Abstract
Treatment of acute otitis media (AOM) with azithromycin results in apparent clinical success, but tympanocentesis performed 4 to 6 days after initiation of therapy in children with nontypeable Haemophilus influenzae (NTHI) recovered from initial middle ear cultures demonstrates persistence of infection in more than 50% of episodes. We sought to determine the effect of azithromycin at different doses on the density of middle ear infection due to NTHI to provide additional understanding of this dichotomy between clinical and microbiologic outcome measures in AOM. In a chinchilla model of experimental otitis media (EOM), animals treated with placebo were compared to animals receiving a single daily dose 30 or 120 mg of azithromycin per kg of body weight per day for 5 days. Microbiologic outcome was assessed by obtaining quantitative cultures from the middle ear during a 5-day course and for 1 week following therapy. Azithromycin concentrations were measured to ascertain whether a concentration-dependent effect was present. Azithromycin at 30 and 120 mg/kg/day demonstrated a dose-dependent effect on the quantitative assessment of middle ear infection due to NTHI. A 30-mg/kg dose of azithromycin daily resulted in levels in serum and areas under the serum concentration-time curve at 24 h comparable to published data obtained with children given azithromycin at 5 to 10 mg/kg in multiday regimens. Increased doses of azithromycin (120 mg/kg) achieved 2.5- to 4-fold-higher levels in serum and 3- to 6-fold-higher total levels and levels in extracellular middle ear fluid as well as more rapid reduction in bacterial density and a greater proportion of middle ears with complete sterilization than either placebo or the 30-mg/kg/day regimen.
Nontypeable Haemophilus influenzae (NTHI) is one of the two most common bacterial causes of acute otitis media (AOM) in children (2, 18). Previously, investigators have demonstrated that eradication of bacterial pathogens from middle ear fluid (MEF) correlates with clinical outcome (4, 7, 8). Studies of azithromycin have demonstrated good outcomes in children with AOM using clinical endpoints despite the persistence of NTHI in the middle ear observed in “double-tap” (tympanocentesis) protocols. (1, 15, 16, 22, 24). Azithromycin demonstrates high intracellular and tissue concentrations and slow elimination from infected tissue but lower concentrations in extracellular MEF and serum (11, 23). The low extracellular concentration in the middle ear potentially explains the persistence of culture-positive disease in azithromycin-treated AOM due to NTHI at day 4 to 6 in children (6) and through day 7 in chinchillas as reported by Chan et al. (5).
Data obtained with the chinchilla model of experimental otitis media (EOM) have consistently been found to correlate with subsequent clinical trials in children (10). In the model used in our laboratory, a low inoculum (5 to 50 CFU in 0.1 ml) is injected directly into the middle ear via the superior bulla (21). Middle ear disease in the animals is evaluated longitudinally by otomicroscopic examination and quantitative microbiology of the middle ear space. Serum and middle ear samples are obtained at various time points to evaluate pharmacokinetic parameters and drug penetration into the middle ear. We sought to determine the microbiologic outcome in EOM due to NTHI in chinchillas treated with two different doses of azithromycin by assessing quantitative middle ear cultures during and following antimicrobial therapy. Placebo animals were compared to animals receiving 30 or 120 mg of azithromycin per kg of body weight per day for 5 days each. We chose a substantially higher dose (120 mg/kg/day) to ensure that increased concentrations in serum would be achieved and we could determine whether there were differences between low- and high-dose regimens. Azithromycin concentrations were measured to determine the relevance of the dosing schedule used in current treatment regimens in children and to ascertain whether concentration-dependent killing occurred.
(This work was presented in part at the International Conference on Antimicrobial Agents and Chemotherapy in Toronto, Canada, in August 2000.)
MATERIALS AND METHODS
Bacterial isolate and MIC.
