Abstract
The majority of the 90 capsule types made by the gram-positive pathogen Streptococcus pneumoniae are assembled by a block-type mechanism similar to that utilized by the Wzy-dependent O antigens and capsules of gram-negative bacteria. In this mechanism, initiation of repeat unit formation occurs by the transfer of a sugar to a lipid acceptor. In S. pneumoniae, this step is catalyzed by CpsE, a protein conserved among the majority of capsule types. Membranes from S. pneumoniae type 2 strain D39 and Escherichia coli containing recombinant Cps2E catalyzed incorporation of [14C]Glc from UDP-[14C]Glc into a lipid fraction in a Cps2E-dependent manner. The Cps2E-dependent glycolipid product from both membranes was sensitive to mild acid hydrolysis, suggesting that Cps2E was catalyzing the formation of a polyprenyl pyrophosphate Glc. Addition of exogenous polyprenyl phosphates ranging in size from 35 to 105 carbons to D39 and E. coli membranes stimulated Cps2E activity. The stimulation was due, in part, to utilization of the exogenous polyprenyl phosphates as an acceptor. The glycolipid product synthesized in the absence of exogenous polyprenyl phosphates comigrated with a 60-carbon polyprenyl pyrophosphate Glc. When 10 or 100 μM UMP was added to reaction mixtures containing D39 membranes, Cps2E activity was inhibited 40% and 80%, respectively. UMP, which acted as a competitive inhibitor of UDP-Glc, also stimulated Cps2E to catalyze the reverse reaction, with synthesis of UDP-Glc from the polyprenyl pyrophosphate Glc. These data indicated that Cps2E was catalyzing the addition of Glc-1-P to a polyprenyl phosphate acceptor, likely undecaprenyl phosphate.
In the gram-positive pathogen Streptococccus pneumoniae, 90 different capsule types have been identified, each of which varies in its sugar composition and/or the complexity of its glycosidic linkages (25, 52). Capsule is essential for pneumococcal virulence and provides a barrier against recognition by phagocytic receptors of complement bound to the bacterial surface (5, 10, 20, 23, 57, 58). In addition, the capsule can affect the amount of complement that binds to the bacterial surface (1), and it is required for the asymptomatic colonization of the nasopharynx (38). The majority of capsule types in S. pneumoniae are synthesized by a block-type mechanism similar to that used in Wzy-dependent O-antigen synthesis in gram-negative bacteria. In this mechanism, the capsule repeat units are built upon a lipid acceptor on the intracellular face of the cytoplasmic membrane, subsequently transported across the membrane, and polymerized (56). Capsular polysaccharides synthesized by this mechanism in gram-positive bacteria are transferred onto the cell wall following polymerization (15, 50). In S. pneumoniae, the latter step is independent of the size of the polymer (8).
The enzymes uniquely required for capsule synthesis in S. pneumoniae are encoded within a single locus on the chromosome (3, 16, 17, 21, 35). The genes are organized in a cassette structure with type-specific genes needed to synthesize each capsule being flanked by genes that are common to all capsule types (3, 17, 21, 41, 42). CpsA, -B, -C, and -D, encoded by the upstream common genes, are highly conserved among serotypes and have been proposed to play a role in modulating capsule amounts and chain length (8, 37, 41-43). CpsE, the initiating glycosyltransferase, is encoded by the next gene in most S. pneumoniae capsule loci and has been designated a type-specific gene (21). However, it also is highly conserved among serotypes, with the majority of CpsE proteins sharing 70 to 98% amino acid identity (28, 32, 37, 41, 54), and should be reclassified as a common protein. Using high-stringency Southern blots, Morona et al. have demonstrated that cpsA and cpsB are highly conserved among capsule serotypes but that cpsE, along with cpsC and cpsD, can be grouped into two classes (41). This result suggests that the functions of the proteins encoded by cpsC, cpsD, and cpsE are interrelated. CpsC and CpsD together encode an autophosphorylating tyrosine kinase involved in controlling the amount or size of the capsule (9), while CpsE shares homology with glycosyltransferases, including WbaP from Salmonella enterica serovar Typhimurium (33), CpsE (formerly designated CpsD) from Streptococcus agalactiae (49), and GumD from Xanthomonas campestris (29). CpsE in S. pneumoniae capsule types 14 and 9v is a 455-amino-acid membrane-localized protein that initiates repeat unit formation by adding the first sugar to a lipid acceptor (35, 36, 45, 54). Based on its homology with WbaP, which initiates repeat unit formation in Salmonella enterica O-antigen synthesis (33, 44) and Escherichia coli group 1 capsule synthesis (18), CpsE is thought to catalyze the addition of a hexose-1-phosphate to an undecaprenyl phosphate lipid acceptor (21). Although Cps14E, Cps9vE, and Cps8E from S. pneumoniae have been shown to utilize UDP-Glc to synthesize a Glc-containing glycolipid, neither the lipid acceptor nor the precise activity of the enzyme has been demonstrated (35, 36, 45, 54).
To further characterize CpsE activity and the lipid acceptor, we used membranes isolated from the S. pneumoniae type 2 strain D39 and an E. coli recombinant expressing Cps2E. The type 2 capsular repeat unit is a branched hexasaccharide containing glucose, rhamnose, and glucuronic acid (Fig. 1) (30). Although the initiating sugar for the type 2 capsule repeat unit is not known, Kolkman et al. demonstrated that membranes from type 2 S. pneumoniae can incorporate [14C]Glc into a lipid product, suggesting that Glc is the first sugar added to the repeat unit (37). We demonstrate here that Cps2E catalyzes the reversible addition of Glc-1-phosphate from UDP-Glc to a polyprenyl phosphate acceptor.
FIG. 1.
The repeating structure of S. pneumoniae type 2 capsular polysaccharide (30). Glc, glucose; Rha, rhamnose; GlcUA, glucuronic acid.
MATERIALS AND METHODS
Materials.
UDP-[14C]Glc (257 mCi/mmol) was obtained from Andotek, and UDP-[3H]Glc (1 Ci/mmol) was obtained from Sigma. Econo-safe scintillation cocktail was from Research Products International, Corp. Heptaprenyl phosphate (C35-P), dodecaprenyl phosphate (C60-P), hexadecaprenyl phosphate (C80-P), dolichyl phosphate (Dol-P; C85-C105-P), mutanolysin, and UDP-Glc, were obtained from Sigma. Nonidet P-40 (NP-40) was from Calbiochem, and Todd-Hewitt broth, yeast extract, and tryptone were from Difco. 3MM chromatography paper was from Whatman. Silica gel 60 F254 thin-layer chromatography (TLC) plates were from Merck.
