Abstract
The transcriptome responses to hydrogen peroxide, H2O2, of the facultatively phototrophic bacterium Rhodobacter sphaeroides grown under semiaerobic conditions were investigated. At 7 min after the addition of 1 mM H2O2, the expression of approximately 9% of all genes (total, 394) was changed reliably by at least twofold. At 30 min, the number of genes (total, 88) and the magnitude of expression changes were much lower, indicating rapid recovery from stress. Two types of responses were observed: (i) an H2O2 stress response per se and (ii) a shift to high-oxygen metabolism. The former response involved the upregulation of genes for H2O2 detoxification, protein folding and proteolysis, DNA damage repair, iron transport and storage, iron-sulfur cluster repair, and the downregulation of genes for protein translation, motility, and cell wall and lipopolysaccharide synthesis. The shift to high-oxygen metabolism was evident from the differential regulation of genes for aerobic electron transport chain components and the downregulation of tetrapyrrole biosynthesis and photosystem genes. The abundance of photosynthetic complexes was decreased upon prolonged exposure of R. sphaeroides to H2O2, thus confirming the physiological significance of the transcriptome data. The regulatory pathways mediating the shift to high-oxygen metabolism were investigated. They involved the anaerobic activator FnrL and the antirepressor-repressor AppA-PpsR system. The transcription of FnrL-dependent genes was down at 7 min, apparently due to the transient inactivation by H2O2 of the iron-sulfur cluster of FnrL. The transcription of the AppA-PpsR-dependent genes was down at 30 min, apparently due to the significant decrease in appA mRNA.
In natural environments, microorganisms have to cope with oxidative stress caused by reactive oxygen species (ROS). In aerobically growing bacteria, the autooxidation of the respiratory chain components is believed to be one of the main sources of endogenous ROS (16, 20, 30); however, additional sources exist (48). ROS are also produced by exposure of aerobically grown cells to metals, redox-active chemicals, or radiation. To cope with damage inflicted by ROS, bacteria induce oxidative stress defense systems. These include superoxide dismutases, catalases, and peroxidases directly involved in ROS detoxification and repair mechanisms. The ROS-oxidized cysteine and methionine residues of proteins are repaired (reduced) by the thioredoxin, glutathion/glutaredoxin, and methionine sulfoxide reductase systems. DNA is protected from damage by specific ROS-induced DNA-binding proteins, while damaged DNA is repaired via the SOS response (1, 8, 52).
Rhodobacter sphaeroides is a facultatively phototrophic bacterium belonging to the alpha division of the proteobacteria that can use various pathways for energy generation. At high O2 tension, it uses aerobic respiration and lacks photosynthetic complexes. When O2 tension drops, the expression of the photosystem (PS) genes increases, resulting in the production of photosynthetic complexes. However, aerobic respiration remains the means to generate energy for as long as O2 is present. As the PS is highly sensitive to oxidative damage, anoxygenic photosynthesis is used for energy generation only under anaerobic conditions in the presence of sunlight (54).
In nature, R. sphaeroides is likely to be challenged by oxidative stress derived from the inevitable interactions of its PS with O2 and encounters with ROS from the environment. R. sphaeroides efficiently detoxifies ROS. For example, the wild-type strain grown semiaerobically degrades 3.5 mM exogenously added H2O2 within approximately 3 min (57). We have investigated roles of the individual oxidative stress systems in R. sphaeroides (26-28, 57). In this study, we employed whole-genome transcriptome profiling (38) to gain a comprehensive view of the candidate mechanisms involved in H2O2 tolerance in R. sphaeroides. The H2O2-dependent transcriptome data are available for γ-proteobacteria, Escherichia coli (58), Salmonella enterica (40), Pseudomonas aeruginosa (37), firmicute Bacillus subtilis (17), and unicellular cyanobacterium Synechocystis sp. (25).
The transcriptome analysis of the H2O2 stress in the semiaerobically grown R. sphaeroides revealed two distinct types of responses. One may be classified as the H2O2 stress response per se. Many general trends in transcriptome changes within this response appeared to be similar to what has been observed in other species. However, some unique features of R. sphaeroides have also been uncovered. The regulatory pathways controlling this type of response will be discussed elsewhere. The second type of response may be characterized as a shift toward high-oxygen metabolism. In this work, we identify regulatory pathways involved in the shift toward high-oxygen metabolism and assess the physiological relevance of the transcriptome data.
MATERIALS AND METHODS
Microbiological methods.
R. sphaeroides wild-type strain 2.4.1 was cultivated at 32°C in 50-ml Erlenmeyer flasks containing 40 ml malate minimal salt medium (10) with continuous shaking at 140 rpm. The concentration of dissolved O2 in the medium was approximately 0.85 to 0.90 mg liter−1 (approximately 10% of saturation) at the time when the cultures were treated with exogenously added H2O2, 1 mM final concentration. At time points 0, 7, and 30 min, aliquots were withdrawn for viable cell count and/or RNA extraction. The number of viable cells was calculated based on the number of colonies on agar medium of the same composition after 2 days of incubation. For assessment of the effects of H2O2 on the abundance of photosynthetic complexes, H2O2 was periodically added to the cultures for the total duration of 3 or 6 h.
Spectroscopy.
Whole-cell suspensions of H2O2-treated or control cultures were adjusted to equal A660. The abundance of photosynthetic complexes in cell suspensions was assayed using UV-VIS spectroscopy on a Lambda 12 Spectrometer (Perkin Elmer).
RNA extraction, reverse transcription-PCR, and DNA microarrays.
At time points 0, 7, and 30 min after the addition of 1 mM H2O2, RNA was extracted from cultures by the hot phenol method as described earlier (53, 57). High-density oligonucleotide R. sphaeroides microarrays (Affymetrix gene chips corresponding to the whole 4.6-Mb genome) were used for transcriptome profiling. The microarray contains 4,292 open reading frames, 47 rRNA and tRNA genes, and 394 intergenic regions (presented by both DNA strands); its construction and performance analysis have been described earlier (38). Genomic DNA contamination from RNA samples was removed by DNase treatment, followed by purification on RNeasy minicolumns (QIAGEN, Chatsworth, Calif.). The lack of DNA was verified by quantitative reverse transcription-mediated real time PCR (qPCR), using SYBR Green chemistry and an iCycler iQ (Real Time PCR Detection System; Bio-Rad, Calif.) as described earlier (38). cDNA synthesis, fragmentation, labeling, genechip hybridizations, and scanning were performed according to specifications from Affymetrix (Santa Clara, Calif.) exactly as described earlier (38). RNA and cDNA qualities were tested at each step using capillary electrophoresis (Bioanalyzer; Agilent Technologies). The cDNA aliquots prior to fragmentation were used for quantification by qPCR of the expression of selected genes using primers and conditions described earlier (2, 32, 38).
Microarray data analysis.
We recorded and analyzed transcriptome profiles from three independent cultures per every time point. The experimental reproducibility (r values) between replicates from the same growth conditions ranged from 0.95 to 0.99. Robust multiarray analysis with quantile normalization (21 and http://www.stat.berkeley.edu/users/bolstad/RMAExpress/RMAExpress.html) and the GeneSpring 7.2 software package (Silicon Genetics) were used for gene chip data analysis and representation.
To filter out unreliably measured and unchanged genes, we used two criteria. (i) To filter out unreliable changes, we retained only genes whose expression values from each replicate at a given time point (ai) differed by at least a factor of 1.15 from values for the same gene from each replicate at time point 0 min (aj), i.e., either ai ≥ 1.15 aj or ai ≤ 0.87 aj (38). For genes that passed the first criterion, average values from all replicates were derived. Fold changes, i.e., ratios of expression values at a given time point and at 0 min, were calculated based on these average values. (ii) To filter out potentially insignificant changes among genes that passed the reliability criterion, we applied a twofold cutoff, i.e., we retained those genes whose average expression value at a given time point (āi) compared to the average value at time point 0 min (āj) was either āi ≥ 2.0 āj or āi ≤ 0.50 āj.
The fold changes are shown in the text in parentheses preceded by the RSP numbers of their corresponding genes. When expression of several genes is discussed, the lower and upper fold changes are shown, e.g., a range of two- to fivefold increase is shown as “2.0-5.0.” The expression data obtained here were deposited in the Gene Expression Omnibus (GEO) database of the National Center for Biotechnology Information (www.ncbi.nih.gov/geo), platform GPL162, under the “Oxidative stress” series.