An isolate of NTHI originally obtained in pure culture from the transtracheal aspirate of an adult with pneumonia was utilized. The isolate was confirmed as NTHI from physical appearance on chocolate agar and its requirement for X and V factors. The MIC of azithromycin was 1.5 μg/ml as determined by E test on Haemophilus test medium, per NCCLS guidelines. The range of MICs in our lab using this method for NTHI is between 1 and 2 μg/ml.
Experimental infection.
Prior to the experiment the area surrounding the superior bulla was shaved and cleaned with Betadine. Both ears were examined before inoculation with bacteria and before surgery. Both middle ears were inoculated by injection of 0.1 ml of balanced Gey's solution containing 10 to 50 CFU of H. influenzae through a 25-gauge tuberculin needle into both superior bullae. Daily and at each subsequent surgery, animals were examined for general health and well-being as determined by their activity level. Forty-eight hours after inoculation, animals were inspected by direct otomicroscopy. After anesthesia, the middle ear cavity was accessed following topical disinfection with Betadine, through a small incision 0.5 to 1 cm in length over the superior bulla. A small hole was made in the bullar bone with a scalpel. The middle ear cavity was examined with an operating microscope. MEF was obtained with a calcium alginate swab and by aspiration through a 20-gauge angiocatheter.
Experiments were performed as comparisons between placebo (17 animals) and 120-mg/kg/day (23 animals) dosing and 30-mg/kg/day (19 animals) and 120-mg/kg/day (17 animals) cohorts (see timeline in Table 1). For analysis, the two 120-mg/kg/day cohorts were combined. Azithromycin was administered suspended in sterile water via an orogastric feeding tube. On days 3 (after the third dose of azithromycin) and 5 (after the fifth dose of azithromycin) after the beginning of therapy the animals were examined by direct otomicroscopy and quantitative middle ear cultures were obtained. Three to five days after completion of therapy, MEF was obtained for quantitative culture. Thereafter, all animals were euthanized. Although both ears were inoculated, not all ears became infected. Ears that remained uninfected were excluded from the calculation of infected ears compared to sterilized ears. The number of animals decreased during the course of the experiment mainly due to anesthesia complications or aspiration during orogastric intubation to administer the study drug. However, deaths (overall mortality of 12%) were equally distributed between the azithromycin group (7 of 59 animals died) and the placebo group (2 of 12 animals died).
TABLE 1.
Timeline of comparison of high and low doses of azithromycin with placebo in EOM due to NTHIa
| Day | Step |
|---|---|
| −3 | Shave and examine superior bullae of chinchillas |
| −2 | Inoculate middle ears with 10-50 CFU of H. influenzae |
| 1 | Access bullae; obtain MEF for culture; administer azithromycin at 30 or 120 mg/kg or placebo via orogastric intubation |
| 2 | Obtain blood by superior cranial sinus puncture (selected animals); administer azithromycin at 30 or 120 mg/kg or placebo via orogastric intubation; obtain blood by superior cranial sinus puncture for pharmacodynamic study (selected animals) |
| 3 | Obtain blood by superior cranial sinus puncture (selected animals); access bullae; obtain MEF for culture and azithromycin levels (intracellular and total); administer azithromycin at 30 or 120 mg/kg or placebo via orogastric intubation |
| 4 | Administer azithromycin at 30 or 120 mg/kg or placebo via orogastric intubation |
| 5 | Obtain blood by superior cranial sinus puncture (selected animals); access bullae; obtain MEF for culture azithromycin at 30 or 120 mg/kg or placebo via orogastric intubation |
| 9 | Access bullae; obtain MEF for culture and azithromycin level (intracellular and total) |
| 11 | Access bullae; obtain MEF for culture |
For details, see Materials and Methods.
Serum sample collection.
Blood samples were obtained on days 2, 3, and 5 and 4 days after completion of therapy in selected animals to assess antibiotic concentrations in serum. About 1.5 to 2 ml of blood was obtained by aspiration from the superior cranial sinus and spun at 2,000 rpm (Jouan MR22 centrifuge) for 5 min. The serum was separated and frozen at −80°C.
MEF collection.