Bacterial strains, growth conditions, and membrane preparations.
The S. pneumoniae strains listed in Table 1 were grown in Todd-Hewitt broth supplemented with 0.5% yeast extract or on blood agar plates (BAP) containing blood agar base no. 2 (Difco) and 3% defibrinated sheep blood (Colorado Serum Company). Membranes from S. pneumoniae cells were isolated as previously described (12), except that the final membrane preparations were washed and suspended in 100 mM Tris-acetate (pH 7.5) containing 10% glycerol.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Relevant properties | Source or reference |
|---|---|---|
| Strains | ||
| S. pneumoniae | ||
| D39 | Type 2 parent strain, Cps+ | 4 |
| KA1521 | pKA211 × D39, Δcps2E, Cps− | This study |
| KA1522 | pKA211 × D39, Δcps2E, Cps− | This study |
| E. coli | ||
| BL21-AI | F−ompT hsdSB(rB− mB−) gal dcm araB::T7 RNAP-tetA | Invitrogen |
| DH5αF′ | F′φ80dlacZΔM15 Δ(lacZYA-argF)U169 deoR recA1 endA1 hsdR17(rK− mK+) phoA supE44 λ−thi-1 gyrA96 relA1 | Life Technologies |
| MB091 | BL21-AI(pMB091); C-terminal portion of Cps2E with C-terminal-His6 tag | This study |
| RC123 | BL21-AI(pRC123); Cps2E full length | This study |
| RC124 | BL21-AI(pET20b) | This study |
| TOP10 | F−mcrA Δ(mrr-hsdRMS-mcrBC) φ80dlacZΔM15 ΔlacX74 recA1 deoR araD139 Δ(ara-leu)7697 galU galK rpsL (Strr) endA1 nupG | Invitrogen |
| Plasmids | ||
| pCRII-TOPO | Cloning vector for PCR products, Apr Kmr | Invitrogen |
| pET20b | Protein expression vector, Apr | Novagen |
| pGEM-T Easy | Cloning vector for PCR products, Apr | Promega |
| pJY4163 | Lacks origin of replication for S. pneumoniae, Emr | 61 |
| pKA211 | pJY4163 derivative containing PCR fragments from the amplification of D39 chromosomal DNA using primer pairs cps2E-5/cps2T-1 and cps2D-3/cps2E-6; used to delete cps2E | This study |
| pMB091 | pET20b containing DNA encoding C-terminal portion of Cps2E with C-terminal His6 tag, used for Cps2E expression for antiserum production | This study |
| pRC123 | pET20b containing full-length cps2E, used for Cps2E expression in E. coli |
E. coli strains listed in Table 1 were grown in L broth (10 g tryptone, 5 g yeast extract, 5 g NaCl, and 1 g glucose per liter) or on L agar plates (L broth containing 15 g of agar per liter) containing 100 μg/ml ampicillin or 300 μg/ml erythromycin. For membrane preparations, E. coli strains were grown in 250-ml cultures to a cell density of approximately of 1 × 109 CFU/ml. Arabinose (2% final concentration) was added to induce expression of cps2E, and the cultures were incubated for an additional 30 min at 37°C. Bacteria were harvested by centrifugation at 10,000 × g for 5 min and washed twice with 250 ml phosphate-buffered saline (PBS; 140 mM NaCl, 3 mM KCl, 5 mM Na2HPO4 [pH 7.4]) containing 10% glycerol. Cell pellets were frozen at −80°C and then thawed at room temperature; suspended in 2 ml of spheroplast buffer (20% sucrose, 10 mM EDTA, 10 mM Tris-HCl [pH 7.5]) containing 400 μg/ml lysozyme, 0.5 μg/ml pepstatin, 0.7 μg/ml leupeptin, 1 mM dithiothreitol, and 1 mM phenylmethylsulfonyl fluoride (PMSF); and incubated at 4°C with constant mixing for 40 min. Spheroplasts were sedimented by centrifugation at 12,000 × g for 5 min and suspended in 2 ml sterile water containing 10 mM EDTA and 1 mM PMSF. Suspensions were sonicated five times for 20 s with a Heat Systems-Ultrasonics sonicator equipped with a microtip on a setting of 4. Intact cells were sedimented by centrifugation at 5,000 × g for 10 min. The supernatant was collected, and membranes were sedimented by centrifugation at 100,000 × g for 30 min. Membranes were washed three times with 4 ml of 100 mM Tris (pH 7.5) containing 10% glycerol. The final membrane pellet was suspended in 1 ml of wash buffer.
Construction of cps2E mutants.
cps2E deletion mutants were generated in S. pneumoniae D39 by previously described techniques (23). Briefly, PCR fragments flanking cps2E were generated using type 2 S. pneumoniae strain D39 chromosomal DNA as a template and primer pairs cps2E-5/cps2T-1 and cps2D-3/cps2E-6, whose sequences and locations in the published type 2 capsule sequence are indicated in Table 2. The resulting two PCR products were cloned separately into pGEM-T Easy (Promega) and maintained in DH5αF′. The cps2E-5/cps2T-1 product was excised by digestion with SacII and SacI, while the cps2D-3/cps2E-6 product was excised by digestion with SpeI and SacII. Both excised products were ligated together into pJY4163 digested with SpeI and SacI. The resulting plasmid, designated pKA211, was used to transform competent D39 (22). Transformants were plated on blood agar plates without selection. Small colonies, indicative of loss of capsule production, were isolated and screened for the cps2E deletion using PCR amplification with primers cps2D-3 and cps2T-1. Deletion mutants yielded a 1,173-bp PCR product, in contrast to the 2,480-bp product of the parent D39. Mutants from two independent transformations were designated KA1521 and KA1522. Using indirect enzyme-linked immunosorbent assays performed as previously described (9), no capsule was detected in these strains.
TABLE 2.
Primers used in this study
| Primera | Sequence | Positionb |
|---|---|---|
| Cps2D-3 (+) | CTCACAGGCAAAATTGGATTTTG | cps2D4368-4380 |
| Cps2E-8 (+) | CATATGAATGGAAAAACAGTAAAGTC | cps2E5046-5068 |
| Cps2E-6 (−) | GGCCAATGAAGACTTTACTG | cps2E5078-5059 |
| Cps2E-12 (+) | CATATGGGGGGCTCTGCTATTTTTGC | cps2E5919-5938 |
| Cps2E-5 (+) | GTAGTATTTATGAGAGATGGAG | cps2E6384-6405 |
| Cps2E-9 (−) | CTCGAGCTTCGCTCCATCTCTCATAA | cps2E6411-6391 |
| Cps2E-11 (−) | CTCGAGCTACTTCGCTCCATCTCTC | cps2E6414-6395 |
| Cps2T-1 (−) | CTCATGACCATCTGGATTTAC | cps2T6849-6829 |
Cloning of the full-length and C-terminal portion of Cps2E.