RESULTS AND DISCUSSION
Experimental design and dynamics of H2O2-dependent gene expression changes in R. sphaeroides. Earlier, we analyzed the expression of selected R. sphaeroides genes in response to H2O2 by Northern blots and semiquantitative reverse transcription-PCR (26, 27, 28). As most genes showed a fast response, which was maximal 5 to 10 min after the addition of H2O2, we chose 7 min as the time point for detecting primary changes in gene expression. Given the fast degradation by R. sphaeroides of exogenously added H2O2, we chose 30 min as the time point corresponding to an adapted culture which was anticipated to reveal the long-term consequences of H2O2 stress.
We and others have shown that changes in the R. sphaeroides transcriptome correlate well with the metabolic and physiological changes occurring in this bacterium (38, 44). To ensure that the observed responses are stimulus specific, we used H2O2 concentrations that do not severely impair cellular survival and growth. We have determined that 1 mM H2O2 does not affect cell viability. Cell survival was 100% ± 2% at 7 and 30 min after H2O2 addition, where 100% corresponds to the viable cell count at 0 min. Further, we detected no significant difference in growth between the treated and control cultures.
At 7 min, the expression of 394 genes was changed by ≥2-fold compared to that of the untreated cells; the expression of 179 genes was lower, and that of 215 was higher. This comprises approximately 9% of all genes present on the gene chip, suggesting that moderate H2O2 stress results in modification of the R. sphaeroides transcriptome that is fast and significant in scope (Table S1 in the supplemental material). As expected, many fewer genes (88 total) were affected at 30 min, and expression of the most highly upregulated genes had drastically decreased (Table S2).
Primary responses to H2O2 deduced from the R. sphaeroides transcriptome changes.
Below we discuss major trends characterizing early (7 min) cellular responses to H2O2 deduced from the transcriptome analysis. We were able to place most highly H2O2-responsive genes into several functional categories. We also uncovered highly responsive genes of unknown or poorly understood function. These genes are of interest for subsequent functional analyses (Table 1).
TABLE 1.
Major categories of H2O2-responsive elements in R. sphaeroidesa
| Category and RSP no. | Gene | Change atb:
|
Description | |
|---|---|---|---|---|
| 7 min | 30 min | |||
| H2O2 detoxification | ||||
| RSP0899 | ahpC | 2.2 | 1.8 | Alkyl hydroperoxide reductase |
| RSP0900 | ahpF | 1.8 | 1.2 | Alkyl hydroperoxide reductase |
| RSP2389 | 3.2 | 1.6 | Glutathione peroxidase | |
| RSP2395 | 0.22 | 1.0 | Cytochrome c peroxidase | |
| RSP2779 | katE | 69.2 | 4.5 | Catalase |
| Protein synthesis, maintenance, and repair | ||||
| RSP0166 | dksA | 0.05 | 0.63 | DnaK suppressor protein homolog |
| RSP0554 | 4.1 | 1.3 | HtpX protease | |
| RSP0559 | 3.6 | 1.5 | Peptide methionine sulfoxide reductase | |
| RSP1016 | 3.4 | 3.8 | Hsp20-type heat shock protein | |
| RSP1207 | 2.8 | 1.5 | Hsp33-type heat shock protein | |
| RSP1489 | 2.9 | 1.3 | Thioredoxin-domain-containing protein | |
| RSP1490 | 2.8 | 1.2 | ATP-dependent Lon-type protease | |
| RSP1529 | trxA | 1.9 | 2.4 | Thioredoxin |
| RSP1572 | 2.0 | 1.5 | Hsp20-type heat shock protein | |
| RSP1742 | 2.1 | 1.3 | Putative serine protease | |
| RSP2247 | 0.33 | 0.91 | Translation elongation factor EF-G | |
| RSP2248 | 0.56 | 0.56 | Translation elongation factor EF-G | |
| RSP2293 | clpA | 2.7 | 1.3 | ATPase with chaperone activity |
| RSP2310 | groES | 1.4 | 2.2 | GroES chaperonin |
| RSP3747 | 2.6 | 1.6 | Metallopeptidase | |
| RSP4127 | 7.9 | 1.1 | ATP-dependent Lon-type protease | |
| DNA damage repair/SOS response | ||||
| RSP0353 | dnaE2 | 6.5 | 2.8 | DNA polymerase III alpha subunit |
| RSP0452 | 2.8 | 3.2 | Homologous recombinase and LexA | |
| RSP0553 | ruvB | 2.0 | 1.7 | Holliday junction helicase |
| RSP0556 | ruvC | 1.9 | 2.0 | Holliday junction |
| RSP1997 | lexA | 3.7 | 4.0 | SOS-response repressor |
| RSP2354 | 4.0 | 1.4 | Vacuolar ATPase/ archaeal ATP synthase | |
| Iron transport and metabolism | ||||
| RSP0434 | sufD | 3.6 | 2.3 | FeS-cluster assembly/repair |
| RSP0435 | 5.4 | 2.8 | Possible frame-shift with sufD | |
| RSP0436 | 5.1 | 2.8 | Possible frame-shift with sufD | |
| RSP0437 | sufC | 8.3 | 3.4 | FeS-cluster assembly/repair |
| RSP0438 | 3.5 | 1.5 | Unknown | |
| RSP0439 | 8.1 | 2.5 | Conserved hypothetical protein | |
| RSP0440 | sufB | 9.6 | 2.6 | FeS-cluster assembly/repair |
| RSP0441 | sufB | 10.0 | 3.2 | FeS-cluster assembly/repair |
| RSP0442 | sufA | 9.4 | 1.2 | FeS-cluster assembly/repair, cysteine |
| RSP0443 | 11.2 | 1.6 | Conserved hypothetical protein | |
| RSP0862 | 1.9 | 0.83 | 3-Isopropylmalate dehydratase | |
| RSP0863 | 3.1 | 1.1 | large subunit | |
| RSP0904 | sitA | 4.0 | 1.1 | ABC-type Fe (III) transport system |
| RSP0905 | sitB | 2.4 | 0.91 | ATPase |
| RSP0906 | sitC | 1.8 | 1.0 | transporter |
| RSP0920 | exbB | 14.8 | 2.5 | Biopolymer transport protein |
| RSP0921 | exbD | 17.8 | 2.8 | Biopolymer transport protein |
| RSP0922 | tonB | 9.6 | 2.4 | Protein involved in iron transport |
| RSP1546 | bfr | 3.7 | 1.7 | Bacterioferritin |
| RSP1547 | bfd | 73.6 | 4.6 | Bacterioferritin-associated ferredoxin |
| RSP1806c | acnA | 5.4 | 0.91 | Aconitase |
| RSP1818 | feoB | 0.56 | 0.83 | Fe2+ uptake system protein |
| RSP1819 | feoA | 0.22 | 0.45 | Fe2+ uptake system protein |
| RSP2646 | edo | 6.0 | 1.3 | Phosphogluconate dehydratase |
| RSP2913 | 31.2 | 2.0 | Periplasmic iron binding protein | |
| RSP3074 | 2.2 | 0.91 | Dihydroxyacid dehydratase | |
| RSP3079 | 2.6 | 1.4 | Ferrichrome-binding periplasmic protein | |
| Metabolic changes | ||||
| RSP0446 | icdA | 4.7 | 1.1 | Isocitrate dehydrogenase |
| RSP0962 | 0.45 | 2.0 | 2-Oxoglutarate dehydrogenase | |
| RSP0964 | 0.42 | 1.8 | E2 component | |
| RSP0965 | 0.63 | 1.6 | E1 component | |
| RSP1078 | fdsC | 6.5 | 1.0 | NAD-dependent formate dehydrogenase |
| RSP1079 | fdsB | 8.4 | 1.2 | β subunit |
| RSP1256 | 0.31 | 1.3 | Enoyl-(acyl-carrier-protein) reductase | |
| RSP1257 | pbh | 0.24 | 1.2 | Poly-beta-hydroxyalkanoate (PHB) |
| RSP1806 | acnA | 5.4 | 0.91 | Aconitase |
| RSP1994 | gltA | 2.9 | 0.83 | Citrate synthase |
| Cell wall, lipo, and exopolysaccharide biosynthesis | ||||
| RSP0331 | bcsA | 0.40 | 0.91 | Cellulose synthase |
| RSP0332 | bcsB | 0.32 | 0.77 | Putative regulatory subunit |
| RSP0513 | 0.27 | 1.1 | Putative glycosyl hydrolase | |
| RSP0650 | 0.48 | 0.83 | Lytic murein transglycosylase | |
| RSP1461 | 2.1 | 1.0 | 3-Deoxy-d-manno-octulosonic-acid transferase | |
| RSP1462 | 1.9 | 1.0 | Tetraacyldisaccharide 4′-kinase UDP-3-O-3-hydroxymyristoyl N-acetylglucosamine deacetylase | |
| RSP2115 | 14.6 | 1.2 | UDP-3-O-3-hydroxymyristoyl N-acetylglucosamine deacetylase | |
| RSP2116 | 10.9 | 0.91 | Glucosamine-fructose-6-phosphate | |
| RSP2502 | 2.6 | 1.1 | UDP-N-acetylglucosamine uridyl transferase | |
| RSP2503 | 4.6 | 0.83 | ||
| RSP2550 | 2.4 | 1.3 | Endo-beta-1,3-1,4-glycanase | |
| RSP2716 | 0.48 | 0.91 | Lipid A disaccharide synthetase | |
| RSP3565 | 0.43 | 0.77 | Putative lysozyme | |
| RSP3838 | 0.48 | 0.83 | Cell surface polysaccharide export | |
| Highly regulated genes of unknown functiond | ||||
| RSP0557 | 9.0 | 2.3 | ||
| RSP0775 | 0.20 | 0.71 | ||
| RSP0820 | 0.23 | 0.71 | ||
| RSP0850 | 9.1 | 3.1 | ||
| RSP1170 | 5.2 | 0.45 | ||
| RSP1543 | 5.9 | 1.2 | ||
| RSP1544 | 6.2 | 1.0 | ||
| RSP1545 | 7.3 | 1.1 | ||
| RSP1548 | 73.0 | 4.9 | ||
| RSP1852 | 13.4 | 1.3 | ||
| RSP1948 | 6.0 | 1.9 | ||
| RSP2218 | 5.6 | 1.3 | ||
| RSP2234 | 5.1 | 1.8 | ||
| RSP2337 | 0.11 | 0.91 | ||
| RSP2624 | 34.1 | 28.5 | ||
| RSP2641 | 2.3 | 8.0 | ||
| RSP3078 | 8.5 | 1.7 | ||
| RSP3163 | 3.6 | 4.0 | ||
| RSP3361 | 0.19 | 0.56 | ||
| RSP3750 | 0.06 | 1.0 | ||
| RSP3751 | 0.05 | 1.2 | ||
| RSP3752 | 0.18 | 0.77 | ||
| RSP4160 | 0.11 | 0.50 | ||
| RSP4162 | 0.19 | 0.43 | ||
| RSP4164 | 0.24 | 0.77 | ||
Genes whose products are involved in energy generation, tetrapyrrole biosynthesis, and PS genes (shown in Fig. 1 and 2) are not included in this table.