A calcium alginate swab of MEF from both ears was streaked on culture plates for presence of viable bacteria. When MEF could be obtained by aspiration, quantitative cultures were performed on the aspirate of the left ear by plating 0.1 ml from successive 10-fold dilutions on chocolate agar plates to calculate number of CFU of NTHI per milliliter. In order to calculate the mean CFU per milliliter, middle ears that were culture positive on direct qualitative assessment but not by quantitative culture were assigned a value of 10 CFU/ml. Those that were negative by both qualitative and quantitative culture were assigned a value of 5 CFU/ml. The remaining MEF was split. One half was spun at 10,000 rpm at 4°C, and the supernatant was frozen at −80°C; the other half was repeatedly frozen and thawed to disrupt cell elements and also frozen at −80°C.
Pharmacokinetic studies.
Blood samples for the pharmacodynamic studies were drawn from three animals in the high-dose and the low-dose azithromycin groups each on the second day of dosing. Specimens were obtained at 0 h (prior to dosing of azithromycin) and at 1, 2, 4, 6, 8, 12, and 24 h after the second azithromycin dose. Serum and middle ear azithromycin levels were analyzed in batch by high-pressure liquid chromatography assay (BAS Analytics, West Lafayette, Ind.). Azithromycin and its N-propargyl derivative (CP-67094) were extracted by a liquid-liquid extraction at alkaline pH. CP-67094 served as an internal standard. After the addition of carbonate solution and internal standard, the macrolides were extracted into methyl-t-butyl ether. The ether layer was transferred to a clean tube, evaporated under nitrogen, and reconstituted with a pH 6 buffer-acetonitrile mixture. The extract was injected into an LCEC system set up with a zirconium oxide stationary phase and an alkaline buffer-acetonitrile mobile phase. The area under the curve (AUC) was calculated with the linear trapezoidal rule.
Animal care.
All procedures were performed using sedation analgesia with a mixture of 30 mg of ketamine and 4 mg of xylazine per kg injected intramuscularly for long procedures or isoflurane inhalation for brief procedures. After receiving anesthesia, all animals were monitored until they recovered from anesthesia, as judged by their ability to respond to stimuli. The study was approved by and conducted under the auspices of the Boston University School of Medicine IACUC committee.
Statistical analysis.
Statistical analysis of microbiologic outcome for MEF (see Fig. 1) was performed with chi-square and Fisher's exact tests. For analysis of the longitudinal log10 CFU means by treatment group (see Fig. 2), we used mixed linear models, an analogue to repeated-measures analysis of variance (9). Following the result of a significant “time-by-group” interaction, we performed a separate one-factor analysis of variance at each time point to examine differences between groups. A Bonferroni correction was applied in interpreting the P values from these time-specific analyses. MEF samples with no growth were assigned a value of 5 CFU/ml (half the value for the lower limit of detection). We transformed CFU values to the base 10 log scale prior to statistical analysis.
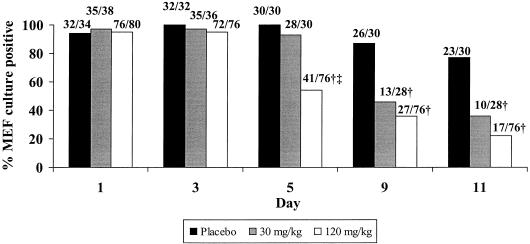
FIG. 1.
Microbiologic outcome in MEF of experimental AOM due to NTHI. Numbers above the bars are the numbers of infected ears over the total numbers of ears cultured (number of animals decreased due to deaths during the experiment; see the text). †, P < 0.002 treatment group versus the placebo group; ‡, P < 0.0001 120-mg/kg group versus the 30-mg/kg group.
