Abstract
In enteric bacteria, adaptation to a number of different stresses is mediated by the RpoS protein, one of several sigma factors that collectively allow a tailored transcriptional response to environmental cues. Stress stimuli including low temperature, osmotic shock, nutrient limitation, and growth to stationary phase (SP) all result in a substantial increase in RpoS abundance and activity. The mechanism of regulation depends on the specific signal but may occur at the level of transcription, translation, protein activity, or targeted proteolysis. In both Escherichia coli and Salmonella enterica, SP induction of RpoS in rich medium is >30 fold and includes effects on both transcription and translation. Recently, we found that SP control of rpoS transcription in S. enterica involves repression of the major rpoS promoter during exponential phase by the global transcription factor Fis. Working primarily with E. coli, we now show that 24 nucleotides of the rpoS ribosome-binding site (RBS) are necessary and sufficient for a large part of the increase in rpoS translation as cells grow to SP. Genetic evidence points to an essential role for the leader nucleotides just upstream of the Shine-Dalgarno sequence but is conflicted on the question of whether sequence or structure is important. SP regulation of rpoS is conserved between E. coli and S. enterica. When combined with an fis mutation to block transcriptional effects, replacement of the rpoS RBS sequence by the lacZ RBS eliminates nearly all SP induction of RpoS.
Bacteria maintain intricate signaling networks that sense the environment and adjust cellular physiology accordingly. In Salmonella enterica and Escherichia coli, unfavorable growth conditions (including nutrient limitation, outright starvation, low temperature, osmotic shock, as well as other stresses) initiate a generalized stress response by triggering increased abundance of the RNA polymerase sigma factor RpoS (σS) (for a review, see reference 15). In association with RNA polymerase, RpoS directs transcription of as much as 10% of the E. coli genome, including genes necessary for stress resistance and virulence (12, 45). RpoS thereby serves as the central regulator of the general protective response (15).
The transition to stationary phase (SP) is accompanied by morphological and physiological changes resulting in a nondividing and multiple-stress-resistant state. Growth into SP in rich media such as Luria-Bertani (LB) leads to a dramatic increase of >30 fold in RpoS abundance (13, 16, 17, 20, 27, 31, 36). In recent work, we characterized transcriptional regulation of rpoS in S. enterica as cells enter SP (17). The mechanism involves Fis, a DNA-binding protein which acts globally as a transcription factor. Fis is itself growth-phase regulated in an inverse relationship to RpoS: the Fis protein is undetectable in SP but rapidly increases to a level of >40,000 dimers per cell upon dilution into fresh medium (1, 3, 33). A strong Fis-binding site near the major rpoS promoter (PrpoS) is required for this regulation. Fis likely binds to this site specifically during exponential growth, resulting in repression of rpoS transcription (17). As cells enter SP, Fis disappears, and rpoS transcription increases nearly 10 fold (1, 17).
The rpoS transcript contains a 565-nucleotide (565-nt) 5′ untranslated leader region which includes the ribosome-binding site (RBS) (15, 40). Here, we use this term to refer to a region including a short stretch of nucleotides upstream of the Shine-Dalgarno (SD) sequence and extending to the initiation codon. The rpoS leader includes an antisense element (leader nt 461 to 478) that can pair with the rpoS RBS and inhibit translation, presumably by blocking ribosome access (7). The antisense element is the reported target of three regulatory RNAs that are thought to alter conformation of the RBS to an “open” position, increasing translation (21, 22, 24, 37, 35, 43; for a review, see reference 14). The best-characterized example of regulation of rpoS translation occurs at low temperature and relies on the direct pairing of the antisense element with the 85-nt regulatory RNA, DsrA (39). This interaction activates rpoS translation 5 to 10 fold and is mediated by the RNA-binding protein Hfq (24, 35).
In the present study, we show that 24 nt of the rpoS RBS are necessary and sufficient for a nearly 10-fold increase in rpoS translation as cells grow to SP. Replacement of this sequence by the RBS of lacZ in a fis mutant background virtually eliminates SP induction of RpoS.
MATERIALS AND METHODS
Bacterial strains and construction.
Most strains used in this study are derived from the wild-type Escherichia coli K-12 strain MG1655 (Table 1). The parental strain was CF7968, which is MG1655 that has been corrected to rph+ (19) and deleted for lacIZ, obtained from M. Cashel. Phage P1 vir was used for transduction in E. coli by standard methods (38). The katE-lac operon ([op]) fusion used in this work has been described previously and is a reporter of RpoS activity (6, 8, 17). All fusions in E. coli are located in single copy in the trp region of the bacterial chromosome as described previously (11).
TABLE 1.
Bacterial strains
| Strain | Genotypea |
|---|---|
| E. coli | |
| CF7968 | MG1655 Δ(lacIZ) rph+ |
| DY330 | W3110 lacU169 [λ cI857ts Δ(cro-bioA)] |
| TE8402 | DY330 trpDC700::putPA1303::Kanr-rpoS (ClaI, codon 8)-lac [pr] |
| TE8403 | DY330 trpDC700::putPA1303::Kanr-rpoS (ClaI, codon 8)-RNAse III site-lac [op] |
| TE8419 | CF7968 trpDC700::putPA1303::tetAR-lacUV5p-rpoS (+1, codon 8)-lac [pr] |
| TE8420 | CF7968 trpDC700::putPA1303::tetAR-lacUV5p-rpoS (542, codon 8)-lac [pr] |
| TE8448 | CF7968 trpDC700::putPA1303::tetAR-lacUV5p-lac (TCACACAGGAACAGCT)-rpoS (ATG, codon 8)-lac [pr] |
| TE8483 | CF7968 trpDC700::putPA1303::tetAR-lacUV5p-rpoS (542, 558)-lac (AACAGCT)-rpoS (ATG, codon 8)-lac [pr] |
| TE8520 | CF7968 trpDC700::putPA1303::tetAR-lacUV5p-(TCACAC)-rpoS (554, codon 8)-lac [pr] |