Significant changes (according to criteria described in Materials and Methods) are in boldface. Selected genes that narrowly missed the cutoffs are included in this table to fully represent functional groups discussed.
Present in more than one category in this table.
Relative change (compared to no-stress conditions), >4 or <0.25.
(i) H2O2 detoxification.
The expression of several genes whose products are directly involved in the degradation of H2O2 was increased. The catalase KatE is important for H2O2 detoxification in R. sphaeroides, as shown by us earlier (57). The katE transcript level was increased by 69-fold (RSP2779, 69.2). However, the expression of the second catalase gene, katC (RSP2380), was at or below reliable detection levels and was not changed in response to H2O2. This is consistent with our earlier observations (57), and, given the low expression, we anticipate that the role of KatC in H2O2 detoxification under these conditions is limited.
What additional mechanisms of H2O2 detoxification may be involved? Expression of the putative alkyl hydroperoxide reductase ahpFC (RSP0899-0900, 1.8-2.2) was increased approximately twofold, implying its involvement in H2O2 detoxification. In Escherichia coli, AhpFC is believed to be the main scavenger of H2O2 produced endogenously, whereas inducible catalases are the main scavengers at high levels of H2O2, e.g., exogenous H2O2 (47). It is peculiar, however, that the induction of R. sphaeroides ahpFC is much lower than that observed in the γ-proteobacterial species or in Synechocystis sp., where it was 10- to 25-fold (25, 37, 40, 58). A gene for glutathione peroxidase (RSP2389, 3.2) was also upregulated, revealing another potential contributor to H2O2 detoxification in R. sphaeroides. Unexpectedly, the cytochrome c peroxidase gene (RSP2395, 0.22) was significantly downregulated. Cytochrome c peroxidases degrade H2O2 by using ferrocytochrome c as an electron donor. In other species, these enzymes are believed to be important for H2O2 stress defense, and their genes are induced by oxidative stress (7, 49). In contrast, this route of H2O2 removal apparently does not operate in R. sphaeroides. Therefore, in R. sphaeroides, catalase KatE, glutathione peroxidase, and AhpFC appear to be primarily responsible for H2O2 degradation.
(ii) Protein synthesis, maintenance, and repair.
The protein synthesis, maintenance, and repair group includes genes whose products are involved in protein translation, folding, the reduction of oxidized Cys and Met residues, and the removal of damaged proteins. The expression of translation elongation factor EF-G (RSP2247-2248, 0.33-0.56) was decreased compared to that of the untreated cells, suggesting potentially lower translation efficiency. One of the most significantly downregulated genes was RSP0166, which is predicted to encode the DnaK suppressor protein (RSP0166, 0.05), a DksA homolog. DksA is an RNA polymerase binding factor recently shown to affect efficiency of rRNA operon transcription depending on environmental conditions (39). It is therefore possible that decreased RSP0166 levels contribute to reduction in protein translation by lowering rRNA transcription. A DksA homolog from P. aeruginosa has been shown to exert translation control over specific genes (22), and a dksA mutant of Shigella flexneri is more sensitive to oxidative damage (31). RSP0166 is predicted to contain a Zn finger, where Zn is coordinated by Cys residues. Interestingly, R. sphaeroides contains yet another, constitutively expressed DksA homolog, RSP2654, that lacks conserved Cys residues. The downregulation of transcript levels for one of the dksA homologs may represent a novel regulatory mechanism involved in oxidative stress. The significance of this phenomenon and the interplay between two DksA homologs in R. sphaeroides warrants further investigation.
Genes encoding chaperones from several heat shock protein families were induced, revealing cellular machinery involved in protein misfolding caused by H2O2 stress: Hsp20 type (RSP1016, 3.4; and RSP1572, 2.0) and Hsp33 type (RSP1207, 2.8). A host of proteases was upregulated to remove damaged proteins, including homologs of E. coli Lon (RSP1490, 2.8; and RSP4127, 7.9), ClpA (RSP2293, 2.7), HtpX (RSP0554, 4.1), and other proteases (RSP1742, 2.1; and RSP3747, 2.6). While some transient inhibition of translation and induction of selected chaperone and protease genes in response to H2O2 stress have been reported in other species (25, 37, 40, 58), the coordinated upregulation of the genes described here is most evident.
Cellular systems involved in the reversal of oxidative damage to Cys and Met residues, which are particularly susceptible to oxidation by H2O2, were activated as judged by upregulation of the genes for peptide methionine sulfoxide reductase (RSP0559, 3.6), a putative thioredoxin-domain-containing protein (RSP1489, 2.9), and thioredoxin trxA (RSP1529, 1.9). The latter gene narrowly missed our cutoff criteria, but its induction by H2O2 has been observed earlier by Northern hybridizations (27).
(iii) DNA damage repair.
H2O2 is a potent DNA damage agent. Therefore, it is not surprising that SOS response components were activated (2, 24, 52), i.e., recA (RSP0452, 2.8), encoding homologous recombinase and coprotease of the SOS regulon repressor LexA (6), Holliday junction resolvase genes ruvBC (RSP0553, 2.0; and RSP0556, 1.9), and dnaE2 (RSP0353, 6.5), encoding a DNA polymerase III α subunit involved in DNA repair. Induction of the recA and ruvBC genes by H2O2 and the involvement of their products in H2O2-induced DNA damage repair have been demonstrated in γ-proteobacteria (37, 40, 58). The lexA gene (RSP1997, 3.7) was also upregulated, apparently as a feedback mechanism (51).
(iv) Iron transport and metabolism.
Protein iron-sulfur clusters, especially of the Fe4S4 type, are susceptible to oxidative degradation that could result in the release of iron. Fe2+ reacts with H2O2 (Fenton reaction) to produce ·OH radicals, whose oxidizing potential and target range supersedes those of H2O2 (18). The observed transcriptome changes in R. sphaeroides reveal the consequences of iron-sulfur cluster destruction and the mechanisms of coping with a potential increase in free iron and pose questions regarding iron transport during H2O2 stress.