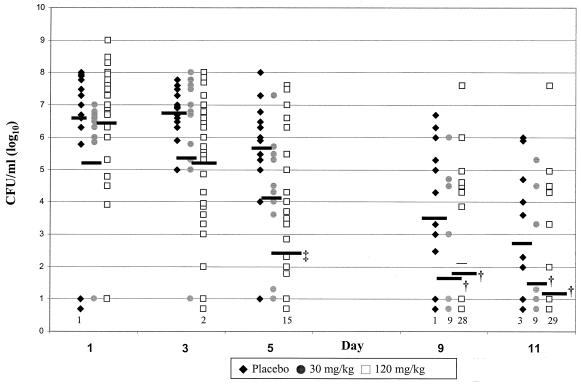
FIG. 2.
Mean infection densities in EOM due to NTHI. Sterile ears were counted as having 100.7 CFU/ml, and the numbers of animals with sterile ears are identified at the bottom of the columns in the figure. †, P < 0.0001 treatment group versus the placebo group; ‡, P < 0.05 120-mg/kg group versus the 30-mg kg group.
RESULTS
The microbiologic outcome of experimental AOM due to NTHI is shown in Fig. 1. On day 0 of the study (before commencing therapy), 95 to 100% of middle ear cultures were positive for NTHI. On day 5 (after 4 days of placebo or azithromycin therapy), 100% of middle ears were positive in the placebo group, 93% were positive in the low-dose group, and 54% were positive in the high-dose group. The high-dose group had a significantly lower culture positivity rate than the placebo and low-dose groups (P < 0.0001 for both comparisons). On day 10 to 11, 36% in the low-dose and 23% in the high-dose treatment groups remained culture positive. Both azithromycin treatment groups were significantly different from the placebo group but not from each other (P < 0.0001 for 120 mg/kg versus placebo; P = 0.002 for 30 mg/kg versus placebo; P = 0.17 for 30 mg/kg versus 120 mg/kg). The infection density (number of CFU per milliliter) for each treatment group at each day of observation is shown in Fig. 2. No significant difference was observed in MEF at day 1 between treatment groups or between day 3 and day 1 in any of the groups. However, by day 5 a significant decrease in density in both treatment groups compared to placebo as well as between the high-dose and low-dose groups (P for 30 versus 120 mg/kg, <0.05). At day 9 the mean density of middle ear infection in the placebo group declined to 103.6 CFU/ml, while in both treatment groups it fell to less than 102 CFU/ml. At day 11, the mean density of middle ear infection in the placebo group declined to 102.9 CFU/ml, whereas in both high- and low-dose treatment groups, the mean densities in the MEF cultures were less than 102 CFU/ml (P for group effect, <0.004).
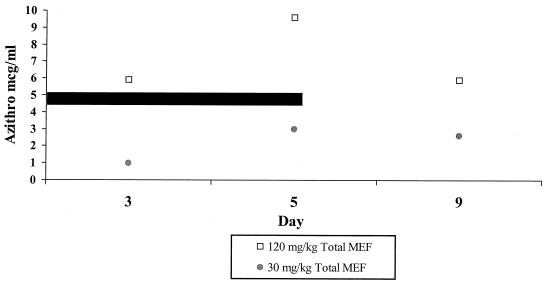
The total azithromycin concentrations in MEF (after disruption of cells) are shown in Fig. 3 and Table 2. In the low-dose group the total azithromycin concentration rose from 0.96 μg/ml on day 3 (i.e., prior to dose 3) to 2.98 μg/ml on day 5 (i.e., prior to dose 5) and was 2.5 μg/ml on day 9 (4 days after completion of therapy). Total azithromycin levels in the high-dose group rose from 5.99 μg/ml on day 3 to 9.62 μg/ml on day 5 and decreased to 6.0 μg/ml on day 9. The extracellular azithromycin concentration in middle ear rose from 0.26 μg/ml prior to dose 3 to 0.41 μg/ml prior to dose 5 in the low-dose group and from 1.20 μg/ml prior to dose 3 to 2.50 μg/ml prior to dose 5 in the high-dose group.
FIG. 3.