| TE8999 | CF7968 trpDC700::putPA1303::tetAR-lacUV5p-rpoS (549, codon 8)-lac [pr] |
| TE9024 | CF7968 trpDC700::putPA1303::tetAR-lacUV5p-rpoS (542, codon 8; G550C, G551C)-lac [pr] |
| TE9030 | CF7968 trpDC700::putPA1303::tetAR-lacUV5p-rpoS (545, codon 8)-lac [pr] |
| TE9036 | CF7968 trpDC700::putPA1303::tetAR-lacUV5p-rpoS (553, codon 8)-lac [pr] |
| TE9059 | DY330 trpDC700::putPA1303::Kanr-lacUV5p-lac [op] |
| TE9042 | CF7968 trpDC700::putPA1303::tetAR-lacUV5p-rpoS (+1,542)-(TCACACAGGAACAGCT)-rpoS (ATG, codon 8)-lac [pr] |
| TE9145 | CF7968 trpDC700::putPA1303::Kanr-katE-lac [op] |
| TE9146 | CF7968 trpDC700::putPA1303::tetAR-lacUV5p-rpoS (542, 565)-lac (ATG) [pr] |
| TE9200 | CF7968 trpDC700::putPA1303::tetAR-lacUV5p-rpoS (542, codon 8; G550C, G551C, C563G, T564G)-lac [pr] |
| TE9249 | CF7968 trpDC700::putPA1303::tetAR-lacUV5p-rpoS (542, 558)-(AACAGCT)-lac [op] |
| TE9251 | CF7968 trpDC700::putPA1303::tetAR-lacUV5p-GGGTTCCACCAGGAGTGGGCACT-lac [pr] |
| TE9270 | CF7968 trpDC700::putPA1303::tetAR-lacUV5p-ATCGATTGAGAGGATTTGA-lac [op] |
| TE9274 | DY330 trpDC700::putPA1303::Kanr-rpoS (claI, codon 8)-cat-lac [op] |
| TE9277 | DY330 trpDC700::putPA1303::Kanr-rpoS (claI, codon 8)-lac [op] |
| TE9284 | CF7968 trpDC700::putPA1303::Kanr-katE-lac [op] rpoS(541)-AATTTCACACAGGAAACAGCT-rpoS (ATG) |
| TE9300 | CF7968 trpDC700::putPA1303::tetAR-lacUV5p-rpoS (542, codon 8)-lac [op] |
| TE9302 | CF7968 trpDC700::putPA1303::Kanr-katE-lac [op] fis::cat |
| TE9303 | CF7968 trpDC700::putPA1303::Kanr-katE-lac [op] rpoS (541)-AATTTCACACAGGAAACAGCT-rpoS (ATG) fis::cat |
| TE9304 | CF7968 trpDC700::putPA1303::tetAR-lacUV5p-GGGTTCCACCAGGAGTGGGCACT-lac [op] |
| TE9305 | CF7968 trpDC700::putPA1303::tetAR-lacUV5p-rpoS (542, codon 8; G550C, G551C, C563G, T564G)-lac [op] |
| TE9335 | CF7968 trpDC700::putPA1303::tetAR-lacUV5p-rpoS (542, codon 8; G550C, G551C)-lac [op] |
| TE9379 | CF7968 trpDC700::putPA1303::tetAR-lacUV5p-rpoS (542, codon 8; C563G, T564G)-lac [op] |
| TE9380 | CF7968 trpDC700::putPA1303::tetAR-lacUV5p-rpoS (542, codon 8; C563G, T564G)-lac [pr] |
| S. enterica | |
| LT2 | Wild type (mviA V102G) |
| LT2A | LT2 mviA+ |
| TE9195 | LT2A putPA1303::tetAR-lacUV5p-rpoS (542, codon 8)-lac [pr] |
| TE9196 | LT2 putPA1303::tetAR-lacUV5p-rpoS (542, codon 8)-lac [pr] |
| TE9294 | LT2A putPA1303::tetAR-lacUV5p-lac (TCACACAGGAACAGCT)-rpoS (ATG, codon 8)-lac [pr] |
| TE9295 | LT2 putPA1303::tetAR-lacUV5p-lac (TCACACAGGAACAGCT)-rpoS (ATG, codon 8)-lac [pr] |
All numbers shown in parentheses are base pairs (unless otherwise indicated) relative to the first nucleotide of the transcript originating from PrpoS. In this description, the first nucleotide of the rpoS transcript corresponds to nt 2866139 of GenBank AE000111 for E. coli and nt 12589 of GenBank AE008833.1 for S. enterica serovar Typhimurium. The standard abbreviations [op] and [pr] represent transcriptional and translational fusions, respectively. Kanr, kanamycin resistance.
We also investigated the behavior of some rpoS-lacZ constructs in Salmonella enterica serovar Typhimurium. The parental strain was LT2, obtained from J. Roth, or LT2A (9, 17). To this end, constructs in E. coli were transduced into a galE mutant of S. enterica serovar Typhimurium by using P1 vir as described previously (9, 11). The phage P22 mutant HT105/1 int-201 was then used for transduction in S. enterica serovar Typhimurium by standard methods (10). All fusions in S. enterica serovar Typhimurium are located in single copy at the putPA locus (11).
Media and growth conditions.
Bacteria were grown at 37°C in LB medium (38) and on nutrient agar (NB) plates containing 5 g of NaCl per liter, except where indicated. Minimal agar was prepared with NCE medium containing 0.2% glucose (4). Liquid minimal medium was morpholinepropanesulfonic acid (MOPS) medium (32) with modifications as described previously (5), supplemented with 0.2% glucose as the carbon and energy source. Antibiotics were added to final concentrations in selective media as follows: 20 μg of chloramphenicol/ml, 50 μg of kanamycin sulfate/ml, and 20 μg of tetracycline hydrochloride/ml. X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) was used at 50 μg/ml.
Fusion construction.
We took advantage of our previously described method for making lac transcriptional and translational constructs in which a region of interest is inserted between the promoter PlacUV5 and the lacZYA genes (16). This method relies on the high-efficiency λ Red recombination system of Yu et al. (46). A chromosomal template with the tetAR cassette immediately upstream of PlacUV5 was amplified by PCR, using the following primer design (all primer elements are listed in order from 5′ to 3′): (i) a common upstream 60-mer oligonucleotide that contains 40 nt of kan homology to mediate upstream recombination, followed by 20 nt of a tetA priming sequence; and (ii) construct-specific 80-mer oligonucleotides with 40 nt of either lac or rpoS homology for downstream recombination, followed by a variable region of interest preceding priming homology within the lacUV5 promoter. The resulting PCR products have the following structure: kan-tetAR-PlacUV5-region of interest-rpoS codons 1 to 8-lacZYA. PCR products with downstream homology to rpoS were used to transform TE8402 or TE9277 to generate translational or transcriptional fusions, respectively. When recombination directly to the lacZ coding sequence was desired, strain TE9059 served as the recipient and PCR products had a slightly different arrangement: kan-tetAR-PlacUV5-region of interest-lacZYA. Transformants were selected on NB plates containing tetracycline. The region extending from upstream of PlacUV5 through the downstream recombination site (codon 12 of lacZ) was sequenced. All fusions were then backcrossed into E. coli CF7968 (MG1655 ΔlacIZ). The sequences for primers used in this study are available upon request.
Unselected chromosomal mutations.