To clone cps2E, the 1.2-kb open reading frame was amplified from D39 chromosomal DNA using primers cps2E-8 and cps2E-11. The resulting product was gel purified, cloned into pCRII-TOPO (Invitrogen), and maintained in TOP10 cells. The cloned product was excised by digestion with NdeI and XhoI and subcloned into pET20b, generating pRC123, which was maintained in E. coli strain BL21-AI.
A 492-bp product encoding the C-terminal portion of Cps2E (amino acids 291 to 455) was amplified from D39 chromosomal DNA using primers cps2E-9 and cps2E-12. As above, the PCR product was cloned into pCRII-TOPO and excised with NdeI and XhoI. The excised product was cloned into pET20b in frame with the His6 tag of the vector, generating pMB091, which was maintained in E. coli BL21-AI.
Cps2E antiserum preparation and immunoblot analyses.
Two liters of MB091 was grown to a cell density of 1 × 109 CFU/ml and induced with arabinose as described above. Bacteria were pelleted by centrifugation at 7,000 × g, suspended in 25 ml of pH 8.0 denaturing buffer (8 M urea, 100 mM NaH2PO4, and 10 mM Tris), and mixed at room temperature for 1 h. Insoluble cell debris was pelleted by centrifugation at 10,000 × g for 10 min, and the supernatant was mixed with 4 ml of a 50% Ni-nitrilotriacetic acid (QIAGEN) slurry for 1 h at room temperature. The lysate resin was allowed to settle in a column and washed twice with 4 ml of denaturing buffer (pH 6.3). Proteins bound to the resin were eluted by washing 4 times with 0.5 ml of denaturing buffer (pH 5.9). The eluted samples were separated on a sodium dodecyl sulfate-10% polyacrylamide gel and visualized by Coomassie staining. The protein band corresponding to the size of the C-terminal portion of Cps2E (26 kDa) was excised. The polyacrylamide gel fragment was placed in 2 ml of PBS, and the protein was extracted by grinding the gel with a mortar and pestle. The acrylamide fragments were removed by centrifugation, and the resulting protein (1 mg) was used for antiserum production in rabbits by Gemini Research (Odenville, AL).
To detect Cps2E in S. pneumoniae and E. coli, membranes prepared as described above and containing 5 to 15 μg of total protein were separated on a sodium dodecyl sulfate-10% polyacrylamide gel. Immunoblots were performed as previously described (62), except that membranes were blocked for 1 h at room temperature in 5% nonfat dried milk, 1% bovine serum albumin, and 0.05% Tween 20 in PBS. The primary antibody was a 1:5,000 dilution of the rabbit polyclonal antiserum directed against the C-terminal portion of Cps2E, prepared as described above, that had been absorbed against the cps2E deletion strain KA1522.
Chromatography.
Silica gel G plates were chromatographed in butanol-ethanol-water (5:3:2) or butanol-acetic acid-water (50:12:24) followed by autoradiography. Samples spotted on paper were chromatographed in ethanol-1 M ammonium acetate (pH 5.5) (65:35). Paper chromatograms were cut into 1-cm strips, and the amount of radioactivity in the strips was determined by liquid scintillation counting.
Glycosyltransferase assays.
Cps2E activity was determined as previously described for Cps14E (35). Briefly, membranes (amounts indicated in relevant figure legends) were incubated at 10°C in 100-μl reaction mixtures containing 5 mM Tris-acetate (pH 7.5), 10 mM MgCl2, and UDP-[14C]Glc or UDP-[3H]Glc (label and amount indicated in figure legends). For assays containing exogenous lipids, 10 μl of 1% NP-40 was added to 100-μl aliquots of the lipid (5 mg/ml), which were then dried under a stream of nitrogen. The dried lipids were suspended in 100 μl of H2O, and dilutions were made in 0.1% NP-40. Ten microliters of the concentrated or diluted lipids was added to the reaction mixtures. All reactions were stopped by the addition of 1 ml chloroform-methanol (2:1) and vortexed briefly, and the phases were allowed to separate. The upper phase was removed and the organic phase was extracted three times with 200 μl pure solvent upper phase (PSUP; 1.5 ml chloroform, 25 ml methanol, 23.5 ml H2O, and 0.183 g KCl). The organic phase was dried, and the amount of radioactivity was determined by liquid scintillation counting. For analysis by TLC, the lipid-containing products were dried under a stream of nitrogen and then solublized in 20 μl chloroform-methanol (1:1).
Assay for Cps2E-catalyzed formation of UDP-Glc.
Labeled glycolipid was synthesized as described above for the glycosyltransferase assay using either 1 μM UDP-[3H]Glc or UDP-[14C]Glc. For reactions containing UDP-[3H]Glc, the glycolipids were labeled in 200-μl reaction mixtures containing D39 membranes (80 μg of total protein) and 20 μg of C60-P for 10 min at 10°C. The labeled glycolipids were extracted as described for the glycosyltransferase assay. The organic phase containing the labeled glycolipids was dried under a stream of nitrogen and suspended in 100 μl of chloroform-methanol (1:1). Ten microliters of 1% NP-40 was added to the lipid extract, and the sample was dried again under a stream of nitrogen. The labeled glycolipids were suspended in 100 μl H2O. Ten microliters of the 3H-labeled glycolipid was incubated in 100-μl reaction mixtures containing 5 mM Tris-acetate (pH 7.5), 10 mM MgCl2, 100 μM UMP, and membranes from D39 or KA1522 for 30 min at 10°C. Reactions were stopped by the addition of 1 ml of chloroform-methanol (2:1), and the phases were allowed to separate. The upper aqueous phase was removed and saved. The lower organic phase was extracted twice with 200 μl PSUP, and the aqueous phases were combined with the initial aqueous phase. The combined aqueous phases were extracted once with 200 μl chloroform, and the organic phase was combined with the initial organic phase. The aqueous and organic phases were dried, and the amount of radioactivity present in both phases was determined by liquid scintillation counting.
