haigh, and I. B. Holland. 1989. A novel C-terminal signal sequence targets Escherichia coli haemolysin directly to the medium. J. Cell Sci. Suppl. 11:45-57. . [DOI] [PubMed] [Google Scholar]
- 19.Gray, L., N. Mackman, J.-M. Nicaud, and I. B. Holland. 1986. The carboxy-terminal region of haemolysin 2001 is required for secretion of the toxin from Escherichia coli. Mol. Gen. Genet. 205:127-133. [DOI] [PubMed] [Google Scholar]
- 20.Higgins, M. K., E. Bokma, E. Koronakis, C. Hughes, and V. Koronakis. 2004. Strucure of the periplasmic component of a bacterial drug efflux pump. Proc. Natl. Acad. Sci. USA 101:9994-9999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holland, I. B., H. Benabdelhak, J. Young, A. L. Pimenta, L. Schmitt, and M. A. Blight. 2003. Bacterial ABC transporters involved in protein translocation, p. 209-241. In I. B. Holland, S. P. C. Cole, K. Kuchler, and C. F. Higgins (ed.), ABC proteins: from bacteria to men. Elsevier Science, Ltd., London, United Kingdom.
- 22.Holland, I. B., M. A. Blight, and B. Kenny. 1990. The mechanism of secretion of hemolysin and other polypeptides from gram-negative bacteria. J. Bioenerg. Biomembr. 22:473-491. [DOI] [PubMed] [Google Scholar]
- 23.Humphreys, G. O., G. A. Willshaw, H. R. Smith, and E. S. Anderson. 1976. Mutagenesis of plasmid DNA with hydroxylamine: isolation of mutants of multi-copy plasmids. Mol. Gen. Genet. 145:101-108. [DOI] [PubMed] [Google Scholar]
- 24.Issartel, J.-P., V. Koronakis, and C. Hughes. 1991. Activation of Escherichia coli prohaemolysin to the mature toxin by acyl carrier protein-dependent fatty acylation. Nature 351:759-761. [DOI] [PubMed] [Google Scholar]
- 25.Johnson, J. M., and G. M. Church. 1999. Alignment and structure prediction of divergent protein families: periplasmic and outer membrane proteins of bacterial efflux pumps. J. Mol. Biol. 287:695-715. [DOI] [PubMed] [Google Scholar]
- 26.Kenny, B., R. Haigh, and I. B. Holland. 1991. Analysis of the haemolysin transport process through the secretion from Escherichia coli of PCM, CAT or β-galactosidase fused to the Hly C-terminal signal domain. Mol. Microbiol. 5:2557-2568. [DOI] [PubMed] [Google Scholar]
- 27.Kenny, B., S. Taylor, and I. B. Holland. 1992. Identification of individual aminoacids required for secretion within the haemolysin (HlyA) C-terminal targeting region. Mol. Microbiol. 6:1477-1489. [DOI] [PubMed] [Google Scholar]
- 28.Koronakis, V., M. Cross, and C. Hughes. 1988. Expression of the E. coli hemolysin secretion gene hlyB involves transcript anti-termination within the hly operon. Nucleic Acids Res. 16:4789-4800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koronakis, V., E. Koronakis, and C. Hughes. 1989. Isolation and analysis of the C-terminal signal directing export of Escherichia coli hemolysin protein across both bacterial membranes. EMBO J. 8:595-605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koronakis, V., A. Sharff, E. Koronakis, B. Luisi, and C. Hughes. 2000. Crystal structure of the bacterial membrane protein TolC central to multidrug efflux and protein export. Nature 405:914-919. [DOI] [PubMed] [Google Scholar]
- 31.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-683. [DOI] [PubMed] [Google Scholar]
- 32.Lupas, A., M. V. Dyke, and J. Stock. 1991. Predicting coiled-coils from protein sequences. Science 252:1162-1164. [DOI] [PubMed] [Google Scholar]
- 33.Mackman, N., and I. B. Holland. 1984. Functional characterization of a cloned haemolysin determinant from E. coli of human origin, encoding information for the secretion of a 107K polypeptide. Mol. Gen. Genet. 196:129-134. [DOI] [PubMed] [Google Scholar]
- 34.Mackman, N., and I. B. Holland. 1984. Secretion of a 107 K dalton polypeptide into the medium from a haemolysin E. coli K12 strain. Mol. Gen. Genet. 193:312-315. [DOI] [PubMed] [Google Scholar]
- 35.Mackman, N., J.-M. Nicaud, L. Gray, and I. B. Holland. 1985. Genetical and functional organisation of the Escherichia coli haemolysin determinant 2001. Mol. Gen. Genet. 201:282-288. [DOI] [PubMed] [Google Scholar]
- 36.Mackman, N., J.-M. Nicaud, L. Gray, and I. B. Holland. 1986. Secretion of haemolysin by Escherichia coli. Curr. Top. Microbiol. Immunol. 125:159-181. [DOI] [PubMed] [Google Scholar]
- 37.Morelli, G. 1989. A plasmid extraction procedure on a miniprep escale. Focus 11:7. [Google Scholar]