To replace the rpoS RBS by that of lacZ in the rpoS native context (i.e., at the rpoS locus), a strain with λ Red was utilized that carries the katE-lac [op] fusion. First, 24 nt including the rpoS RBS (reading from 5′ to 3′), GGGATCACGGGTAGGAGCCACCTT, were replaced by tetAR. Transformants had a Lac− phenotype on NB plates containing tetracycline and X-Gal. Next, a 188-bp PCR product was generated from a wild-type template with an upstream rpoS primer (position 410 of the rpoS transcript) and a downstream primer that contained (i) 40 nt of homology to allow recombination downstream of the ATG initiation codon of rpoS, (ii) 17 nt of the lacZ RBS (AATTTCACACAGGAAACAGCT), and (iii) 19 nt of priming homology to the rpoS leader (nt 541 to 522). This PCR product was used to transform the Tetr strain constructed in the first step, followed by dilution into fresh medium for overnight growth. Cultures were then diluted and plated on NB containing kanamycin and X-Gal. The phenotype of desired transformants was Lac+; these were recovered at a frequency of >10−4, and the substitution was confirmed by DNA sequencing. This unmarked mutation was then transduced into a MG1655 background by using a recipient strain which contains the katE-lac [op] fusion, ΔrpoS::cat (Camr) and ΔcysC::tetAR. The cysC locus is ≈6 kb from rpoS and tightly linked to it by P1 transduction. Cys+ Lac+ transductants were selected on minimal plates containing X-Gal, and the lacZ RBS replacement in the rpoS leader was confirmed again by sequencing.
Typically, our lac [op] constructs contain a RNase III processing site (6, 23), which insulates lacZ expression from variations due to differences in upstream sequences. For the experiments described here, this property is not desirable. A similar nonselective transformation method to that described above was employed to eliminate the processing site from a strain containing the kan-PlacUV5-rpoS (codon 8) transcriptional fusion (TE8403). Briefly, the cat gene was used to make an insertion-deletion with loss of 76 bp, including the RNase III cleavage site, located in the 170-nt spacer region between rpoS codon 8 and the lacZ RBS. The desired transformants were Lac− (TE9274). Next, a PCR product was generated with a structure of rpoS codons 6 to 8, followed by 31 nt of the spacer region, the lacZ RBS, and 144 nt of the adjacent lacZ coding sequence. Transformation of TE9274 with this PCR product was followed by screening for Lac+, resulting in a strain that is deleted for the processing site and sensitive to chloramphenicol. The relevant region was confirmed by DNA sequencing (TE9277).
The fis gene of E. coli was deleted and replaced by cat by standard methods (17). Regions surrounding the sites of recombination at fis codon 22 and immediately following the fis termination codon were confirmed by DNA sequencing.
Assay of β-galactosidase.
Cells were centrifuged, resuspended in Z buffer (100 mM NaPO4 [pH 7.0], 10 mM KCl, 1 mM MgSO4), and then permeabilized by treatment with sodium dodecyl sulfate and chloroform (28). The samples from the exponential phase were concentrated before assay to be approximately equal in density to samples obtained at later times. For all experiments, exponential phase is defined as optical density at 600 nm (OD600) of 0.25 and SP is 24 h after inoculation. Assays were performed with Z buffer containing 50 mM β-mercaptoethanol by a kinetic method with a plate reader (Molecular Dynamics). In all experiments, β-galactosidase activity (change in OD420 per min) was normalized to cell density (OD650) and was always compared to appropriate controls assayed at the same time. The values shown are averages of at least four experiments with a standard deviation of <17%, unless otherwise indicated.
Immunological detection of proteins.
For Western blots, cultures were grown as described in the text. Electrophoresis and protein transfer were as described previously (6, 9). After transfer to a Sequi-Blot polyvinylidene difluoride membrane (Bio-Rad), blots were blocked in 5% nonfat milk and incubated in phosphate-buffered saline (PBS)-0.05% Tween containing the anti-RpoS monoclonal antibody R12 (6). After 60 min of incubation, blots were washed in PBS-Tween, incubated for 60 min in PBS-0.05% Tween containing biotinylated goat anti-mouse immunoglobulin, and finally incubated in PBS-0.05% Tween containing streptavidin-conjugated horseradish peroxidase (Southern Biotechnology Associates). Detection was by enhanced chemiluminescence (Amersham Biosciences).
RESULTS
The rpoS RBS regulates SP induction of translation.
Growth of E. coli or S. enterica into SP in rich medium (LB) resulted in a >30-fold increase in expression of the rpoS-lac protein (designated [pr]) fusion and an even greater increase in RpoS activity as demonstrated by katE-lac [op] (16, 17). As shown by others (20, 27, 31, 36; for a review, see reference 15) and confirmed by us for both E. coli and S. enterica serovar Typhimurium (16, 17), this increase has components of transcriptional and posttranscriptional regulation. In S. enterica serovar Typhimurium cells, SP induction in LB is independent of the response regulator RssB/SprE/MviA and the energy-dependent protease ClpXP, which together regulate RpoS abundance under other conditions (17, 26, 30, 34, 37). However, evidence suggests some role for proteolysis in SP regulation in E. coli (34). In both species, SP induction is also independent of the Hfq protein and, likewise, the regulatory DsrA RNA (16).
A striking result from previous analyses of rpoS translation in E. coli was that most of the SP induction (in LB) was maintained when a large segment of the 565-nt rpoS leader region was deleted (16), including the antisense element that binds DsrA RNA (24). Nearly 10-fold induction was observed for a rpoS-lac [pr] fusion expressed from PlacUV5 that contained only 48 nt of rpoS: 24 nt of the RBS, including the SD sequence AGGA (underlined) (GGGATCACGGGTAGGAGCCACCTTATG), followed by the first eight codons of the gene (Fig. 1, construct 542; Table 2, construct I). The SP induction that is lost in this construct depends on sequences relatively far away in the leader, upstream of nt 454 (data not shown).
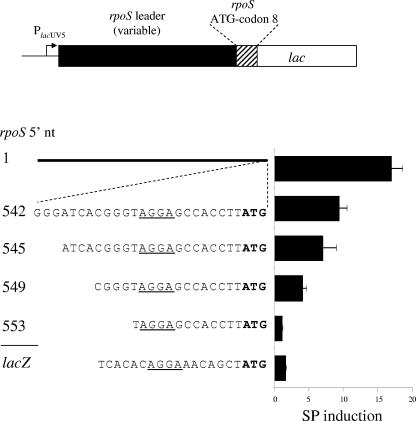
FIG. 1.
Stationary-phase induction of rpoS translation depends on the ribosome-binding sequence. The top line depicts the general fusion context. The lacUV5 promoter drives expression of a variable segment of the rpoS 5′ untranslated region (rpoS leader) followed by the first eight codons of rpoS. At this position, the lacZ gene is joined to rpoS to form a translational fusion. Five different rpoS constructs are shown, labeled according to the first nucleotide of the upstream end of the variable segment. Next to each construct is the SP induction ratio, obtained by dividing SP activity by the exponential-phase activity. The leader region of construct 1 is shown as a black line due to its length, while the broken lines indicate a magnification of the rpoS RBS. The RBS of lacZ served as a control and is labeled lacZ. Strain numbers for these constructs are as follows: 1, TE8419; 542, TE8420; 545, TE9030; 549, TE8999; 553, TE9036.