Azithromycin concentrations in MEF in acute EOM. Total MEF was freeze-thawed to disrupt cells. The black bar indicates the duration of azithromycin therapy.
TABLE 2.
Extracellular and total azithromycin levels in MEF and serum in EOM due to NTHI
| MEF type | Day | Value obtained with azithromycin at:
|
|||||
|---|---|---|---|---|---|---|---|
| 30 (mg/kg/day)
|
120 (mg/kg/day)
|
||||||
| Level (μg/ml) in:
|
Ratio | Level (μg/ml) in:
|
Ratio | ||||
| MEF | Serum | MEF | Serum | ||||
| Extracellulara | 3 | 0.26 | 0.09 | 2.9 | 1.20 | 0.40 | 3.0 |
| 5 | 0.41 | 0.18 | 2.3 | 2.50 | 0.52 | 4.8 | |
| Totalb | 3 | 0.96 | 0.09 | 10.6 | 5.99 | 0.40 | 14.9 |
| 5 | 2.98 | 0.18 | 16.5 | 9.62 | 0.52 | 18.5 | |
MEF supernatant.
Freeze-thawed MEF.
In the 30-mg/kg group, the concentration in serum was measured on day 3 (prior to dose 3) at 0.09 μg/ml and at day 5 (prior to dose 5) at 0.18 μg/ml. In the 120-mg/kg group, the concentration in serum was 0.40 μg/ml on day 3 and 0.52 μg/ml at day 5. The AUC was 5.8 μg · h/ml for low-dose azithromycin and 14.1 μg · h/ml for the high-dose group. The ratio of serum AUC to MIC (AUC/MIC) was ∼4 in the low-dose group and ∼10 in the high-dose group, with an MIC for the NTHI isolate of 1.5 μg/ml. As shown in Table 2, the level in serum in the high-dose group was 4 times the level in the low-dose group on day 3 and 2.8 times that level on day 5. Azithromycin levels in MEF were compared to levels in serum measured at days 3 and 5 to calculate MEF-to-serum ratios (Table 2). The ratio of total azithromycin level in middle ear to that in serum rose from 10.6 to 16.5 prior to dose 5 in the low-dose group and from 14.9 to 18.5 in the high-dose group. The ratio of extracellular azithromycin level in MEF (supernatant of spun MEF) to level in serum in the low-dose group varied between 2.9 and 2.3 and in the high-dose group varied between 3.0 and 4.8 on days 3 and 5, respectively.
DISCUSSION
Azithromycin is an azalide that demonstrates high intracellular and tissue concentrations and slow elimination from infected tissue but relatively lower concentrations in extracellular MEF and serum (11, 23). Azithromycin rapidly achieves high concentrations in polymorphonuclear cells (12), which are present in MEF during AOM. Based on the pharmacokinetic and pharmacodynamic properties of azithromycin, it has been postulated that the drug could be delivered to the site of infection by leukocytes and provides sufficient antimicrobial activity to eradicate middle ear and other localized infections (11).
Azithromycin has been used in pediatric patients for a variety of infections and found to have a lower side effect profile, low rate of discontinuation of therapy caused by side effects, and a low potential for interaction, as well as clinical efficacy similar to that of oral agents such as amoxicillin-clavulanate (1, 15, 16, 22, 24) and amoxicillin (17). However, the definition of satisfactory clinical response varied from study to study. Only a limited number of azithromycin studies documented the specific etiology of AOM as bacterial (6, 7, 10), and fewer demonstrated bacterial eradication by mandatory follow-up tympanocentesis (double tap) (6, 7). Correlation between bacteriologic outcome and clinical outcome has been shown in otitis media (7, 8). Carlin et al. (4) reported agreement between clinical and bacteriologic response in 86% of patients in a large series. Dagan et al. (8) reported clinical failure in 37% of the children with bacteriologic failure, whereas only 3% of patients with bacteriologic eradication had clinical failure.