For glycolipids labeled with 14C, reactions were stopped by being placed on ice, and the membranes were sedimented by centrifugation at 100,000 × g for 30 min. The membranes were washed with 5 ml of wash buffer (100 mM Tris-acetate [pH 7.5], 10% glycerol) to remove unincorporated UDP [14C]Glc and suspended in 100 μl of wash buffer. Ten microliters of the labeled membranes was incubated in 100-μl reaction mixtures containing 5 mM Tris-acetate (pH 7.5), 10 mM MgCl2, and 100 μM UMP or 100 μM TMP for 30 min at 10°C. Reactions were spotted on paper and chromatographed for 16 h in 95% ethanol-1 M ammonium acetate (pH 5.5) (65:35).
Alkaline hydrolysis, acid hydrolysis, and phospholipase D digestion of glycolipid products.
Alkaline hydrolysis of lipid products synthesized as described above was carried out at 35°C for 20 min in 0.4 ml of 80% methanol containing 0.1 N NaOH. The mixture was neutralized with ammonium hydroxide, and the methanol was removed with a stream of nitrogen. Samples were analyzed by TLC. Acid hydrolysis was carried out in 20 mM HCl at 70°C for 20 min. The hydrolysates were neutralized with acetic acid, dried under a stream of nitrogen, and analyzed by TLC. Phospholipase D digestions were conducted in 100-μl reaction mixtures containing 10 mM CaCl2, 100 mM Tris-acetate (pH 8), and 5 U of phospholipase D from Streptomyces chromofuscus. Reaction mixtures were incubated at 30°C for 30 min and analyzed by paper chromatography in butanol-acetic acid-water (44:16:40). A reaction mixture that contained no acid, alkali, or phospholipase D was incubated under the same conditions as the treated samples and used as a negative control.
RESULTS
Cps2E catalyzes the formation of a polyprenyl pyrophosphate Glc.
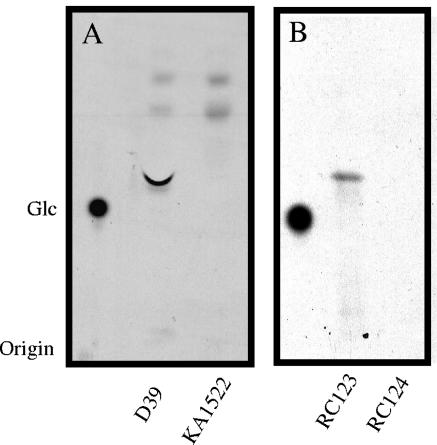
Using a polyclonal antiserum against the C-terminal third of Cps2E, a 52-kDa protein was observed by Western immunoblot analysis in S. pneumoniae D39 membranes and in an E. coli strain expressing recombinant Cps2E (RC123) but not in an S. pneumoniae strain in which cps2E had been deleted (KA1522) (data not shown). The size of the protein was consistent with its predicted molecular mass of 52 kDa. To characterize Cps2E activity, membranes from S. pneumoniae D39 and E. coli RC123 were isolated and incubated in reaction mixtures containing UDP-[14C]Glc. The incorporation of 14C into an organically soluble product was proportional to the amount of membrane added and was linear with time for 20 min (data not shown). Maximum 14C incorporation occurred at a pH of 7 to 7.5 in 50 mM Tris-acetate buffer. Membranes from S. pneumoniae D39 catalyzed the synthesis of four hydrophobic products that migrated more rapidly than Glc upon fractionation by TLC (Fig. 2A). The two fastest migrating products were also observed with membranes from KA1522 (Δcps2E), indicating that these products were not dependent on Cps2E activity. The migration of these products is consistent with the formation of mono- and diglycosyldiacylglycerol, two of the major lipids of S. pneumoniae membranes (11). Only a single product, which comigrated with the major Cps2E-dependent lipid product from S. pneumoniae, was observed with E. coli RC123 membranes (Fig. 2B). No products were observed with the E. coli vector control strain (RC124).
FIG. 2.
Separation of lipid products synthesized using S. pneumoniae and E. coli membranes containing Cps2E. (A) Membranes (300 μg of total protein) from type 2 S. pneumoniae strain D39 or its Δcps2E derivative KA1522 were incubated in 300-μl reaction mixtures containing 10 mM MgCl2, 5 mM Tris-acetate (pH 7.5), and 1 μM UDP-[14C]Glc (257 mCi/mmol) for 20 min at 10°C. Reactions were stopped by the addition of 1 ml chloroform-methanol (2:1), and lipids were extracted as described in Materials and Methods. Samples were applied as spots to a silica plate and chromatographed for 5 h in butanol-ethanol-water (5:3:2). Bands were visualized by autoradiography. [14C]Glc was run as a standard. (B) Membranes (40 μg of total protein) from E. coli strains RC123 (Cps2E+) and RC124 (vector control) were incubated in 100-μl reaction mixtures and processed as described for panel A.
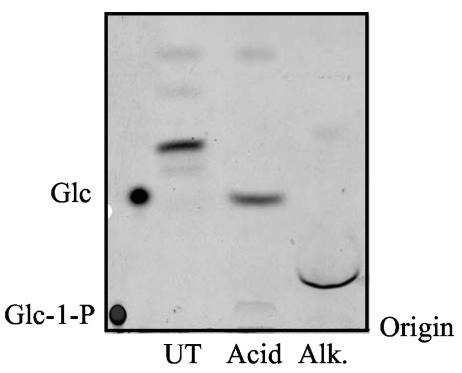
Mild acid hydrolysis of the glycolipids synthesized using D39 and RC123 membranes generated a product that comigrated with Glc (shown for D39 in Fig. 3). The glycolipid products were resistant, however, to phospholipase D digestion (data not shown). These results are consistent with the Cps2E- dependent glycolipid being a polyprenyl pyrophosphate Glc (polyprenyl-P-P-Glc). The Cps2E-dependent glycolipid products were also sensitive to alkaline hydrolysis, which generated a product that migrated slower than that observed with mild acid hydrolysis but faster than Glc-1-P (Fig. 3). The migration of this product is consistent with a sugar cyclic phosphate, which has been shown to be generated upon alkaline hydrolysis of certain polyprenyl phosphate-linked sugars (59).
FIG. 3.
Acid and alkaline hydrolysis of S. pneumoniae lipid products. Lipids (approximately 5,000 cpm) synthesized using D39 membranes as described in the legend to Fig. 2 were hydrolyzed in mild acid (Acid) or alkali (Alk) as described in Materials and Methods. The entire hydrolysate and an untreated sample (UT) were applied as spots to a silica plate, chromatographed, and visualized by autoradiography. [14C]Glc and [14C]Glc-1-phosphate were run as standards.