TABLE 2.
β-Galactosidase activity of various ribosome-binding sequencesa
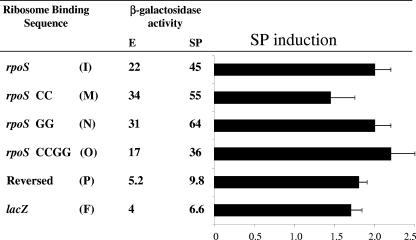
The β-galactosidase activity of various RBSs in a translational lacZ fusion context was determined during exponential growth (E) and during stationary phase (SP). These values were used to determine the SP induction ratio (SP/E) of the listed constructs. All fusions are expressed from the lacUV5 promoter (PlacUV5); a partial promoter sequence, including the −10 hexamer (underlined), is shown in light blue. The transcriptional start site of all constructs is indicated by an asterisk. The fusions are grouped into four categories: (i) hybrid constructs which contain nucleotides from both the rpoS RBS and lacZ RBS, shown in red and blue, respectively; (ii) full-length constructs having the entire rpoS leader region (nt 1 to 565), where a double slash mark symbolizes nt 1 to 541 of the native rpoS transcript, which are not shown (iii) leader deletion constructs shown in Fig. 1; (iv) additional mutant constructs which are based on the rpoS RBS (nucleotides 542 to 565) with mutations affecting the RBS shown in black. Each RBS is linked either to the first eight codons of rpoS and then to lacZ, shown by underlined ATG nucleotides in red, or directly to the lacZ coding sequence (ATG in blue). All strains were grown at 37°C in LB medium. Strain numbers for these constructs are as follows: A, TE9146; B, TE9249; C, TE9248; D, TE8483; E, TE8520; F, TE8448; G, TE8419; H, TE9042; I, TE8420; J, TE9030; K, TE8999; L, TE9036; M, TE9024; N, TE9380; O, TE9200; P, TE9251.
To further define the sequence required for translational regulation of SP induction, three additional constructs with extended deletions of the rpoS leader were analyzed (Fig. 1). Cultures were assayed for β-galactosidase activity during exponential phase (OD600 = 0.25) and after 24 h of growth in LB medium at 37°C (SP). The SP induction ratio (SP activity/exponential-phase activity) for each construct is reported in Fig. 1.
The construct bearing the entire 565-nt leader region of rpoS was induced 17-fold as cells grew to SP (Fig. 1, construct 1; Table 2, construct G). Constructs with sequential 5′ leader deletions to within 21 nt of the rpoS initiation codon each maintained about half of this regulation, with induction ratios of sevenfold to ninefold (Fig. 1, constructs 542 and 545). Removal of an additional 4 nt decreased induction to an intermediate value of fourfold (Fig. 1, construct 549), while SP regulation was completely eliminated in a construct retaining only 13 nt of the rpoS leader (Fig. 1, construct 553). As a negative control for these experiments, 21 nt of the lacZ RBS in the same fusion context (maintaining the first eight codons of rpoS), showed a minimal 1.6-fold increase during SP (Fig. 1, construct lacZ). These results indicate that the 12 nt directly upstream of the SD AGGAG sequence are crucial for SP regulation.
The rpoS mRNA can be activated or inhibited by the action of small RNAs in trans. Because these fusions were studied in a background that contains the wild-type rpoS gene, it seemed possible that the wild-type rpoS message might also act in trans to suppress the effect of some leader sequence deletions. This was tested by measuring the SP induction of construct I (Table 2), in a background with rpoS deleted. No difference from the wild-type background was noted in either the level of expression or the induction ratio.
Conservation of rpoS RBS-mediated induction.
The sequence of the rpoS RBS is completely conserved among several species of enteric bacteria including several strains of E. coli (including MG1655 and O157:H7), serovars of S. enterica (including serovars Typhi and Typhimurium), Shigella flexneri, and Enterobacter cloacae. To determine if RBS-mediated SP induction was specific to E. coli, we investigated the activity of construct I (Table 2) in S. enterica serovar Typhimurium. As cells grew into SP, the activity increased 10 fold in contrast to the 1.5-fold SP induction of the lacZ RBS (data not shown). The nearly identical induction ratios obtained with E. coli MG1655 and S. enterica serovar Typhimurium (LT2A and LT2) suggest a conserved regulatory mechanism.
Dissection of the RBS and its role in SP induction.
The deletion constructs described above also carry the first eight codons of rpoS fused to lacZ. We tested whether this sequence from the rpoS coding region has a regulatory role by determining the β-galactosidase activity of a construct that has just the 24 nt of the rpoS RBS preceding native lacZ (Table 2, construct A). This sequence maintained a SP induction ratio of 8.5 fold, similar to the induction shown by construct 542 (Fig. 1; Table 2, construct I). Together with the deletion results, this clearly demonstrates that the rpoS RBS is necessary and sufficient for the nearly ninefold increase in translation after cells enter SP.
To investigate the regulatory role of the 24 nt rpoS RBS in the context of the full-length rpoS leader, a fusion was constructed in which these bases were replaced by 21 nt of the lacZ RBS (Table 2, construct H). In this case, SP induction decreased to that of lacZ, 1.6 fold, compared to the 17-fold induction of the native leader region (Table 2, compare constructs G and H).
The 24-nt RBS of rpoS includes a 5-base SD sequence near its center (AGGAG), bounded by 12 bases upstream and 7 bases downstream. The lacZ RBS also consists of a nearly centered AGGA bordered by upstream and downstream sequence elements. We exchanged the upstream and downstream elements to construct a panel of hybrid rpoS/lacZ RBS elements in the fusion contexts described above (Table 2). In construct B, the upstream segment is from rpoS and the downstream segment is from lacZ; construct C is identical except that it has the shorter lacZ SD sequence (AGGA). Both of these hybrid RBSs demonstrated significant SP induction, similar to the results obtained with the wild-type rpoS RBS (Table 2, compare constructs A, B, and C). Substitution of the downstream lacZ element was also without effect on SP induction in the context of a fusion bearing the first eight codons of rpoS (construct D).
A quite different result was observed for substitution of the upstream element. Construct E contains the upstream segment from lacZ and the downstream segment from rpoS (Table 2). This hybrid RBS construct was unique in that its relative activity was extremely high (Table 2). This result appears to be due to strongly increased translation because a corresponding transcriptional fusion demonstrates the same activity as the other transcriptional fusions tested (data not shown). Importantly, construct E was not regulated during growth to SP. A similar result was found for the same hybrid RBS expressed from a mutant PlacUV5 (T→A at −12), which was reduced 100-fold in overall transcriptional activity (data not shown). Thus, the bases directly upstream of the SD sequence (rpoS nt 542 to 557) were confirmed to be required for SP induction.