Dihydroxyacid dehydratases represent the class of iron-sulfur-cluster-containing enzymes particularly susceptible to H2O2 damage (5). Genes encoding these enzymes are synchronously upregulated following exposure to H2O2, possibly as a compensatory mechanism for lower enzymatic activities, e.g., phosphogluconate dehydratase edd (RSP2646, 6.0), aconitase acnA (RSP1806, 5.4), and other less characterized enzymes of this class (RSP0862-0863, 1.9-3.1; RSP0176, 2.1; and RSP3074, 2.2).
What do R. sphaeroides transcriptome changes reveal about cellular strategies to solve the “iron problem”? One strategy appears to be increased expression of the suf genes (RSP0434-0443, 3.5-11), whose products are involved in de novo assembly and/or repair of the destroyed iron-sulfur clusters in E. coli (9). Iron uptake may also be downregulated under oxidative stress conditions as a protective mechanism against further damage by ROS. In agreement with this strategy, genes predicted to encode an ABC-type Fe2+ transporter, feoAB (RSP1818-1819, 0.22-0.56), were downregulated. Under anaerobic conditions, the orthologous Feo system of E. coli transports Fe2+ in the uncomplexed form (4). In contrast, expression of a number of systems involved in the uptake of Fe3+-siderophore complexes was upregulated, i.e., exbBD and tonB genes (RSP0920-0922, 9.6-17.8) that energize outer membrane transporters of Fe3+ siderophores, genes for the putative Fe3+-siderophore-binding periplasmic proteins (RSP3079, 2.6; and RSP2913, 31.2), and the sitABC (RSP0904-0906, 1.8-4.0) operon predicted to encode the Fe3+ siderophore ABC transporter. As it is difficult to imagine that Fe3+ uptake really increases during H2O2 stress, it is more likely that increased gene expression represents a mechanism for fast recovery from Fe deficiency after the stress, i.e., similar to the upregulation of Fe-containing dihydroxyacid dehydratases. Consistent with this explanation is the observation that an intracellular bacterioferritin-based iron-scavenging system is activated. Bacterioferritin can safely store excessive iron, which is released from destroyed iron-sulfur clusters (41). The expression of genes encoding bacterioferritin bfr (RSP1546, 3.7) and bacterioferritin-associated ferredoxin (RSP1547, 73.6) was greatly increased upon exposure to H2O2. This system likely plays an important role in limiting iron-dependent oxidative damage.
(v) Energy generation.
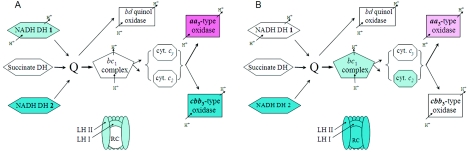
Several key components of the aerobic electron transport chain were synchronously downregulated. Expression levels of genes encoding two type I NADH dehydrogenases (38), the starting points in the electron transport chain, were decreased (RSP0100-0112, 0.2-0.63) and nuo (RSP2517-2531, 0.38-0.77). The cco genes encoding the terminal cytochrome c oxidase cbb3 (RSP0689 and RSP0693-0696, 0.32-0.43) (34, 42) were also downregulated (Fig. 1). One can interpret these changes as a cellular strategy to avoid the additional production of ROS by electron transport chains. However, how do cells generate energy if they downregulate electron flow through the electron transport chains? We suggest that the strategy is not to decrease electron flow but rather to redirect it from one terminal cytochrome c oxidase, cbb3, which predominantly operates under low-oxygen conditions (33), to aa3, which predominantly operates at high-oxygen tension (33). This is supported by the increased expression of the cox/cta genes encoding aa3 cytochrome c oxidase (RSP1826, 7.6; RSP1829, 1.8; and RSP1877, 6.6). Therefore, H2O2 stress results in a shift toward electron transport chains characteristic of the metabolism at high-oxygen tension (38, 54) (Fig. 1).
FIG. 1.
Electron transport chains involved in energy generation under semiaerobic conditions in R. sphaeroides 7 min (A) and 30 min (B) after the addition of 1 mM H2O2. Arrows indicate electron flow. Thicker lines correspond to the anticipated higher electron flow. Known sites for generation of proton-motive force are shown. Expression of genes under no-stress conditions is set as background for comparisons (uncolored). Lack of color corresponds to expression that is not significantly changed from expression under no-stress conditions. Blue color corresponds to decreased expression (light blue, 0.33-0.5; bright blue, <0.33), and pink corresponds to increased expression (light pink, 2-3; pink, >3) of the corresponding genes. Proteins whose genes are expressed below reliable detection are not shown. DH, dehydrogenase; Q, quinone/quinol pool; LH, ligh-harvesting complex; RC, reaction center complex; cyt., cytochrome. The RC, LHI, and LHII complexes comprise the R. sphaeroides PS.
(vi) Metabolic changes.
The genes encoding trichloroacetic acid cycle enzymes involved in acetyl-coenzyme A assimilation, e.g., citrate synthase gltA (RSP1994, 2.9), aconitase acnA (RSP1806, 5.4), and isocitrate dehydrogenase icdA (RSP0446, 4.7), were upregulated. The gene encoding malate dehydrogenase, mdh (RSP0968, 1.8), which is involved in utilization of the main carbon source in the growth medium, might also have been upregulated, although it did not pass our filtering criteria. At the same time, genes involved in the oxidative degradation of acetyl-coenzyme A into CO2 were downregulated, e.g., 2-oxoglutarate dehydrogenase complex lpdA2 and sucABC (RSP0962, 0.45; and RSP0964-0965, 0.42-0.63). This is likely to indicate the decreased production of NADH by the trichloroacetic acid cycle compared to the no-stress levels and is in agreement with the downregulation of the NADH I dehydrogenase genes discussed above. Consistent with the predicted decrease in NADH levels, expression of the pbh genes (RSP1256-1257, 0.24-0.31) is decreased. The pbh genes are involved in biosynthesis of poly-β-hydroxybutyrate, which is the main carbon and reducing equivalents storage material in R. sphaeroides.
Genes encoding two subunits of formate dehydrogenase, fds (RSP1078-1079, 6.5-8.4), were significantly upregulated, whereas the formate-specific subunit (RSP1080) did not change significantly. Formate dehydrogenases oxidize formate to CO2 and may contribute electrons to the respiratory chains. Currently, neither the reason for upregulation nor the differential expression of the formate dehydrogenase subunits is understood.
(vii) Tetrapyrrole biosynthesis and PS genes.
Expression of several tetrapyrrole biosynthesis genes was decreased upon exposure to H2O2. Two functionally redundant oxygen-independent coproporphyrinogen III oxidases, hemZ (RSP0699, 0.07) and hemN (RSP0317, 0.09), were greatly downregulated (Fig. 2). The products of these genes are involved in the biosynthesis of protoporphyrin IX, a common precursor of heme and bacteriochlorophyll (Bchl). Expression of the oxygen-dependent coproporphyrinogen III oxidase encoded by hemF (RSP0682) remained unchanged. Additional genes of protoporphyrin synthesis were also downregulated, although less significantly, e.g., hemA encoding 5-aminolevulinic acid synthase (RSP2984, 0.32) (Fig. 2). Interestingly, the first step specific to heme biosynthesis, i.e., ferrochelation of protoporphyrin IX, was not affected, as judged by the unchanged levels of the ferrochelatase gene, hemH (RSP1197). This observation and the fact that expression of the genes encoding the heme-containing aa3 cytochrome c oxidase is increased suggests that heme synthesis is not limited for the sake of decreasing total heme.
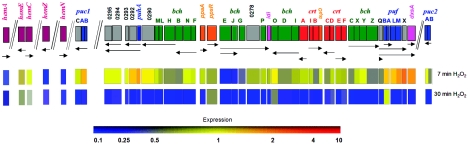
FIG. 2.
Upper panel shows photosystem gene cluster and protoporphyrin IX biosynthesis genes regulated by FnrL and AppA/PpsR. Each gene is represented by a box colored according to its function. bch genes, green; carotenoid crt genes, red; genes encoding structural polypeptides of photocomplexes, blue; genes encoding assembly factors or proteins of unknown function, gray; genes encoding regulatory factors, orange; genes encoding enzymes common to Bchl and ubiquinone biosynthesis, pink; protoporphyrin IX biosynthesis genes, magenta. Putative transcripts are shown as black horizontal arrows. Lower panels show relative expression levels at 7 and 30 min after exposure to H2O2 compared to no-stress conditions. Expression of every gene under the no-stress conditions is assigned 1 (not shown). Expression levels are according to the presented color scheme.