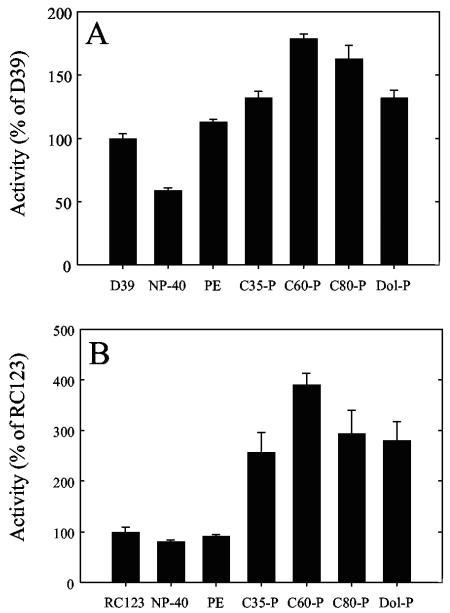
Cps2E activity is stimulated by the addition of exogenous polyprenyl phosphates.
Prior to addition of the exogenous lipids, D39 and RC123 membranes were incubated with 0.005 to 0.01% NP-40. Lipids (20 μg) suspended in 0.1% NP-40 were then added to the membranes to give a final NP-40 concentration of 0.015 to 0.02%. This concentration of NP-40 resulted in an approximately 40% reduction in Cps2E activity from D39 membranes (Fig. 4A). Addition of the polyprenyl phosphates heptaprenyl phosphate (C35-P), dodecaprenyl phosphate (C60-P), hexadecaprenyl phosphate (C80-P), and dolichyl phosphate (Dol-P) increased Cps2E activity above that observed when no exogenous lipid or NP-40 was added (Fig. 4A). Addition of phosphatidylethanolamine (PE) to D39 membranes also increased 3H incorporation above that observed with NP-40 (Fig. 4A). The addition of NP-40 to E. coli membranes had little effect on Cps2E activity, while the addition of the exogenous polyprenyl phosphates increased 3H incorporation (Fig. 4B). Unlike S. pneumoniae membranes, the presence of PE did not increase 3H incorporation with recombinant E. coli membranes (Fig. 4B). No increase in 3H incorporation was observed in the presence of exogenous polyprenyl phosphates with membranes from either S.pneumoniae strain KA1522 (Δcps2E) or the E. coli vector control strain RC124, indicating the increases in 3H incorporation observed with Cps2E-containing membranes from S. pneumoniae and E. coli were due to Cps2E activity (data not shown).
FIG. 4.
Addition of exogenous polyprenyl phosphates stimulates Cps2E activity. (A) Membranes (40 μg of total protein) from strain D39 or KA1522 (Δcps2E) were incubated in 100-μl reaction mixtures containing 10 mM MgCl2, 5 mM Tris-acetate (pH 7.5), and 1 μM UDP-[3H]Glc. For reaction mixtures containing NP-40 and the indicated exogenous lipids, D39 membranes were incubated with 0.005 to 0.01% NP-40 prior to the addition of either 10 μl of 0.1% NP-40 or the indicated lipid (2 mg/ml) dissolved in 0.1% NP-40. The reaction mixtures were incubated for 10 min at 10°C and processed as described in Materials and Methods. Lipids were extracted, and the amount of radioactivity in the organic phase was determined by liquid scintillation counting. The data are the mean of duplicate samples ± the standard error. All samples except PE were significantly different from D39 (P < 0.005 for C60-P; P < 0.05 for all others). All samples were significantly different from NP-40 (P < 0.001 for C60-P; P < 0.01 for all others). (B) Membranes (40 μg of total protein) from E. coli strain RC123 or RC124 (vector control) were incubated for 10 min at 10°C in reaction mixtures processed as described for panel A. C60-P and Dol-P were significantly different from RC123 (P < 0.01 and < 0.05, respectively). All samples except PE were significantly different from NP-40 (P = 0.005 for C60-P; P ≤ 0.05 for all others). Results were compared using Student's t test. Dol-P, dolichyl phosphate (C85-105-P).
To determine if the increase in 3H incorporation observed in the presence of the exogenous lipids was due to utilization of the lipids as acceptors, the organically soluble products were separated by TLC. Only a single Cps2E-dependent glycolipid product was observed with S. pneumoniae membranes (Fig. 5A, D39, NP-40, and PE) and E. coli membranes (Fig. 5B, RC123, NP-40, and PE) incubated in the absence of exogenous polyprenyl phosphates or in the presence of PE. In the presence of the C35-P, however, two products were observed: one that comigrated with the endogenous glycolipid and a slower migrating glycolipid. Two glycolipids were also synthesized in the presence of the C80-P and Dol-P (C85-105): one that comigrated with the endogenous glycolipid and a faster migrating glycolipid (Fig. 5A and B). These data indicate that Cps2E is utilizing these lipids as acceptors and likely accounts for a portion of the stimulation in Cps2E activity observed in Fig. 4. In reaction mixtures containing C60-P, a single glycolipid that comigrated with the endogenous glycolipid was observed. The amount of this glycolipid, however, appeared to be greater than that observed when no exogenous lipids were added, consistent with the increased 3H incorporation shown in Fig. 4. These data suggest that the endogenous lipid acceptor for Cps2E activity is similar in size to the C60-P.
FIG. 5.
Separation of glycolipid products by TLC. Labeled lipids were synthesized using (A) D39 membranes or (B) E. coli RC123 membranes in the presence or absence of exogenous lipids as described in the legend to Fig. 4, except the nucleotide sugar was 1 μM UDP-[14C]Glc. Lipids were isolated as described in the legend to Fig. 1, applied as spots on a silica plate, and chromatographed for 6 h in butanol-acetic acid-water (50:12:25). Bands were visualized by autoradiography.
Cps2E catalyzes synthesis of UDP-Glc from UMP and a polyprenyl pyrophosphate Glc.
To determine whether specific compounds could inhibit Cps2E activity, membranes from D39 were incubated in reaction mixtures containing UDP-[14C]Glc and 10 μM and 100 μM of the compounds indicated in Table 3. These concentrations are equivalent to or 10-fold above the UDP-Glc concentration, respectively. Among various nucleotides, sugars, and sugar nucleotides, only UMP showed strong inhibition of Cps2E activity. This result is indicative of a glycosyltransferase that transfers Glc-1-P to a polyprenyl phosphate, whereas strong inhibition by UDP would be consistent with transfer of Glc to the polyprenyl phosphate (59). Like the observations with S. pneumoniae membranes, Cps2E activity in E. coli membranes was inhibited by UMP (data not shown). The kinetics of this inhibition suggested that UMP was acting as a competitive inhibitor of UDP-Glc (Fig. 6), with an apparent Ki of 3 μM. The apparent Km for UDP-Glc in the absence of any exogenous polyprenyl phosphates was 3.5 μM.