Testing the role of potential trans-regulators.
We considered the possibility that trans-acting factors recognize the rpoS RBS and repress activity during exponential phase or activate translation during SP. Three proteins implicated in control of rpoS translation were investigated: DksA, the RNA-binding protein Hfq, and the transiently expressed subunit (β) of the DNA-binding HU dimer (2, 6, 29, 44). Normal SP induction of construct 542 occurred in a dksA, hfq, or hupB mutant background (data not shown). Additionally, it seems unlikely that induction is mediated by a small regulatory RNA, since most are dependent on Hfq for action (35).
We investigated whether known variations in ribosome composition as cells grow to SP confer a higher affinity for the rpoS RBS. The genes encoding four transiently expressed, ribosome-associated proteins (YfiA, YhbH, Sra, and Rmf) (18, 25, 42) were individually inactivated, and the SP induction of construct 542 in the mutant backgrounds was determined. In each case, SP regulation was not significantly different than that of the wild type (data not shown).
Secondary structure and SP induction.
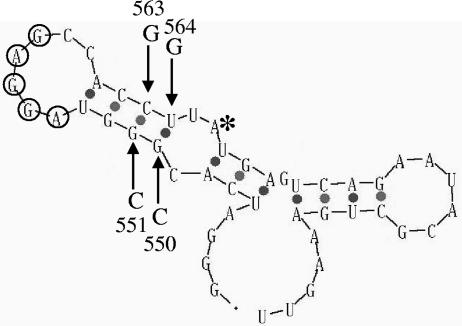
Another model posits that the secondary structure of the rpoS RBS acts directly as a regulatory signal. The thermodynamically favored secondary structure of construct 542 (48 nt of rpoS) was predicted using the mfold algorithm of M. Zuker (Fig. 2) (47). In this structure, the rpoS SD sequence (AGGAG) is positioned within a single-stranded loop flanked by a 4-bp stem—we will refer to this as the stem-loop RBS model. Disruption of the stem with a targeted double mutation eliminated much of the SP induction observed for the wild-type rpoS RBS (Table 2, construct M; Fig. 2, G550C/G551C). A construct containing two compensatory mutations (Table 2, construct O; Fig. 2, G550C/G551C/C563G/T564G), which restore the predicted structure of the wild-type RBS, was made. For this construct, SP induction was also restored and actually elevated compared to the wild type (Table 2, compare constructs I, M, and O). However, when the downstream double substitution was made (C563 and T564 both changed to G), it showed normal regulation, contrary to expectations (Table 2, construct N). This result is not consistent with the stem-loop RBS model for regulation.
FIG. 2.
Predicted RNA secondary structure of the rpoS ribosome-binding sequence. Mfold was used to predict the RNA secondary structure of a 48-nt region of the rpoS transcript: 24 nt directly preceding the rpoS start codon (labeled with an asterisk) extending to rpoS codon 8 (47). The bases of the extended Shine-Dalgarno sequence of rpoS are individually circled. Arrows indicate positions of directed mutations; the substituted nucleotides are also shown, as described in the text.
Fortuitously, the downstream double substitution was made last. Prior to this, and having reasonable evidence for the model, a further test construct was designed in which the sequence of the rpoS RBS was reversed while maintaining the extended SD sequence (Table 2, construct P). The reversed RBS demonstrated robust SP induction, with an induction ratio even higher than that of wild-type rpoS RBS (Table 2, compare constructs P and I). Because the spatial configuration of the nucleotides is completely different in a 5′-to-3′ sequence from the reverse, this result supports the idea that the structure of the RBS, and not its primary sequence, functions as a regulatory signal.
RNA stability and SP induction.
To investigate the possibility that differential transcript stability mediates SP induction of rpoS synthesis, we constructed several strains with RBS variants in a transcriptional fusion context. All transcriptional fusions investigated demonstrated a low 1.5- to 2-fold SP induction ratio, similar to the lacZ control, regardless of translational regulation (Fig. 3). This small increase in transcriptional activity did not account for the large differences in translational induction seen among the various RBSs, indicating that altered RNA stability does not regulate SP induction.
FIG. 3.
Stationary-phase regulation of various ribosome-binding sequences at the transcriptional level. The SP induction ratios of various transcriptional fusions containing the indicated RBSs were investigated. A description of each RBSs is given, and the actual sequences correspond to the letter designations of Table 2. The β-galactosidase activity of each construct was determined during exponential growth (E) and SP, and the SP induction ratio (SP/E) is shown. Strain numbers for these constructs are as follows: I, TE9300; M, TE9335; N, TE9379; O, TE9305; P, TE9304; F, TE9270.
Regulation at the native rpoS locus.
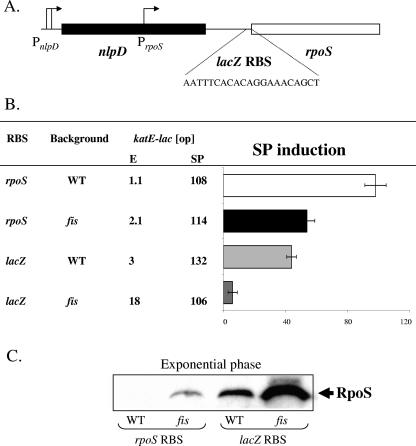
SP induction of RpoS in LB is regulated at both the transcriptional and translational levels. We tested the effect of altering the RBS of rpoS in the native context (i.e., the wild-type gene at its normal position on the chromosome, with no lac fusion). The 24-nt sequence of the rpoS RBS region was replaced by the RBS of lacZ (Fig. 4A). In this background, RpoS activity as measured by the katE-lac [op] reporter increased nearly threefold specifically during exponential phase, thereby reducing SP induction (Fig. 4B). Increased RpoS abundance was confirmed by Western blotting during exponential phase (Fig. 4C, rpoS RBS versus lacZ RBS).
FIG. 4.
SP regulation of RpoS depends on the rpoS ribosome-binding sequence and Fis. (A) The 24 nt preceding the rpoS initiation codon were replaced by the depicted lacZ RBS in the native rpoS context. (B) The SP induction ratio of the katE-lac reporter fusion was determined in a wild type and a fis mutant background (rpoS RBS). SP induction of katE-lac was also determined in the context depicted in panel A, with or without a fis deletion (lacZ RBS). (C) Western analysis of RpoS protein during exponential growth in the indicated backgrounds. Strain numbers for the constructs (reading from top to bottom in panel B) are TE9145, TE9302, TE9284, and TE9303.