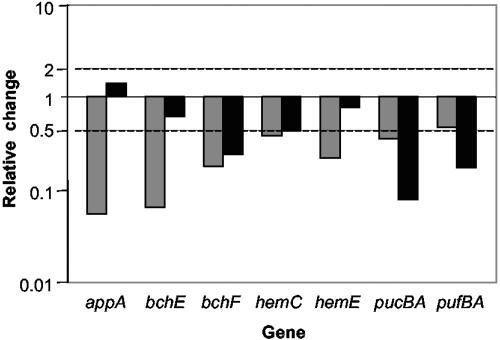
We suggest that the main purpose of decreasing protoporphyrin IX synthesis is to suppress Bchl synthesis. In agreement with this assumption, the expression of several Bchl biosynthesis genes (bch), most significantly the bchEJG operon (RSP0281-0279, 0.11-0.33), was downregulated (Fig. 2). It is worth noting that the expression of additional bch genes was also decreased at this time point, albeit in most cases below our selection criteria. However, at 30 min, all bch genes were downregulated, and so were most other PS genes involved in formation of the photosynthetic apparatus (Fig. 2). To verify gene chip data, we tested expression levels of selected tetrapyrrole and PS genes using qPCR. We found that the qPCR data were in good agreement with the gene chip data, except that the former technique proved to be more sensitive than gene chips (Fig. 3), a trend that we and others have noticed earlier (3, 32, 38). These data suggest that H2O2 suppresses PS formation, which is characteristic of R. sphaeroides grown at high-oxygen tension.
FIG. 3.
Relative change in expression compared to no-stress (0 min) conditions measured by qPCR. The average values of two independent qPCR amplifications performed in triplicate are presented. Gray bars, 7 min; black bars, 30 min.
(viii) Flagellum biosynthesis and chemotaxis.
Expression of numerous flagellum biosynthesis and chemotaxis genes was decreased. These include two large gene clusters (RSP0032-0086, 0.2-0.67; and RSP1582-1589, 0.22-0.67). Apparently, oxidative stress impairs cellular motility and chemotactic abilities. Interestingly, another cluster of flagellum genes present in the R. sphaeroides genome was irresponsive to H2O2, i.e., RSP1302-RSP1332.
(ix) Cell wall, exopolysaccharide biosynthesis, and lipopolysaccharide biosynthesis.
Expression of some genes of the cell wall, exopolysaccharide biosynthesis, and lipopolysaccharide biosynthesis functional category was downregulated, i.e., bsc genes for cellulose synthase (RSP0331-0332, 0.32-0.4), a putative glycosyl hydrolase (RSP0513, 0.27), a soluble lytic murein transglycosylase (RSP0650, 0.48), a putative lysozyme (RSP3565, 0.43), a lipid A disaccharide synthetase (RSP2716, 0.48), and a putative cell surface polysaccharide export protein (RSP3838, 0.48). At the same time, expression of other genes was upregulated, e.g., 3-deoxy-d-manno-octulosonic-acid transferase and tetraacyldisaccharide 4′-kinase (RSP1461-1462, 1.9-2.1), UDP-3-O-3-hydroxymyristoyl N-acetylglucosamine deacetylase (RSP2115-2116, 10.9-14.6), glucosamine-fructose-6-phosphate aminotransferase (isomerizing), and UDP-N-acetylglucosamine UDP transferase (RSP2502-2503, 2.6-4.6) and endoglycanase (RSP2550, 2.4). It is clear that remodeling of the outer membrane and cell wall takes place in response to H2O2. Although it is known that H2O2 and ·OH radicals can increase cell wall permeability (19, 45, 46), the significance and functional consequences of changes in the expression of genes involved in cell wall, exopolysaccharide biosynthesis, and lipopolysaccharide biosynthesis revealed here remain to be investigated.
Adaptation to H2O2 stress revealed by the R. sphaeroides transcriptome changes.
After 30 min of exposure to H2O2, the expression of 88 genes was changed by ≥2-fold (56 down, 32 up) compared to that of the no-stress cells. This is a much smaller fraction of changed transcripts compared to the 394 that responded at 7 min. No new categories of genes whose expression were changed at 30 min of exposure to H2O2 but not at 7 min could be identified. The magnitude of most expression changes at 30 min was also significantly lower compared to that at 7 min. These observations suggest that by 30 min R. sphaeroides has essentially overcome the immediate consequences of H2O2 stress, although the long-term mechanisms of adaptation to H2O2 are likely to be present. This conclusion underscores the importance of early time points for the analysis of primary responses to stresses.
As shown below, we could deduce functional roles of many genes whose expression was changed at 30 min. However, several highly upregulated genes were of unknown or poorly known function, e.g., RSP1548, 4.9; RSP2624, 28.5; and RSP2641, 8.0. These genes may be important for long-term survival in the presence of H2O2 and, therefore, are good candidates for functional analysis.
(i) H2O2 detoxification.
The catalase katE (RSP2779, 4.5) transcript remained upregulated compared to no-stress conditions; however, not nearly as much was upregulated as at 7 min. Expression of other systems predicted to be directly involved in H2O2 degradation returned to the no-stress levels.
(ii) Protein synthesis, maintenance, and repair.
At 30 min after addition of H2O2, translation must have essentially recovered from stress. Expression of most chaperones and protease genes was no longer significantly increased, except for the Hsp20-type protein (RSP1016, 3.8) and groES (RSP2310, 2.2). Transcript levels of the genes involved in reduction of oxidized cysteine and methionine residues were decreased to the no-stress levels, except for that of thioredoxin (trxA) (RSP1529, 2.4).
(iii) DNA damage repair.
The levels of recA (RSP0452, 3.2), ruvBC (RSP0553, 1.7; RSP0556, 2.0), and lexA (RSP1997, 4.0) transcripts remained upregulated at approximately the same levels as they were at 7 min, whereas the extent of upregulation of dnaE2 (RSP0353, 2.8) was lower. Whether this indicates that DNA repair has not yet been completed by 30 min or simply reflects the high stability of the SOS response gene transcripts is unknown.
(iv) Iron transport and metabolism.
None of the dihydroxyacid dehydratase genes was upregulated ≥2-fold at 30 min. This suggests that these enzymes are now functional due to the repair of iron-sulfur clusters by the action of the suf gene products (RSP0434-0443, 1.2-3.4), whose expression remained increased. Expression of feoAB (RSP1818-1819) was close to the control level, whereas expression of another iron transporter, fhu (RSP1437-1440, 2-6), which did not respond at 7 min, was increased. Expression of the exbBD tonB genes (RSP0920-0922, 2.4-2.8) was higher than that at 0 min; however, it was significantly lower than that at 7 min. The above-proposed explanation for the dynamics of iron transport during and after the H2O2 stress warrants further investigation.
(v) Energy generation.
Expression of the cco and rdx genes encoding cbb3 cytochrome c oxidase and its accessory proteins returned to the no-stress level, as did expression of the cta/cox genes encoding aa3 cytochrome c oxidase (Fig. 1). The expression levels of genes encoding two kinds of NADH dehydrogenase I behaved differently, in that one remained low (RSP0100-0112, 0.22-0.71) while the other, nuo (RSP2517-2531), recovered to no-stress levels. The differential expression of the two NADH dehydrogenase complexes has been observed by us in another study (38) but is currently not understood. The expression of additional electron transport genes may have decreased at 30 min (below our criteria), i.e., genes encoding bc1 complex, petAB (RSP1394-1395, 0.53-0.63), and the periplasmic cytochrome c2, cycA (RSP0296, 0.53), none of which was downregulated at 7 min (Fig. 1).
(vi) Metabolic changes, (viii) flagellum biosynthesis and chemotaxis, and (ix) cell wall and lipopolysaccharide biosynthesis.
Expression of most genes in the large categories of metabolic changes, flagellum biosynthesis and chemotaxis and cell wall and lipopolysaccharide biosynthesis, was restored to no-stress levels or slightly higher (Table 1).
(vii) Tetrapyrrole biosynthesis and PS genes.
The hem genes belonging to the central part of tetrapyrrole synthesis remained slightly downregulated at 30 min. However, most of the bch genes were downregulated to a greater extent at 30 min than at 7 min, thus indicating an inhibition of Bchl synthesis (Fig. 2 and 3). In fact, all PS genes were significantly downregulated, including those that encode structural polypeptides of light-harvesting and reaction center complexes, puf, puh, puc1, and puc2 operons, as well as carotenoid synthesis (crt) (Fig. 2 and 3). The decreased PS gene expression is consistent with the shift toward high-oxygen metabolism discussed above.
Regulatory pathways involved in the shift to high-oxygen metabolism after exposure to H2O2.