In two prospective randomized double-tap studies of otitis media (6, 7) azithromycin demonstrated a high bacteriologic failure rate in children infected with NTHI. In both studies azithromycin was administered for 5 days (10 mg/kg on day 1 and then 5 mg/kg daily on days 2 through 5). When efficacy of cefaclor and azithromycin in AOM were assessed, bacteriologic failure after 3 to 4 days of treatment occurred in a high proportion of H. influenzae culture-positive patients with azithromycin (53%) and with cefaclor (52%) (7). In a comparison of the bacteriologic and clinical efficacy of amoxicillin-clavulanate and azithromycin in AOM with tympanocentesis before the first dose and repeated on days 4 to 6, culture-positive OM persisted in 61% of children receiving azithromycin with disease due to NTHI at the initial visit (6). However, even in children with persistent culture-positive disease due to NTHI, evidence of clinical response was present by days 4 to 6, as evidenced by significantly lower scores of disease intensity (from entry).
The effect of azithromycin in EOM was also evaluated previously with a chinchilla model. The results indicate that azithromycin at 30 mg/kg/day had a significantly higher rate of sterilization than azithromycin at 15 mg/kg/day. However, 6 days after treatment was begun, 37% of ears were still culture positive in the 30-mg/kg/day group, and 67% were positive in the 15-mg/kg/day group (5). As in children, EOM due to NTHI resolves spontaneously over time in the chinchilla, albeit over a longer time course (time to 50% sterilization is estimated to be 4 to 6 days in children and 13 to 15 days in chinchillas) (3, 14).
Our data indicate that administration of azithromycin at 120 and 30 mg/kg/day each results in a reduction in density of middle ear infection due to NTHI compared to placebo. In addition, the rapidity of sterilization and the proportion of animals achieving sterilization is greater in the 120-mg/kg/day group than in either the placebo or 30-mg/kg/day cohorts. Increasing the serum AUC/MIC ratio from 4 to 10 results in more rapid and a higher rate of sterilization. In children, increasing severalfold the AUC/MIC ratio currently achieved with dosing at 10 mg/kg on day 1 and 5 mg/kg on days 2 to 5 AUC (approximately 2 to 3 μg · h/ml) would be desirable and potentially improve the microbiologic outcome. However, based on prior experimental studies in our laboratory with β-lactam antibiotics, sterilization with azithromycin is less rapid (14). These observations may indicate that in otitis media due to H. influenzae a decline in the number of CFU per milliliter occurs, although middle ear cultures obtained during double-tap studies are “positive” at 4 to 6 days (5, 6), and if follow-up tympanocentesis was performed at a later time point, eradication might be observed.
When azithromycin was given to children once daily for 5 days (10 mg/kg on day 1 and 5 mg/kg on days 2 to 5), the maximum concentration of azithromycin in serum and the AUC at 24 h were 0.224 μg/ml and 1.841 μg · h/ml, respectively (19). Pediatric studies indicate that increased azithromycin at 12 mg/kg results in proportionately higher concentrations in serum and presumably higher AUCs at 24 h than lower doses (5 mg/kg) (25). Human immunodeficiency virus-infected children received azithromycin at 5 mg/kg daily with a minimum concentration in serum of 0.052 μg/ml, a maximum concentration in serum of 0.230 μg/ml, and an AUC of 2.32 μg · h/ml (20).
Azithromycin levels in intracellular and extracellular compartments of MEF were assessed in pediatric patients with AOM after administration of 10 mg/kg (23). MEF was obtained by tympanocentesis 4, 12, and 24 h after dosing and divided into with-cell and cell-free fractions. Azithromycin concentrations in the cell-free fraction were 0.11, 0.12, and 0.23 μg/ml at 4, 12, and 24 h, respectively. Concentrations in the fraction with cells were 0.38, 0.9, and 1.05 μg/ml at the same time points.