TABLE 3.
Inhibitors of Cps2E activitya
| Compound | % Activity (P) at:
|
|
|---|---|---|
| 10 μM | 100 μM | |
| None | 100 ± 2.1 | 100 ± 2.1 |
| UMP | 68.3 ± 1.3 (<0.01)b | 20.3 ± 0.3 (<0.001)b |
| UDP | 94.6 ± 2.4 | 60.5 ± 0.5 (<0.01)b |
| TMP | 102 ± 2.8 | 101 ± 1.8 |
| Glc | 98.2 ± 1.0 | 99.5 ± 1.1 |
| Glc-1-P | 101 ± 0.3 | 100 ± 1.2 |
| UDP-Gal | 93.8 ± 4.2 | 75.0 ± 8.1 |
| UDP-Xyl | 99.3 ± 0.7 | 82.7 ± 0.7 (<0.05)b |
| TDP-Glc | 96.9 ± 1.4 | 95.1 ± 0.5 |
Cps2E activity was measured in 100-μl reaction mixtures containing 5 mM Tris-acetate, 10 mM MgCl2, 10 μM UDP-[14C]Glc, 10 μg C60-P, and either 10 μM or 100 μM of the indicated compounds. Reaction mixtures were incubated at 10°C for 10 min, and the amounts of radioactivity present in the lipid fraction were determined as described in Materials and Methods. Results are presented as the percent activity of reaction mixtures that contained no addition. The data for Glc, Glc-1-P, and UDP-Gal are the mean of triplicate reactions ± standard error. All the remaining data are the result of duplicate reactions. Results were compared using Student's t test.
Significantly different from no addition. P values are indicated in parentheses.
FIG. 6.
UMP is a competitive inhibitor of UDP-Glc. (A) Membranes from RC123 were incubated in 100-μl reaction mixtures containing 10 mM MgCl2, 50 mM Tris-acetate, 1 to 21 μM UDP-[14C]Glc, 10 μg of C60-P, and either no UMP (•), 5 μM UMP (○), or 10 μM UMP (▾) for 10 min at 10°C. Reactions were stopped and processed as described for glycosyltransferase assays in Materials and Methods. The amount of radioactivity in the organic phase was determined by liquid scintillation counting. (B) Plot of the reciprocal data from panel A.
Many glycosyltransferases catalyzing the addition of sugar-1-phosphates to polyprenyl phosphate acceptors are also capable of catalyzing the reverse reaction (2, 6, 34, 44). WbaP, the Cps2E homolog from S. enterica serovar Typhimurium, catalyzes synthesis of undecaprenyl pyrophosphate Gal as well as the reverse reaction, forming UDP-Gal from the glycolipid and UMP (44). Since UMP was a strong inhibitor of Cps2E activity, we wanted to determine if UMP could stimulate Cps2E-dependent catalysis of the reverse reaction. [3H]Glc-labeled glycolipid was synthesized using D39 membranes, isolated, and added to reaction mixtures containing UMP and either D39 or KA1522 (Δcps2E) membranes. In the presence of D39 membranes, a portion of the 3H was shifted from the organic phase to the aqueous phase. This shift was dependent on the amount of membranes added (Fig. 7A) and was linear with time for 30min (data not shown). Only 0.7% of the 3H was shifted to the aqueous phase when the labeled glycolipid was added to KA1522 membranes, indicating that Cps2E was required for the reaction. Similar results were obtained when labeled glycolipid was added to Cps2E-containing E. coli membranes (data not shown).
FIG. 7.
UMP stimulates synthesis of UDP-Glc from polyprenyl-P-P-Glc by Cps2E-containing membranes. (A) [3H]Glc-labeled glycolipids were synthesized and extracted as described in Materials and Methods. Ten microliters of the labeled glycolipid was added to reaction mixtures containing 10 mM MgCl2, 5 mM Tris-acetate (pH 7.5), 100 μM UMP, and either increasing concentrations of D39 membranes or 40 μg of KA1522 membranes for 30 min at 10°C. Reactions were processed as described in Materials and Methods, and the amounts of radioactivity present in the organic and aqueous phases were determined by liquid scintillation counting. The amount of radioactivity present in the aqueous phase of reactions that contained no membranes was subtracted as background. Data are the mean of duplicate samples ± standard errors. (B) Endogenous lipid acceptor was labeled with [14C]Glc as described in the legend to Fig. 2. The membranes were sedimented by centrifugation and washed to remove unincorporated UDP-[14C]Glc. The membranes were then incubated as described above with 100 μM UMP (•), 100 μM TMP (○), or no nucleotide (▪) for 30 min at 10°C. The reaction mixtures were applied as spots on paper and chromatographed overnight in ethanol-1 M ammonium acetate (pH 5.5) (65:35). The chromatogram was cut into 1-cm strips, and the amount of radioactivity was determined. The migration of a UDP-Glc standard visualized under UV is indicated on the graph.
To determine if the compound shifted to the aqueous phase in the presence of Cps2E was UDP-Glc, D39 membranes were incubated with UDP-[14C]Glc to generate [14C]Glc-labeled glycolipid and then washed to remove unincorporated label. The membranes were then incubated in the presence of UMP, TMP, or buffer. In the presence of UMP, 41% of the labeled glycolipid that migrated faster than Glc was converted to a product that comigrated with UDP-Glc (Fig. 7B). Thus the inhibition of Cps2E activity observed in the presence of UMP could be due to both preventing the binding of UDP-Glc to the active site of the enzyme and to stimulating Cps2E to catalyze the reverse reaction.
DISCUSSION
In this study, we analyzed the initiation and formation of the lipid repeat unit utilized to produce type 2 capsule in S. pneumoniae. This activity was first linked to the cpsE gene product in the S. pneumoniae type 14, 9V, and 8 capsule loci, although detailed analyses of the activity were not performed (35, 45, 54). We have demonstrated here that the native and recombinant Cps2E, contained in S. pneumoniae and E. coli membranes, respectively, catalyzes the formation of a glycolipid product using an endogenous lipid acceptor that has properties consistent with undecaprenyl pyrophosphate Glc. The biosynthetic repeat unit for type 2 capsule therefore has the structure shown in Fig. 8, and the repeat units are linked via a β(1→3) bond catalyzed by the type 2 polymerase.