The quantitative effect of this substitution was not as large as in the context of the rpoS-lacZ protein fusion. It seemed possible that feedback on the SP control of rpoS transcription could be a factor. In S. enterica serovar Typhimurium, Fis controls SP induction of rpoS transcription by binding near PrpoS and repressing activity during exponential phase (17). We investigated Fis regulation of RpoS in E. coli by determining the SP induction of katE-lac [op] in a fis mutant background. Due to increased expression during exponential phase, SP induction of RpoS decreased twofold in the fis mutant (Fig. 4B), an effect that correlated with a direct measure of RpoS abundance (Fig. 4C, fis rpoS RBS).
Finally, the SP induction of RpoS was determined in an E. coli background that was defective in both transcriptional control (fis mutant) and translational control (rpoS RBS replaced by that of lacZ). In this context, the near-100-fold SP induction of katE-lac [op] decreased to 5 fold. This result was due to a large increase in RpoS protein during exponential phase (Fig. 4B and C, compare WT rpoS RBS versus fis lacZ RBS).
DISCUSSION
Regulation of RpoS in enteric bacteria is striking for its complexity, in both the signals that increase the level of the protein and the mechanisms by which the increase is achieved. RpoS is strongly induced under a variety of conditions: in response to different stresses, during nutrient limitation, and during growth to stationary phase in either minimal or rich medium (including LB, used here). The mechanisms of induction by different stimuli are also remarkably varied and include both transcriptional and posttranscriptional effects on RpoS synthesis, as well as changes in protein turnover and in vivo activity (for a review, see reference 15). Perhaps the most dramatic difference in RpoS abundance occurs between exponential phase and SP in rich medium and is established by a combination of transcriptional and translational controls (16, 17). We have shown that there is no regulatory role for protein stability in this process in S. enterica (17), but the situation is unclear in E. coli (e.g., see reference 34).
In this study, we explored posttranscriptional control of RpoS in E. coli during growth into SP in rich medium. Primarily, lac [pr] (translational) fusions were employed that contain sequences derived from the rpoS leader and expressed from the control PlacUV5 promoter. We started with a short stretch of 48 nt from the rpoS RBS region—24 nt from the leader and 24 nt extending to codon 8 of the gene. This sequence was previously shown to confer ≈9-fold SP induction on lacZ (Fig. 1, construct 542; Table 2, construct I) (16). This compares with the 35-fold induction observed for a native construct expressed from PrpoS and the 15- to 17-fold induction for the identical full-length leader expressed from PlacUV5 (Fig. 1, construct 1; Table 2, construct G) (16).
A set of deletions was constructed to further define the sequences involved. On the downstream side, the rpoS coding sequence could be completely eliminated with no effect on SP regulation (Table 2, construct A). Results for a series of deletions extending into the RBS region from the upstream side (Fig. 1) indicated that a block of 12 nt just upstream of the SD AGGAG sequence was essential for regulation. Sequential deletions showed a pattern of increasing loss until there was no regulation with construct 553, which was missing all but the rpoS SD sequence (Table 2, construct L). The importance of this region was confirmed by experiments in which the same 24-nt sequence was replaced by the RBS region of lacZ in the native rpoS context and combined with a fis mutation to block SP transcriptional regulation at PrpoS. SP induction of rpoS was reduced 20-fold (Fig. 4). Also, substitution of the lacZ RBS region into a full-length leader construct expressed from PlacUV5 eliminated regulation (Table 2, construct H). Together, these results indicate that the rpoS RBS region is both necessary and sufficient for most SP control of rpoS that operates posttranscriptionally. This short sequence is highly conserved among enteric bacteria, and the induction phenomenon was shown to occur in S. enterica serovar Typhimurium as well.
For this analysis, we have compared the SP induction ratios (SP expression divided by exponential-phase expression). It should be noted that there is substantial variation for the actual data values between constructs (e.g., Table 2, compare the deletion constructs I through L). We think that the ratios rather than the individual data values are appropriate for comparison. Expression of a particular construct might be differentially affected through subtle effects on RNA folding (speed, final state, or equilibrium between multiple states) or even affinity for nucleases. The last possibility can explain some differences between sequences, as shown by lac [op] fusions (Fig. 3). However, some differences cannot be explained in this way. Comparing deletions in RNA is clearly different than comparing deletions in DNA, and using the ratio as the basis for comparison should normalize potentially confounding effects. It would be interesting to know whether the regulatory mechanism works positively in SP or negatively during exponential phase. We argue that variation between constructs makes it difficult to know the answer from these experiments. Some less- or unregulated constructs (e.g., F, H, and M) had elevated exponential-phase expression, but this may be misleading.
Several point mutations and block substitutions were made to elucidate the role played by the rpoS RBS region in SP regulation. Some results pointed to a role for secondary structure, possibly the one predicted by an mfold analysis that forms a stem-loop including AGGAG in the loop region (Fig. 2). A double substitution of two adjacent G residues (G550C and G551C) in the upstream part of the stem strongly reduced SP regulation. As predicted, the compensatory “double” mutant with these two changes plus two more affecting the predicted pairing partners (C563G and T564G) restored regulation to a level even greater than that of the original parental construct (Table 2, constructs I, M, and O). Another construct was made with a reversed sequence for the stems adjacent to the AGGAG sequence, which is predicted to maintain pairing but eliminate sequence-specific interactions; this construct maintained and actually elevated SP induction (Table 2, construct P). The combined results suggested the possibility that any stem loop including the AGGAG might show regulation by SP.
Nevertheless, there is also substantial evidence against this model. Two constructs with well-studied structured RBS regions, derived from cbiA and rpoH, were made and tested (data not shown). One (cbiA) showed some SP regulation: fivefold upon entry into SP, but this decreased to twofold at our normal SP time point (24 h). The other (rpoH) showed no regulation. More directly, the downstream double substitution was made (C563 and T564 both changed to G) but, contrary to expectation, showed normal regulation (Table 2, construct O). This result is not consistent with the stem-loop RBS model for regulation. However, we note that these bases are not irrelevant for regulation. In formal genetic terms, the downstream CT → GG substitution is epistatic to the upstream GG → CC substitution because the “double” mutant shows normal regulation, hiding the effect of the upstream mutation by itself.
Two constructs bearing substitutions of a block of six to seven bases, either downstream or upstream of the AGGAG sequence, also had surprising effects. The downstream substitution (Table 2, construct D) cannot easily be reconciled with the stem-loop RBS model. It clearly eliminated the predicted paired structure but still showed normal regulation. The upstream substitution (Table 2, construct E) nearly eliminated regulation but also had a second, remarkable effect. Basal expression was elevated 10 to 50 fold (Table 2, compare construct E with constructs A, I, K, and L). The same pattern of high-level expression with minimal regulation was also seen for a construct like E but with the rpoS coding sequences removed and lacZ starting from its native ATG start codon (data not shown). There is also little effect on transcription—an [op] fusion version of construct E (like those shown in Fig. 3) showed comparable expression to the other constructs (data not shown). The lack of regulation of construct E is not due to its very high expression. A variant designed with a mutation in the lac promoter showed low expression but little regulation (data not shown).