After administration of azithromycin at 30 mg/kg as single daily doses in our chinchilla model of EOM due to NTHI, we were able to achieve levels in serum and AUCs approximately twice those observed in children treated with 10 mg/kg or with 10, 5, 5, 5, and 5 mg/kg as single daily doses (13, 19, 20) and concentrations in MEF comparable to those reported for children with AOM. Our observations provide evidence that current doses of azithromycin administered to children are likely to have a modest antibacterial effect on AOM due to NTHI, characterized by a reduction in density of infection. Maximizing the dosing of azithromycin in children has the potential to improve the microbiologic outcome. The results also suggest that follow-up tympanocentesis may need to be performed at a somewhat later time point to observe this effect in clinical trials.
Acknowledgments
The study was funded through a grant from Pfizer Pharmaceutical Company. Stephen I. Pelton is a member of the Pfizer Otitis Media Advisory Panel.
REFERENCES
- 1.Aronovitz, G. 1996. A multicenter, open label trial of azithromycin vs. amoxicillin-clavulanate for the management of acute otitis media in children. Pediatr. Infect. Dis. J. 15(Suppl.):S15-S19. [DOI] [PubMed] [Google Scholar]
- 2.Block, S. L. 1997. Causative pathogens, antibiotic resistance and therapeutic considerations in acute otitis media. Pediatr. Infect. Dis. J. 16:449-456. [DOI] [PubMed] [Google Scholar]
- 3.Bolduc, G. R., P. G. Tam, and S. I. Pelton. 1996. Therapeutic approaches to experimental otitis media due to penicillin-resistant Streptococcus pneumoniae, p. 518-519. In Recent advances in otitis media. Proceedings of the Sixth International Symposium. B. C. Decker, Hamilton, Ontario, Canada.
- 4.Carlin, S. A., C. D. Marchant, P. A. Shurin, C. E. Johnson, D. M. Super, and J. M. Rehmus. 1991. Host factors and early therapeutic response in acute otitis media. J. Pediatr. 118:178-183. [DOI] [PubMed] [Google Scholar]
- 5.Chan, K. H., J. D. Swarts, W. J. Doyle, J. Tanpowpong, and D. R. Kardatzke. 1988. Efficacy of a new macrolide (azithromycin). For acute otitis media in the chinchilla model. Arch. Otolaryngol. Head Neck Surg. 114:1266-1269. [DOI] [PubMed] [Google Scholar]
- 6.Dagan, R., C. E. Johnson, S. McLinn, N. Abughal, J. Feris, E. Leibovitz, D. J. Burch, and M. R. Jacobs. 2000. Bacteriologic and clinical efficacy of amoxicillin/clavulanate vs. azithromycin in acute otitis media. Pediatr. Infect. Dis. J. 19:95-104. [DOI] [PubMed] [Google Scholar]
- 7.Dagan, R., E. Leibovitz, D. M. Fliss, A. Leiberman, M. R. Jacobs, W. Craig, and P. Yagupsky. 2000. Bacteriologic efficacies of oral azithromycin and oral cefaclor in treatment of acute otitis media in infants and young children. Antimicrob. Agents Chemother. 44:43-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dagan, R., E. Leibovitz, D. Greenberg, P. Yagupsky, D. M. Fliss, and A. Leiberman. 1998. Early eradication of pathogens from middle ear fluid during antibiotic treatment of acute otitis media is associated with improved clinical outcome. Pediatr. Infect. Dis. J. 17:776-782. [DOI] [PubMed] [Google Scholar]
- 9.Diggle, P. J., K. Y. Lang, and S. L. Zeyer. 1994. Analysis of longitudinal data. Clarendon Press, Oxford, United Kingdom.