FIG. 8.
The biosynthetic repeat unit of S. pneumoniae type 2 capsular polysaccharide. The repeat unit is shown attached to the lipid anchor.
The sensitivity to mild acid hydrolysis of the glycolipid synthesized using both S. pneumoniae and E. coli membranes containing Cps2E and comigration of the resulting product with Glc suggested that the glycolipid was a polyprenyl-P-P-Glc. Furthermore, the glycolipid synthesized using the endogenous lipid acceptor comigrated with C60-P-P-Glc, indicating that the lipid acceptor was similar in size to C55 undecaprenyl phosphate. Cps2E was also capable of using polyprenyl phosphates ranging in size from 35 to 105 carbons. A lack of size specificity for the lipid acceptor has also been demonstrated for the dolichyl phosphate mannose synthase from rat liver, which could catalyze transfer of mannose to Dol-P as well as to the water-soluble phenyl phosphate (31). In addition, MurG, the enzyme responsible for the addition of GlcNAc to lipid I (undecaprenyl-P-P-MurNAc-pentapeptide) in peptidoglycan synthesis, is able to utilize lipid acceptors of various chain lengths and double-bond geometries (13).
Like the undecaprenyl pyrophosphate-linked repeat units of the Salmonella enterica serovar Newington (14) and Salmonella enterica serovar Anatum (59) O antigens, the xanthan repeat units from X. campestris (29), and dolichyl monophosphate Glc involved in protein glycosylation in rat liver cells (7), the polyprenyl pyrophosphate Glc synthesized by Cps2E was sensitive to treatment with mild alkali. This is in contrast to what has been reported for the undecaprenyl pyrophosphate-linked repeat units of enterobacterial common antigen (48), mannosyl monophosphoryl undecaprenol (39), and peptidoglycan (27). Alkaline hydrolysis has commonly been used to separate glycerophosphate lipids, which are saponified in mild alkali, from polyprenyl-based glycolipids, which are in general thought to be resistant to alkaline hydrolysis (24). Since some polyprenyl phosphate-linked sugars are sensitive to the same alkaline hydrolysis conditions used in saponification, care should be taken in using this method for purification or identification of polyprenyl phosphate-linked glycolipids. Wright proposed that the different susceptibilities of polyprenyl-linked sugars are due to both the isomeric arrangement of the sugar and its anomeric configuration (59). If the hydroxyl group on carbon 2 of the sugar is cis to the hydroxyl on the adjoining phosphate, then the linkage is susceptible to alkaline hydroysis, while the trans arrangement is less susceptible. Since Cps2E catalyzes the transfer of Glc-1-P, the anomeric configuration of UDP-Glc is retained during the transfer, making the hydroxyl groups cis and thus susceptible to alkaline hydrolysis.
The level of undecaprenyl phosphate in the membranes of bacteria is estimated to be extremely low (40). Based on the Vmax determined in our kinetic studies of Cps2E, S. pneumoniae and E. coli contain approximately 500 to 1,000 molecules of the polyprenyl phosphate acceptor per bacterium. The use of undecaprenyl phosphate as the lipid acceptor for polysaccharide synthesis has been confirmed through mass spectral or nuclear magnetic resonance analysis for only a few polymers. These include S. enterica serovar Newington O antigens (60), enterobacterial common antigen (48), and colanic acid (34) in gram-negative bacteria; peptidoglycan in gram-negative (51) and gram-positive bacteria (26, 27); and teichoic acid in Bacillus licheniformis (55). Many of the capsules of E. coli are also thought to use undecaprenyl phosphate as an acceptor for repeat unit formation, due to the homology of the initiating glycosyltransferase WbaP with the initiating glycosyltransferase involved in O-antigen synthesis (56).
Since the synthesis of several different polysaccharides in bacteria, including essential polymers like peptidoglycan, draws from a limited pool of lipid acceptor, it may be important for cell viability that the lipid acceptor not be sequestered into the repeat units of any one polysaccharide. Yuasa et al. demonstrated that mutations in S. enterica LT2 that impaired synthesis of the side chain of the O-antigen repeat unit and resulted in the accumulation of incomplete repeat units were toxic to the bacterium, likely due to sequestration of undecaprenyl phosphate in O-antigen repeat units at the expense of peptidogycan synthesis (63). Similar toxic effects were observed when certain genes involved in the synthesis of succinoglycan from Sinorhizobium meliloti were mutated (19, 46) as well as when the E. coli enterobacterial common antigen flippase gene, wzxE, was mutated (47). The toxic effect of these mutations was also attributed to the buildup of polyprenyl pyrophosphate-linked intermediates. In peptidoglycan synthesis, the formation of lipid I by MraY is reversible and the lipid accumulates to approximately 700 copies per cell, even in E. coli strains that contain mutations inhibiting the subsequent steps in peptidoglycan synthesis (40, 53). This result suggests that the maximum level at which lipid I can accumulate in the bacterium is limited. The reversibility of the addition of the first sugar to the lipid acceptor in capsule synthesis, as well as the synthesis of all polysaccharides that utilize undecaprenyl phosphate as an acceptor, may be one way in which the cell ensures a constant pool of lipid acceptor. Factors that influence the direction of this reaction may thus be important in determining the ratio of cellular polysaccharides under different environmental conditions.
Acknowledgments
This work was supported by Public Health Service Grants GM53017, AI28457, T32 HL07553, and T32 GM08111 from the National Institutes of Health.