- 38.Nicaud, J.-M., N. Mackman, L. Gray, and I. B. Holland. 1985. Characterisation of HlyC and mechanism of activation and secretion of haemolysin E. coli 2001. FEBS Lett. 187:339-344. [DOI] [PubMed] [Google Scholar]
- 39.Nicaud, J. M., N. Mackman, L. Gray, and I. B. Holland. 1986. The C-terminal 23K peptide of E. coli haemolysin 2001 contains all information necessary for its secretion by the haemolysin (hly) export machinery. FEBS Lett. 204:331-335. [DOI] [PubMed] [Google Scholar]
- 39a.Nikaido, H. 1994. Prevention of drug access to bacterial targets: permeability barriers and active efflux. Science 264:382-388. [DOI] [PubMed] [Google Scholar]
- 40.Pimenta, A. L., J. Young, I. B. Holland, and M. A. Blight. 1999. Antibody analysis of the localisation, expression and stability of HlyD, the MFP component of the E. coli haemolysin translocator. Mol. Gen. Genet. 261:122-132. [DOI] [PubMed] [Google Scholar]
- 41.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 42.Sapriel, G., C. Wandersman, and P. Delepelaire. 2002. The N terminus of the HasA protein and the SecB chaperone cooperate in the efficient targeting and secretion of HasA via the ATP-binding cassette transporter. J. Biol. Chem. 277:6726-6732. [DOI] [PubMed] [Google Scholar]
- 43.Schmitt, L., H. Benabdelhak, M. A. Blight, I. B. Holland, and M. T. Stubbs. 2003. Crystal structure of the nucleotide-binding domain of the ABC-transporter haemolysin B: identification of a variable region within ABC helical domains. J. Mol. Biol. 330:333-342. [DOI] [PubMed] [Google Scholar]
- 44.Schülein, R., I. Gentschev, S. Schlör, R. Gross, and W. Goebel. 1994. Identification and characterization of two functinal domains of the haemolysin translocator protein HlyD. Mol. Gen. Genet. 245:203-211. [DOI] [PubMed] [Google Scholar]
- 45.Schülein, R., I. Gentshev, H.-J. Mollenkopf, and W. Goebel. 1992. A topological model for the haemolysin translocator protein HlyD. Mol. Gen. Genet. 234:155-163. [DOI] [PubMed] [Google Scholar]
- 46.Stanley, P., V. Koronakis, and C. Hughes. 1991. Mutational analysis supports a role for multiple structural features in the C-terminal secretion signal of Escherichia coli haemolysin. Mol. Microbiol. 5:2391-2403. [DOI] [PubMed] [Google Scholar]
- 47.Stanley, P., L. C. Packman, V. Koronakis, and C. Hughes. 1994. Fatty acylation of two internal lysine residues required for the toxic activity of Escherichia coli hemolysin. Science 266:1992-1996. [DOI] [PubMed] [Google Scholar]
- 48.Stanley, P. L. D., P. Diaz, M. J. A. Bailey, D. Gygi, A. Juarez, and C. Hughes. 1993. Loss of activity in the secreted form of Escherichia coli haemolysin caused by an rfaP lesion in core lipopolysaccharide assembly. Mol. Microbiol. 10:781-787. [DOI] [PubMed] [Google Scholar]
- 49.Thanabalu, T., E. Koronakis, C. Hughes, and V. Koronakis. 1998. Substrate-induced assembly of a contiguous channel for protein export from E. coli: reversible bridging of an inner-membrane translocase to an outer membrane exit pore. EMBO J. 17:6487-6496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vakharia, H., G. J. German, and R. Misra. 2001. Isolation and characterization of Escherichia coli tolC mutants defective in secreting enzymatically active alpha-hemolysin. J. Bacteriol. 183:6908-6916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wandersman, C., and P. Delepelaire. 1990. TolC an E. coli outer membrane protein required for hemolysin secretion. Proc. Natl. Acad. Sci. USA 87:4776-4780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang, R., S. J. Seror, M. A. Blight, J. M. Pratt, J. Broome-Smith, and I. B. Holland. 1991. Analysis of the membrane organization of an E. coli protein translocator HlyB, a member of a large family of prokaryote and eukaryote surface transport proteins. J. Mol. Biol. 217:441-454. [DOI] [PubMed] [Google Scholar]
- 53.Wolff, N., G. Sapriel, C. Bodenreider, A. Chaffotte, and P. Delepelaire. 2003. Antifolding activity of the SecB chaperone is essential for secretion of HasA, a quickly folding ABC pathway substrate. J. Biol. Chem. 278:38247-38253. [DOI] [PubMed] [Google Scholar]
- 54.Young, J. 1999. Analyse biochimique des protéines du translocateur de l'hémolysine et leurs intéractions in vivo. Ph.D. thesis. Université Paris XI, Orsay, France.