- 10.Giebink, G. S. 1999. Otitis media: the chinchilla model. Microb. Drug Resist. 5:57-72. [DOI] [PubMed] [Google Scholar]
- 11.Girard, A. E., C. R. Cimochowski, and J. A. Faiella. 1996. Correlation of increased azithromycin concentrations with phagocyte infiltration into sites of localized infection. J. Antimicrob. Chemother. 37(Suppl. C):9-19. [DOI] [PubMed] [Google Scholar]
- 12.Gladue, R. P., G. M. Bright, R. E. Isaacson, and M. F. Newborg. 1989. In vitro and in vivo uptake of azithromycin (CP-62 993) by phagocytic cells: possible mechanism of delivery and release at sites of infection. Antimicrob. Agents Chemother. 33:277-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ishida, Y., K. Kida, and M. Matsuda. 1996. Pharmacokinetic and clinical evaluation of azithromycin in pediatric infections. Jpn. J. Antibiot. 49:1030-1038. [PubMed] [Google Scholar]
- 14.Karasic, R. B., C. E. Trumpp, H. E. Gnehm, P. A. Rice, and S. I. Pelton. 1985. Modification of otitis media in chinchillas rechallenged with nontypable Haemophilus influenzae and serological response to outer membrane antigens. J. Infect. Dis. 151:273-279. [DOI] [PubMed] [Google Scholar]
- 15.Khurana, C. M. 1996. A multicenter, randomized, open label comparison of azithromycin and amoxicillin-clavulanate in acute otitis media among children attending day care or school. Pediatr. Infect. Dis. J. 15(Suppl.):S24-S29. [DOI] [PubMed] [Google Scholar]
- 16.McLinn, S. 1996. A multicenter, double blind comparison of azithromycin and amoxicillin-clavulanate for the treatment of acute otitis media in children. Pediatr. Infect. Dis. J. 15(Suppl.):S20-S23. [DOI] [PubMed] [Google Scholar]
- 17.Mohs, E., A. Rodriguez-Solares, E. Rivas, and Z. el Hoshy. 1993. A comparative study of azithromycin and amoxycillin in pediatric patients with acute otitis media. J. Antimicrob. Chemother. 31(Suppl. E):73-79. [DOI] [PubMed] [Google Scholar]
- 18.Musher, D., and R. Dagan. 2000. Is the pneumococcus the one and only in acute otitis media? Pediatr. Infect. Dis. J. 19:399-400. [DOI] [PubMed] [Google Scholar]
- 19.Nahata, M. C., K. I. Koranyi, D. R. Luke, and G. Foulds. 1995. Pharmacokinetics of azithromycin in pediatric patients with acute otitis media. Antimicrob. Agents Chemother. 39:1875-1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ngo, L. Y., R. Yogev, and W. M. Dankner for the ACTG 254 Team. 1999. Pharmacokinetics of azithromycin administered alone and with atovaquone in human immunodeficiency virus-infected children. Antimicrob. Agents Chemother. 43:1516-1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pelton, S. I., M. Figueira, R. Albut, and D. Stalker. 2000. Efficacy of linezolid in experimental otitis media. Antimicrob. Agents Chemother. 44:654-657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Principi, N. 1995. Multicentre comparative study of the efficacy and safety of azithromycin compared with amoxicillin-clavulanic acid in the treatment of pediatric patients with otitis media. Eur. J. Clin. Microbiol. 14:669-676. [DOI] [PubMed] [Google Scholar]
- 23.Scaglione, F., G. Demartini, S. Dugnani, M. M. Arcidiacono, J. P. Pintucci, and F. Fraschini. 1999. Interpretation of middle ear fluid concentrations of antibiotics: comparison between ceftibuten, cefixime and azithromycin. Br. J. Clin. Pharmacol. 47:267-271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schaad, U. B. 1993. Multicentre evaluation of azithromycin in comparison with co-amoxiclav for the treatment of acute otitis media in children. J. Antimicrob. Chemother. 31(Suppl. E):81-88. [DOI] [PubMed] [Google Scholar]
- 25.Stevens, R. C., M. D Reed, J. L. Shenep, D. K. Baker, G. Foulds, D. R. Luke, J. L. Blimer, and J. H. Rodman. 1997. Pharmacokinetics of azithromycin after single- and multiple-doses in children. Pharmacotherapy 17:874-880. [PubMed] [Google Scholar]