In summary, crucial nucleotides for rpoS posttranscriptional SP regulation lie just upstream of the SD AGGAG sequence, while all required sequences lie within the rpoS RBS region. Some constructs (e.g., construct P, reversed sequence) are difficult to explain by any model other than a paired structure (stem-loop RBS model) (Fig. 2). On the other hand, several constructs do not behave as predicted by this model. Regulation does not involve the known rpoS regulators DksA or HU. Furthermore, the RNA-binding protein Hfq is not required and, presumably, neither are small RNAs. A simple model would be that the rpoS RBS competes more effectively for ribosomes during SP. If this is true, the increased affinity is not affected by mutations in any one of several proteins associated with the ribosome specifically during SP.
How is control by the antisense element to be integrated into this picture? This question is difficult because the mechanism of SP control is still unclear. Since SP control doesn't require the antisense element (or the Hfq protein), it seems likely that the mRNA activated by SP is not folded with the antisense element paired with the RBS (7). We could imagine that there are two inhibitory structures (antisense-RBS and RBS region with its own inhibitor) but these would be exclusive; they do not form in the same RNA molecule. The first population would be activated by the DsrA and RprA RNAs, while the second would be activated by SP. Nevertheless, if the antisense element really limits expression, one might expect the full-length construct (Table 2, construct G) to be expressed at substantially lower levels than deletion constructs missing the antisense element (e.g., construct A), but it is not. One possibility would be that transcripts of the deletion constructs have lower abundance by instability. Another idea would be that segments of the leader upstream of the antisense element are important for proper folding. We know that deletions ending well upstream (≈100 nt) from the antisense element are blind to Hfq and DsrA (8). It is possible that the folding of a deletion is subtly different from that of rpoS mRNA complexed to DsrA, and this leads to decreased translation efficiency.
The environmental stimulus that triggers RBS-mediated SP induction of rpoS translation also remains unknown, but similar to transcriptional control, regulation is seen in rich undefined media, including LB and either of its individual components tryptone or yeast extract (reference 17 and data cited above in Results). No RBS-mediated SP induction occurs in minimal medium containing various carbon sources even when supplemented with amino acids or putrescine, a polyamine reported to stimulate rpoS translation (41). For example, in minimal glucose medium the exponential-phase activity of construct 542 is 38 units and the SP value is 77 units of activity, while the activity of the control fusion remains unchanged compared to the values determined in LB medium. This suggests that regulation may be primarily a negative effect operating in exponential phase, similar to the transcriptional repression that involves Fis protein. Medium-dependent differential regulation of rpoS expression is apparently not due to altered growth rates because cells grow rapidly in supplemented minimal medium but still show low induction ratios (data not shown).
SP induction of RpoS in rich medium depends on regulation of both transcription and translation (17, 18). During exponential growth, Fis protein binds to a site near PrpoS and blocks transcription (18). As cells grow into SP, Fis abundance is drastically reduced, and expression from PrpoS is released from Fis repression (1, 18). At the translational level, the rpoS RBS enforces SP induction by a presently uncharacterized mechanism, dependent on nucleotides just upstream from the SD sequence (Table 2 and Fig. 4). Collectively, these two regulatory pathways account for approximately 95% of the overall SP induction of RpoS.
Acknowledgments
We are grateful to individuals cited in the text for bacterial strains. We also thank Adam Goodwill for excellent technical support.
This study was supported by Public Health Service grant GM63616.
REFERENCES
- 1.Ali Azam, T., A. Iwata, A. Nishimura, S. Ueda, and A. Ishihama. 1999. Growth phase-dependent variation in protein composition of the Escherichia coli nucleoid. J. Bacteriol. 181:6361-6370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balandina, A., L. Claret, R. Hengge-Aronis, and J. Rouviere-Yaniv. 2001. The Escherichia coli histone-like protein HU regulates rpoS translation. Mol. Microbiol. 39:1069-1079. [DOI] [PubMed] [Google Scholar]
- 3.Ball, C. A., R. Osuna, K. C. Ferguson, and R. C. Johnson. 1992. Dramatic changes in Fis levels upon nutrient upshift in Escherichia coli. J. Bacteriol. 174:8043-8056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berkowitz, D., J. M. Hushon, H. J. Whitfield, J. Roth, and B. N. Ames. 1968. Procedure for identifying nonsense mutations. J. Bacteriol. 96:215-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bochner, B. R., and B. N. Ames. 1982. Complete analysis of cellular necleotides by two-dimensional thin layer chromatography. J. Biol. Chem. 257:9759-9769. [PubMed] [Google Scholar]
- 6.Brown, L., and T. Elliott. 1996. Efficient translation of the RpoS sigma factor in Salmonella typhimurium requires host factor I, an RNA-binding protein encoded by the hfq gene. J. Bacteriol. 178:3763-3770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown, L., and T. Elliott. 1997. Mutations that increase expression of the rpoS gene and decrease its dependence on hfq function in Salmonella typhimurium. J. Bacteriol. 179:656-662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cunning, C., L. Brown, and T. Elliott. 1998. Promoter substitution and deletion analysis of upstream region required for rpoS translational regulation. J. Bacteriol. 180:4564-4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cunning, C., and T. Elliott. 1999. RpoS synthesis is growth rate regulated in Salmonella typhimurium, but its turnover is not dependent on acetyl phosphate synthesis or PTS function. J. Bacteriol. 181:4853-4862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davis, R. W., D. Botstein, and J. Roth. 1980. Advanced bacterial genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 11.Elliott, T. 1992. A method for constructing single-copy lac fusions in Salmonella typhimurium and its application to the hemA-prfA operon. J. Bacteriol. 174:245-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fang, F. C., S. J. Libby, N. A. Buchmeier, P. C. Loewen, J. Switala, J. Harwood, and D. G. Guiney. 1992. The alternative σ factor KatF (RpoS) regulates Salmonella virulence. Proc. Natl. Acad. Sci. USA 89:11978-11982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gentry, D. R., V. J. Hernandez, L. H. Nguyen, D. B. Jensen, and M. Cashel. 1993. Synthesis of the stationary phase sigma factor σS is positively regulated by ppGpp. J. Bacteriol. 175:7982-7989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gottesman, S. 2004. The small RNA regulators of Escherichia coli: roles and mechanisms. Annu. Rev. Microbiol. 58:303-328. [DOI] [PubMed] [Google Scholar]