Based on the scope of transcription changes in response to H2O2, one may predict that multiple regulatory pathways are involved. The fact that the expression of 11 identifiable factors controlling transcript abundance changed at 7 min supports this observation (Tables S1 and S2). The important regulator of long-term survival in the presence of H2O2 in R. sphaeroides is OxyR (57). Interestingly, OxyR turned out to control only a small fraction of H2O2-dependent genes in R. sphaeroides. However, these are genes responsible for major steps in H2O2 stress defense, including catalase katE, iron-sulfur repair suf genes, and bacterioferritin gene bfr (T. Zeller, O. V. Moskvin, K. Li, M. Gomelsky, and G. Klug, unpublished data).
In this study we focus exclusively on the regulatory mechanisms that are responsible for the shift toward high-oxygen metabolism. It is obvious that responses to H2O2 of energy generation pathways, tetrapyrrole, and PS genes resemble their responses to high O2, but it is not obvious why. One possibility is that the breakdown of H2O2 by catalase into H2O and O2 results in an increased intracellular level of O2. Alternatively, similar mechanisms may mediate both O2- and H2O2-dependent regulation of gene expression. Below we discuss the most likely mechanisms involved.
The expression of R. sphaeroides genes involved in electron transport chains, tetrapyrrole biosynthesis, and PS formation is known to be controlled by three major regulatory pathways (54): (i) the anaerobic transcription activator FnrL, a homolog of E. coli FNR (23), (ii) the AppA-PpsR antirepressor-repressor system controlling PS gene expression, and (iii) the PrrBA two-component regulatory system involved in global redox-dependent control. The roles of these systems in H2O2 stress response have not been investigated.
(i) FnrL.
We deduce that the FnrL protein plays a central role in adaptation to H2O2 stress based on the decreased expression of several genes, i.e., hemA, hemN, hemZ, bchEJG, and the cco-rdx cluster (Fig. 1 and 2), known to be dependent on activation by FnrL (33, 35, 43, 55, 56). Further, the cta/cox genes encoding aa3 cytochrome c oxidase, which were upregulated by H2O2 (Fig. 1), contain putative FnrL-binding sites, thus suggesting that FnrL functions as a repressor of these genes (11). DNA binding by E. coli FNR depends on its Fe4S4 cluster, which is sensitive to destruction by O2 and ROS (50). We hypothesize that the oxidative destruction of this cluster in FnrL results in an immediate decrease in DNA binding and subsequent changes in expression of FnrL-dependent genes. FnrL appears to not only function as the key primary regulator of the H2O2 response in R. sphaeroides but also is solely responsible for initiating the shift toward high-oxygen metabolism of energy generation pathways, tetrapyrrole biosynthesis, and PS genes.
It is noteworthy that transcription of the fnrL gene (RSP0698, 3.2) is increased in response to H2O2 at 7 min. However, as the FnrL protein is expected to be inactive at this time point, higher fnrL gene expression is likely to represent a feedback mechanism designed for fast recovery from the stress state after H2O2 is degraded. As judged from the almost complete restoration by 30 min of transcript levels of the FnrL target genes, the Fe4S4 cluster of FnrL must be quickly repaired (due to the products of the suf genes). This is also consistent with the fast recovery of transcripts for the other iron-sulfur-containing enzymes discussed above.
Another noteworthy conclusion based on the response of FnrL-dependent genes to H2O2 is that R. sphaeroides FnrL is active under semiaerobic conditions prior to the addition H2O2. This suggests that its iron-sulfur cluster is more resistant to O2 than that of the E. coli FNR protein. Therefore, the higher tolerance to O2 of R. sphaeroides FnrL compared to that of E. coli FNR proposed earlier by Zeilstra-Ryalls and Kaplan (55) is indirectly confirmed by our gene chip data.
(ii) AppA-PpsR system.
PpsR is a master regulator of PS genes in R. sphaeroides. The PpsR regulon encompasses all PS genes as well as the hemC and hemE genes involved in the central part of tetrapyrrole biosynthesis (32). PpsR repressor activity depends on the oxidation status of two cysteine residues that form an intramolecular disulfide bond as well as on its interaction with the antirepressor AppA (12-15, 29).
The expression of most PS genes did not change at 7 min but decreased significantly at 30 min. The expression of the ppsR gene (RSP0282) was unchanged in response to H2O2; however, that of the appA gene encoding the antirepressor was significantly lower at 7 min (RSP1565, 0.15) (Table 1). According to qPCR data, the appA transcript was decreased even more drastically (Fig. 3). The decreased appA levels should result in stronger repression of the PpsR regulon, similar to the effect of the appA null mutation described earlier (3, 32). One may anticipate then that the response to H2O2 of PpsR-dependent PS genes would be somewhat delayed due to the time required for AppA protein levels to decrease. This is exactly what was observed, i.e., there was much lower PS gene expression at 30 min, long after complete degradation of H2O2, compared to that at 7 min. One may also anticipate a delay in the recovery of PS gene expression compared to the recovery of the appA transcript. This is the case. Whereas the level of appA transcript essentially, but not completely, returned to the prestress levels at 30 min (Fig. 3), the PpsR regulon remained downregulated.
The H2O2 dependence of appA expression presents a previously unknown mechanism of regulation of PS genes. How is appA expression regulated? As no PpsR- or FnrL-binding sites are identifiable upstream of appA, this rules out direct involvement of these regulators. Our unpublished data suggest that the PrrBA pathway is not directly involved either. Therefore, a new regulatory system possibly exists that controls PS gene expression through modulating appA transcript levels.
(iii) PrrBA system.
The expression of the prrB and prrA genes was essentially unchanged during exposure to H2O2. However, activity of the PrrB kinase depends on electron flow to the cbb3 cytochrome c oxidase (36), which may be affected by the lower expression of the cco-rdx cluster (Fig. 1) and result in the lower activity of its cognate response regulator PrrA. The PrrBA system controls numerous genes in R. sphaeroides, some of which overlap with the targets of FnrL and AppA-PpsR (54). Since expression patterns of these genes can be explained by the effect of the FnrL and AppA-PpsR pathways, and no known targets of PrrA but not PpsR appear to be significantly downregulated, we propose that the PrrBA system does not play a major role in the H2O2 response.
In summary, a temporal shift toward high-oxygen metabolism upon exposure to H2O2 apparently originates from the fast inactivation of FnrL, which brings about downregulation of selected tetrapyrrole biosynthesis genes, bchEGJ genes, and cbb3 cytochrome c oxidase as well as upregulation of aa3 cytochrome c oxidase. This is followed by the repression of additional tetrapyrrole biosynthesis genes and virtually all PS genes due to the increased repressor activity of PpsR. This, in turn, is a consequence of lower levels of the antirepressor protein AppA brought about by decreased appA mRNA levels (Fig. 1 and 2).
Physiological consequences of H2O2 stress.
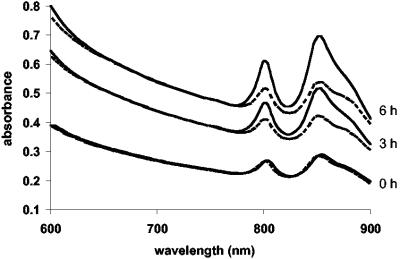
The transcriptome changes in R. sphaeroides in response to H2O2 suggest a physiological shift toward high-oxygen metabolism. To test whether H2O2 results in decreased levels of photosynthetic complexes, a hallmark of growth at high oxygen, we compared photosynthetic complexes in control cultures and in cultures 3 or 6 h after the addition of 1 mM H2O2. In apparent disagreement with the prediction from transcriptome data analysis, no difference was observed (data not shown). However, it is likely that a single dose of H2O2, which was rapidly degraded, was insufficient to produce long-lasting physiological changes. To mimic the conditions of prolonged exposures to ROS, we grew R. sphaeroides cultures and added 1 mM H2O2 every hour. The abundance of photosynthetic complexes after 3 and 6 h of such treatment was significantly decreased as predicted. However, a slowdown in culture growth was also observed (data not shown). To eliminate the differential growth rate effects, we “softened” the imposed H2O2 stress, i.e., we added less H2O2 (0.45 to 0.6 mM) more often (every 20 to 30 min). These treatments did not affect growth, yet they resulted in significant decreases, compared to untreated cultures, in abundance of PS complexes upon extended (3 and 6 h) cultivation (Fig. 4). This validates the transcriptome data presented above.
FIG. 4.
Effect of the periodic (every 20 min) addition of H2O2 (0.45 mM, final concentration) on the abundance of R. sphaeroides photosynthetic complexes. Solid traces, control cultures; dashed traces, H2O2-exposed cultures. Under these conditions, H2O2 did not affect growth rates. Note that the abundance of photosynthetic complexes in the control culture increases with time due to oxygen consumption by the growing culture.
Concluding remarks.
We have deciphered the most significant metabolic and physiological changes that occur in semiaerobically grown R. sphaeroides in response to H2O2 stress. The transcriptome analysis reported here highlights the importance of a carefully designed time course experiment that includes an early time point, within minutes, after the imposition of stress. We have identified two types of responses in R. sphaeroides; one is a response to H2O2 stress per se, and the other one is a shift toward high-oxygen metabolism. Many expression changes characterizing the former response appear to be similar to those described in other bacteria. However, specific responses such as the restructuring of the cell wall, exopolysaccharide biosynthesis, and lipopolysaccharide biosynthesis have thus far been essentially overlooked. Several highly regulated genes of unknown or poorly understood functions identified here present good candidates for functional analysis, as they may play roles in the R. sphaeroides H2O2 stress defense. The shift to high-oxygen metabolism was never identified as part of the response to H2O2. The PS genes belonging to this category are specific to the anoxygenic phototrophic proteobacteria group to which R. sphaeroides belongs. Their downregulation is mediated primarily via the AppA-PpsR regulatory pathway that involves a previously unknown mechanism of regulation of the appA transcript levels. The genes involved in energy generation and their key regulator FnrL is much more common in proteobacteria. Apparently, FnrL plays an important role in the shift toward high-oxygen metabolism induced by H2O2. This work has provided several interesting and unexpected leads for future functional and regulatory studies of oxidative stress in R. sphaeroides.
Supplementary Material
Acknowledgments
This work was supported by DFG Kl563/16-1/16-2/16-3 (G.K.) and NIH NCRR (COBRE) P20 RR15640 (M.G.). T.Z. was the recipient of a fellowship from the “Fonds der Chemischen Industrie” and the BMBF for part of the time.
We thank the staff of the University of Colorado (Denver) Cancer Center Microarray Core Facility, where R. sphaeroides gene chips were processed, and Mark Harpster at UW for proofreading the manuscript.
Footnotes
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1.Almiron, M., A. J. Link, D. Furlong, and R. Kolter. 1992. A novel DNA-binding protein with regulatory and protective roles in starved Escherichia coli. Genes Dev. 6:2646-2654. [DOI] [PubMed] [Google Scholar]
- 2.Boshoff, H. I., M. B. Reed, C. E. Barry III, and V. Mizrahi. 2003. DnaE2 polymerase contributes to in vivo survival and the emergence of drug resistance in Mycobacterium tuberculosis. Cell 113:183-193. [DOI] [PubMed] [Google Scholar]
- 3.Braatsch, S., O. V. Moskvin, G. Klug, and M. Gomelsky. 2004. Reponses of the Rhodobacter sphaeroides transcriptome to blue light under semiaerobic conditions. J. Bacteriol. 186:7726-7735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Braun, V. 2003. Iron uptake by Escherichia coli. Frontiers Biosci. 8:1409-1421. [DOI] [PubMed] [Google Scholar]
- 5.Brown, O. R., E. Smyk-Randall, B. Draczynska-Lusiak, and J. A. Fee. 1995. Dihydroxy-acid dehydratase, a [4Fe-4S] cluster-containing enzyme in Escherichia coli: effects of intracellular superoxide dismutase on its inactivation by oxidant stress. Arch. Biochem. Biophys. 319:10-22. [DOI] [PubMed] [Google Scholar]
- 6.Calero, S., A. R. Fernandez de Henestrosa, and J. Barbe. 1994. Molecular cloning, sequence and regulation of expression of the recA gene of the phototrophic bacterium Rhodobacter sphaeroides. Mol. Gen. Genet. 242:116-120. [DOI] [PubMed] [Google Scholar]
- 7.Campos, É. G., M. Hermes-Lima, J. M. Smith, and R. K. Prichard. 1999. Characterisation of Fasciola hepatica cytochrome c peroxidase as an enzyme with potential antioxidant activity in vitro. Int. J. Parasitol. 29:655-662. [DOI] [PubMed] [Google Scholar]
- 8.Chen, L., L. Keramati, and J. D. Helmann. 1995. Coordinate regulation of Bacillus subtilis peroxide stress genes by hydrogen peroxide and metal ions. Proc. Natl. Acad. Sci. USA 92:8190-8194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Djaman, O., F. W. Outten, and J. A. Imlay. 2004. Repair of oxidized iron-sulfur clusters in Escherichia coli. J. Biol. Chem. 279:44590-44599. [DOI] [PubMed] [Google Scholar]
- 10.Drews, G. 1983. Mikrobiologisches Praktikum. Springer Verlag, Heidelberg, Germany.
- 11.Flory, J. E., and T. J. Donohue. 1997. Transcriptional control of several aerobically induced cytochrome structural genes in Rhodobacter sphaeroides. Microbiology 143:3101-3110. [DOI] [PubMed] [Google Scholar]
- 12.Gomelsky, M., and S. Kaplan. 1995a. Genetic evidence that PpsR from Rhodobacter sphaeroides 2.4.1 functions as a repressor of puc and bchF expression. J. Bacteriol. 177:1634-1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gomelsky, M., and S. Kaplan. 1995b. appA, a novel gene encoding a trans-acting factor involved in the regulation of photosynthesis gene expression in Rhodobacter sphaeroides 2.4.1. J. Bacteriol. 177:4609-4618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gomelsky, M., and S. Kaplan. 1997. Molecular genetic evidence suggesting interactions between AppA and PpsR in regulation of photosynthesis gene expression in Rhodobacter sphaeroides 2.4.1. J. Bacteriol. 179:128-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gomelsky, M., and S. Kaplan. 1998. AppA, a redox regulator of photosystem formation in Rhodobacter sphaeroides 2.4.1, is a flavoprotein. Identification of a novel FAD binding domain. J. Biol. Chem. 273:35319-35325. [DOI] [PubMed] [Google Scholar]
- 16.Gonzalez-Flecha, B., and B. Demple. 1995. Metabolic sources of hydrogen peroxide in aerobically growing Escherichia coli. J. Biol. Chem. 270:13681-13687. [DOI] [PubMed] [Google Scholar]
- 17.Helmann, J. D., M. F. Wu, A. Gaballa, P. A. Kobel, M. M. Morshedi, P. Fawcett, and C. Paddon. 2003. The global transcriptional response of Bacillus subtilis to peroxide stress is coordinated by three transcription factors. J. Bacteriol. 185:243-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Henle, E. S., Y. Luo, and S. Linn. 1996. Fe2+, Fe3+, and oxygen react with DNA-derived radicals formed during iron-mediated Fenton reactions. Biochemistry 35:12212-12219. [DOI] [PubMed] [Google Scholar]
- 19.Higgins, V. J., N. Alic, G. W. Thorpe, M. Breitenbach, V. Larsson, and I. W. Dawes. 2002. Phenotypic analysis of gene deletant strains for sensitivity to oxidative stress. Yeast 19:203-214. [DOI] [PubMed] [Google Scholar]
- 20.Imlay, J. A., and I. Fridovich. 1991. Assay of metabolic superoxide production in Escherichia coli. J. Biol. Chem. 266:6957-6965. [PubMed] [Google Scholar]
- 21.Irizarry, R. A., B. M. Bolstad, F. Collin, L. M. Cope, B. Hobbs, and T. P. Speed. 2003. Summaries of Affymetrix GeneChip probe level data. Nucleic Acids Res. 31:e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jude, F., T. Kohler, P. Branny, K. Perron, M. P. Mayer, R. Comte, and C. van Delden. 2003. Posttranscriptional control of quorum-sensing-dependent virulence genes by dksA in Pseudomonas aeruginosa. J. Bacteriol. 185:3558-3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kiley, P. J., and H. Beinert. 2003. The role of Fe-S proteins in sensing and regulation in bacteria. Curr. Opin. Microbiol. 6:181-185. [DOI] [PubMed] [Google Scholar]
- 24.Konola, J. T., K. E. Sargent, and J. B. Gow. 2000. Efficient repair of hydrogen peroxide-induced DNA damage by Escherichia coli requires SOS induction of RecA and RuvA proteins. Mutat. Res. 459:187-194. [DOI] [PubMed] [Google Scholar]