- 15.Hengge-Aronis, R. 2002. Signal transduction and regulatory mechanisms involved in control of the σS (RpoS) subunit of RNA polymerase. Microbiol. Mol. Biol. Rev. 66:373-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hirsch, M., and T. Elliott. 2002. Role of ppGpp in rpoS stationary-phase regulation in Escherichia coli. J. Bacteriol. 184:5077-5087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hirsch, M., and T. Elliott. 2005. Fis regulates transcriptional induction of RpoS in Salmonella enterica. J. Bacteriol. 187:1568-1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Izutsu, K., C. Wada, Y. Komine, T. Sako, C. Ueguchi, S. Nakura, and A. Wada. 2001. Escherichia coli ribosome-associated protein SRA, whose copy number increases during stationary phase. J. Bacteriol. 183:2765-2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jensen, K. F. 1993. The Escherichia coli K-12 “wild types” W3110 and MG1655 have an rph frameshift mutation that leads to pyrimidine starvation due to low pyrE expression levels. J. Bacteriol. 175:3401-3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lange, R., and R. Hengge-Aronis. 1994. The cellular concentration of the σS subunit of RNA polymerase in Escherichia coli is controlled at the levels of transcription, translation, and protein stability. Genes Dev. 8:1600-1612. [DOI] [PubMed] [Google Scholar]
- 21.Lease, R. A., and M. Belfort. 2000. A trans-acting RNA as a control switch in Escherichia coli: DsrA modulates function by forming alternative structures. Proc. Natl. Acad. Sci. USA 97:9919-9924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lease, R. A., and S. A. Woodson. 2004. Cycling of the Sm-like protein Hfq on the DsrA small regulatory RNA. J. Mol. Biol. 344:1211-1223. [DOI] [PubMed] [Google Scholar]
- 23.Linn, T., and R. St. Pierre. 1990. Improved vector system for constructing transcriptional fusions that ensures independent translation of lacZ. J. Bacteriol. 172:1077-1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Majdalani, N., C. Cunning, D. Sledjeski, T. Elliott, and S. Gottesman. 1998. DsrA RNA regulates translation of RpoS message by an anti-antisense mechanism, independent of its action as an antisilencer of transcription. Proc. Natl. Acad. Sci. USA 95:12462-12467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maki, Y., H. Yoshida, and A. Wada. 2000. Two proteins, YfiA and YhbH, associated with resting ribosomes in stationary phase Escherichia coli. Genes Cells 5:965-974. [DOI] [PubMed] [Google Scholar]
- 26.Mandel, M. J., and T. J. Silhavy. 2005. Starvation for different nutrients in Escherichia coli results in differential modulation of RpoS levels and stability. J. Bacteriol. 187:434-442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McCann, M. P., C. D. Fraley, and A. Matin. 1993. The putative sigma factor KatF is regulated posttranscriptionally during carbon starvation. J. Bacteriol. 175:2143-2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 29.Muffler, A., D. Fischer, S. Altuvia, G. Storz, and R. Hengge-Aronis. 1996. The response regulator RssB controls stability of the σS subunit of RNA polymerase in Escherichia coli. EMBO J. 15:1333-1339. [PMC free article] [PubMed] [Google Scholar]
- 30.Muffler, A., D. D. Traulsen, R. Lange, and R. Hengge-Aronis. 1996. Posttranscriptional osmotic regulation of the σS subunit of RNA polymerase in Escherichia coli. J. Bacteriol. 178:1607-1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mulvey, M. R., J. Switala, A. Borys, and P. C. Loewen. 1990. Regulation of transcription of katE and katF in Escherichia coli. J. Bacteriol. 172:6713-6720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Neidhardt, F. C., P. L. Bloch, and D. F. Smith. 1974. Culture medium for enterobacteria. J. Bacteriol. 119:736-747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Osuna, R., D. Lienau, K. T. Hughes, and R. C. Johnson. 1995. Sequence, regulation, and functions of fis in Salmonella typhimurium. J. Bacteriol. 177:2021-2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pratt, L. A., and T. J. Silhavy. 1996. The response regulator SprE controls the stability of RpoS. Proc. Natl. Acad. Sci. USA 93:2488-2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Repoila, F., N. Majdalani, and S. Gottesman. 2003. Small non-coding RNAs, co-ordinators of adaptation processes in Escherichia coli: the RpoS paradigm. Mol. Microbiol. 48:855-861. [DOI] [PubMed] [Google Scholar]
- 36.Schellhorn, H. E., and V. L. Stones. 1992. Regulation of katF and katE in Escherichia coli K-12 by weak acids. J. Bacteriol. 174:4769-4776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schweder, T., K. H. Lee, O. Lomovskaya, and A. Matin. 1996. Regulation of Escherichia coli starvation sigma factor (σS) by ClpXP protease. J. Bacteriol. 178:470-476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Silhavy, T. J., M. L. Berman, and L. W. Enquist. 1984. Experiments with gene fusions. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 39.Sledjeski, D. D., A. Gupta, and S. Gottesman. 1996. The small RNA, DsrA, is essential for the low temperature expression of RpoS during exponential growth in Escherichia coli. EMBO J. 15:3993-4000. [PMC free article] [PubMed] [Google Scholar]
- 40.Takayanagi, Y., K. Tanaka, and H. Takahashi. 1994. Structure of the 5′ upstream region and the regulation of the rpoS gene of Escherichia coli. Mol. Gen. Genet. 243:525-531. [DOI] [PubMed] [Google Scholar]
- 41.Tkachenko, A. G., and M. S. Shumkov. 2004. Role of putrescine in regulation of the σS subunit of RNA polymerase in Escherichia coli cells on transition to stationary phase. Biochemistry (Moscow) 69:876-882. [DOI] [PubMed] [Google Scholar]
- 42.Wada, A., Y. Yamazaki, N. Fujita, and A. Ishihama. 1990. Structure and probable genetic location of a “ribosome modulation factor” associated with 100S ribosomes in stationary-phase Escherichia coli cells. Proc. Natl. Acad. Sci. USA 87:2657-2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wassarman, K. M., F. Repoila, C. Rosenow, G. Storz, and S. Gottesman. 2001. Identification of novel small RNAs using comparative genomics and microarrays. Genes Dev. 15:1637-1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Webb, C., M. Moreno, M. Wilmes-Riesenberg, R. Curtiss III, and J. W. Foster. 1999. Effects of DksA and ClpP protease on sigma S production and virulence in Salmonella typhimurium. Mol. Microbiol. 34:112-123. [DOI] [PubMed] [Google Scholar]
- 45.Weber, H., T. Polen, J. Heuveling, V. F. Wendisch, and R. Hengge. 2005. Genome-wide analysis of the general stress response network in Escherichia coli: σS-dependent genes, promoters, and sigma factor selectivity. J. Bacteriol. 187:1591-1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yu, D., H. M. Ellis, E. C. Lee, N. A. Jenkins, N. G. Copeland, and D. L. Court. 2000. An efficient recombination system for chromosome engineering in Escherichia coli. Proc. Natl. Acad. Sci. USA 97:5978-5983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zuker, M. 2003. Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res. 31:3406-3415. [DOI] [PMC free article] [PubMed] [Google Scholar]