- 25.Li, H., A. K. Singh, L. M. McIntyre, and L. A. Sherman. 2004. Differential gene expression in response to hydrogen peroxide and the putative PerR regulon of Synechocystis sp. strain PCC 6803. J. Bacteriol. 186:3331-3345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li, K., E. Haertig, and G. Klug. 2003. Thioredoxin 2 is involved in oxidative stress defence and redox-dependent expression of photosynthesis genes in Rhodobacter capsulatus. Microbiology 149:419-430. [DOI] [PubMed] [Google Scholar]
- 27.Li, K., C. Pasternak, and G. Klug. 2003. Expression of the trxA gene for thioredoxin 1 in Rhodobacter sphaeroides during oxidatives stress. Arch. Microbiol. 180:484-489. [DOI] [PubMed] [Google Scholar]
- 28.Li, K., S. Hein, W. Zou, and G. Klug. 2004. The glutathione-glutaredoxin system in Rhodobacter capsulatus: part of a complex regulatory network controlling defense against oxidative stress. J. Bacteriol. 186:6800-6808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Masuda, S., and C. Bauer. 2002. AppA is a blue light photoreceptor that antirepresses photosynthesis gene expression in Rhodobacter sphaeroides. Cell 110:613-623. [DOI] [PubMed] [Google Scholar]
- 30.Messner, K. R., and J. A. Imlay. 1999. The identification of primary sites of superoxide and hydrogen peroxide formation in the aerobic respiratory chain and sulfite reductase complex of Escherichia coli. J. Biol. Chem. 274:10119-10128. [DOI] [PubMed] [Google Scholar]
- 31.Mogull, S. A., L. J. Runyen-Janecky, M. Hong, and S. M. Payne. 2001. dksA is required for intercellular spread of Shigella flexneri via an RpoS-independent mechanism. Infect. Immun. 69:5742-5751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moskvin, O. V., L. Gomelsky, and M. Gomelsky. 2005. Transcriptome analysis of the Rhodobacter sphaeroides PpsR regulon: PpsR as a master regulator of photosystem development. J. Bacteriol. 187:2148-2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mouncey, N. J., and S. Kaplan. 1998. Oxygen regulation of the ccoN gene encoding a component of the cbb3 oxidase in Rhodobacter sphaeroides 2.4.1T: involvement of the FnrL protein. J. Bacteriol. 180:2228-2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oh, J. I., and S. Kaplan. 1999. The cbb3 terminal oxidase of Rhodobacter sphaeroides 2.4.1: structural and functional implications for the regulation of spectral complex formation. Biochemistry 38:2688-2696. [DOI] [PubMed] [Google Scholar]
- 35.Oh, J. I., J. M. Eraso, and S. Kaplan. 2000. Interacting regulatory circuits involved in orderly control of photosynthesis gene expression in Rhodobacter sphaeroides 2.4.1. J. Bacteriol. 182:3081-3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oh, J. I., I. J. Ko, and S. Kaplan. 2004. Reconstitution of the Rhodobacter sphaeroides cbb3-PrrBA signal transduction pathway in vitro. Biochemistry 43:7915-7923. [DOI] [PubMed] [Google Scholar]
- 37.Palma, M., D. DeLuca, S. Worgall, and L. E. N. Quadri. 2004. Transcriptome analysis of the response of Pseudomonas aeruginosa to hydrogen peroxide. J. Bacteriol. 186:248-252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pappas, C. T., J. Sram, O. V. Moskvin, P. S. Ivanov, R. C. Mackenzie, M. Choudhary, M. L. Land, F. W. Larimer, S. Kaplan, and M. Gomelsky. 2004. Construction and validation of the Rhodobacter sphaeroides 2.4.1 DNA microarray: transcriptome flexibility at diverse growth modes. J. Bacteriol. 186:4748-4758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Paul, B. J., W. Ross, T. Gaal, and R. L. Gourse. 2004. rRNA transcription in Escherichia coli. Annu. Rev. Genet. 38:749-770. [DOI] [PubMed] [Google Scholar]
- 40.Porwollik, S., J. Frye, L. D. Florea, F. Blackmer, and M. McClelland. 2003. A non-redundant microarray of genes for two related bacteria. Nucleic Acids Res. 31:1869-1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ringeling, P. L., S. L. Davy, F. A. Monkara, C. Hunt, D. P. Dickson, A. G. McEwan, and G. R. Moore. 1994. Iron metabolism in Rhodobacter capsulatus. Characterisation of bacterioferritin and formation of non-haem iron particles in intact cells. Eur. J. Biochem. 223:847-855. [DOI] [PubMed] [Google Scholar]
- 42.Roh, J. H., and S. Kaplan. 2000. Genetic and phenotypic analyses of the rdx locus of Rhodobacter sphaeroides 2.4.1. J. Bacteriol. 182:3475-3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Roh, J. H., and S. Kaplan. 2002. Interdependent expression of the ccoNOQP-rdxBHIS loci in Rhodobacter sphaeroides 2.4.1. J. Bacteriol. 184:5330-5338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Roh, J. H., W. E. Smith, and S. Kaplan. 2004. Effects of oxygen and light intensity on transcriptome expression in Rhodobacter sphaeroides 2.4.1 Redox active gene expression profile. J. Biol. Chem. 279:9146-9155. [DOI] [PubMed] [Google Scholar]
- 45.Samoilenko, I. I., E. I. Vasil'eva, I. B. Pavlova, and M. A. Tumanian. 1983. Mechanisms of the bactericidal action of hydrogen peroxide. Z. Mikrobiol. Epidemiol. Immunobiol. 12:30-33. [PubMed] [Google Scholar]
- 46.Schweikert, C., A. Liszkay, and P. Schopfer. 2002. Polysaccharide degradation by Fenton reaction- or peroxidase-generated hydroxyl radicals in isolated plant cell walls. Phytochemistry 61:31-35. [DOI] [PubMed] [Google Scholar]
- 47.Seaver, L. C., and J. A. Imlay. 2001. Alkyl hydroperoxide reductase is the primary scavenger of endogenous hydrogen peroxide in Escherichia coli. J. Bacteriol. 183:7173-7181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Seaver, L. C., and J. A. Imlay. 2004. Are respiratory enzymes the primary sources of intracellular hydrogen peroxide? J. Biol. Chem. 279:48742-48750. [DOI] [PubMed] [Google Scholar]
- 49.Seib, K. L., H. J. Tseng, A. G. McEwan, M. A. Apicella, and M. P. Jennings. 2004. Defences against oxidative stress in Neisseria gonorrhoeae and Neisseria meningitidis: distinctive systems for different lifestyles. J. Infect. Dis. 190:136-147. [DOI] [PubMed] [Google Scholar]
- 50.Sutton, V. R., A. Stubna, T. Patschkowski, E. Munck, H. Beinert, and P. J. Kiley. 2004. Superoxide destroys the [2Fe-2S]2+ cluster of FNR from Escherichia coli. Biochemistry 43:791-798. [DOI] [PubMed] [Google Scholar]
- 51.Tapias, A., S. Campoy, and J. Barbe. 2000. Analysis of the expression of the Rhodobacter sphaeroides lexA gene. Mol. Gen. Genet. 2636:957-965. [DOI] [PubMed] [Google Scholar]
- 52.Volkert, M. R., and P. Landini. 2001. Transcriptional responses to DNA damage. Curr. Opin. Microbiol. 4:178-185. [DOI] [PubMed] [Google Scholar]
- 53.von Gabain, A., J. G. Belasco, J. L. Schottel, A. C. Y. Chang, and S. N. Cohen. 1983. Decay of mRNA in Escherichia coli: investigation of the fate of specific segments of transcripts. Proc. Natl. Acad. Sci. USA 80:653-657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zeilstra-Ryalls, J. H., and S. Kaplan. 2004. Oxygen intervention in the regulation of gene expression: the photosynthetic bacterial paradigm. Cell. Mol. Life Sci. 61:417-436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zeilstra-Ryalls, J. H., and S. Kaplan. 1995. Aerobic and anaerobic regulation in Rhodobacter sphaeroides 2.4.1: the role of the fnrL gene. J. Bacteriol. 177:6422-6431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zeilstra-Ryalls, J. H., and S. Kaplan. 1998. Role of the fnrL gene in photosystem gene expression and photosynthetic growth of Rhodobacter sphaeroides. J. Bacteriol. 180:1496-1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zeller, T., and G. Klug. 2004. Detoxification of hydrogen peroxide and expression of catalase genes in Rhodobacter. Microbiology 150:3451-3462. [DOI] [PubMed] [Google Scholar]
- 58.Zheng, M., X. Wang, L. J. Templeton, D. R. Smulski, R. A. LaRossa, and G. Storz. 2001. DNA microarray-mediated transcriptional profiling of the Escherichia coli response to hydrogen peroxide. J. Bacteriol. 183:4562-4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.