REFERENCES
- 1.Abeyta, M., G. G. Hardy, and J. Yother. 2003. Genetic alteration of capsule type but not PspA type affects accessibility of surface-bound complement and surface antigens of Streptococcus pneumoniae. Infect. Immun. 71:218-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson, J. S., M. Matsuhashi, M. A. Haskin, and J. L. Strominger. 1965. Lipid-phosphoacetylmuramyl-pentapeptide and lipid-phosphodisaccharide-pentapeptide: presumed membrane transport intermediates in cell wall synthesis. Proc. Natl. Acad. Sci. USA 53:881-889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arrecubieta, C., E. Garcia, and R. Lopez. 1995. Sequence and transcriptional analysis of a DNA region involved in the production of capsular polysaccharide in Streptococcus pneumoniae type 3. Gene 167:1-7. [DOI] [PubMed] [Google Scholar]
- 4.Avery, O., C. MacLeod, and M. McCarty. 1944. Studies on the chemical nature of the substance inducing transformation of pneumococcal types. Induction of transformation by a desoxyribonucleic acid fraction isolated from pneumococcus type III. J. Exp. Med. 79:137-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Avery, O. T., and R. Dubos. 1931. The protective action of a specific enzyme against type III pneumococcus infections in mice. J. Exp. Med. 54:73-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barr, K., and P. D. Rick. 1987. Biosynthesis of enterobacterial common antigen in Escherichia coli. J. Biol. Chem. 262:7142-7150. [PubMed] [Google Scholar]
- 7.Behrens, N. H., and L. F. Leloir. 1970. Dolichol monophosphate glucose: an intermediate in glucose transfer in liver. Proc. Natl. Acad. Sci. USA 66: 153-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bender, M. H., R. T. Cartee, and J. Yother. 2003. Positive correlation between tyrosine phosphorylation of CpsD and capsular polysaccharide production in Streptococcus pneumoniae. J. Bacteriol. 185:6057-6066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bender, M. H., and J. Yother. 2001. CpsB is a modulator of capsule-associated tyrosine kinase activity in Streptococcus pneumoniae. J. Biol. Chem. 276:47966-47974. [DOI] [PubMed] [Google Scholar]
- 10.Brown, E. J. 1985. Interaction of gram-positive microorganisms with complement. Curr. Top. Microbiol. Immunol. 121:159-187. [DOI] [PubMed] [Google Scholar]
- 11.Brundish, D. E., N. Shaw, and J. Baddiley. 1965. The occurrence of glycolipids in gram-positive bacteria. Biochem. J. 95:158-165. [DOI] [PubMed] [Google Scholar]
- 12.Cartee, R. T., W. T. Forsee, J. S. Schutzbach, and J. Yother. 2000. Mechanism of type 3 capsular polysaccharide synthesis in Streptococcus pneumoniae. J. Biol. Chem. 275:3907-3914. [DOI] [PubMed] [Google Scholar]
- 13.Chen, L., H. Men, S. Ha, X. Ye, L. Brunner, Y. Hu, and S. Walker. 2002. Intrinsic lipid preferences and kinetic mechanism of Escherichia coli MurG. Biochemistry 41:6824-6833. [DOI] [PubMed] [Google Scholar]
- 14.Dankert, M., A. Wright, W. S. Kelley, and P. W. Robbins. 1966. Isolation, purification, and properties of the lipid-linked intermediates of O-antigen biosynthesis. Arch. Biochem. Biophys. 116:425-435. [DOI] [PubMed] [Google Scholar]
- 15.Deng, L., D. L. Kasper, T. P. Krick, and M. R. Wessels. 2000. Characterization of the linkage between the type III capsular polysaccharide and the bacterial cell wall of group B Streptococcus. J. Biol. Chem. 275:7497-7504. [DOI] [PubMed] [Google Scholar]
- 16.Dillard, J., M. Vandersea, and J. Yother. 1995. Characterization of the cassette containing genes for type 3 capsular polysaccharide biosynthesis in Streptococcus pneumoniae. J. Exp. Med. 181:973-983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dillard, J., and J. Yother. 1994. Genetic and molecular characterization of capsular polysaccharide biosynthesis in Streptococcus pneumoniae type 3. Mol. Microbiol. 12:959-972. [DOI] [PubMed] [Google Scholar]
- 18.Drummelsmith, J., and C. Whitfield. 1999. Gene products required for surface expression of the capsular form of the group 1 K antigen in Escherichia coli (O9a:K30). Mol. Microbiol. 31:1321-1332. [DOI] [PubMed] [Google Scholar]
- 19.Glucksmann, M. A., T. L. Reuber, and G. C. Walker. 1993. Genes needed for the modification, polymerization, export, and processing of succinoglycan by Rhizobium meliloti: a model for succinoglycan biosynthesis. J. Bacteriol. 175:7045-7055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Griffith, F. 1928. The significance of pneumococcal types. J. Hyg. 27: 113-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guidolin, A., J. K. Morona, R. Morona, D. Hansman, and J. Paton. 1994. Nucleotide sequence analysis of genes essential for capsular polysaccharide biosynthesis in Streptococcus pneumoniae type 19F. Infect. Immun. 62: 5384-5396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hardy, G. G., M. J. Caimano, and J. Yother. 2000. Capsule biosynthesis and basic metabolism in Streptococcus pneumoniae are linked through the cellular phosphoglucomutase. J. Bacteriol. 182:1854-1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hardy, G. G., A. D. Magee, C. L. Ventura, M. J. Caimano, and J. Yother. 2001. Essential role for cellular phosphoglucomutase in virulence of type 3 Streptococcus pneumoniae. Infect. Immun. 69:2309-2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hemming, F. W. 1974. Lipids in glycan biosynthesis, p. 39-97. In T. W. Goodwin (ed.), Biochemistry of lipids. University Park Press, Baltimore, Md.
- 25.Henrichsen, J. 1995. Six newly recognized types of Streptococcus pneumoniae. J. Clin. Microbiol. 33:2759-2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Higashi, Y., J. L. Strominger, and C. C. Sweeley. 1970. Biosynthesis of the peptidoglycan of bacterial cell walls. J. Biol. Chem. 245:3697-3702. [PubMed] [Google Scholar]
- 27.Higashi, Y., J. L. Strominger, and C. C. Sweeley. 1967. Structure of a lipid intermediate in cell wall peptidoglycan synthesis: a derivative of a C55 isoprenoid alcohol. Proc. Natl. Acad. Sci. USA 57:1878-1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iannelli, F., B. J. Pearce, and G. Pozzi. 1999. The type 2 capsule locus of Streptococcus pneumoniae. J. Bacteriol. 181:2652-2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ielpi, L., R. O. Couso, and M. A. Dankert. 1993. Sequential assembly and polymerization of the polyprenol-linked pentasaccharide repeating unit of the xanthan polysaccharide in Xanthomonas campestris. J. Bacteriol. 175:2490-2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jansson, P. E., B. Lindberg, M. Anderson, U. Lindquist, and J. Henrichsen. 1988. Structural studies of the capsular polysaccharide from Streptococcus pneumoniae type 2, a reinvestigation. Carbohydr. Res. 182:111-117. [DOI] [PubMed] [Google Scholar]
- 31.Jensen, J. W., and J. S. Schutzbach. 1986. Characterization of mannosyl-transfer reactions catalyzed by dolichyl-mannosyl-phosphate-synthase. Carbohydr. Res. 149:199-208. [DOI] [PubMed] [Google Scholar]
- 32.Jiang, S.-M., L. Wang, and P. R. Reeves. 2001. Molecular characterization of Streptococcus pneumoniae type 4, 6B, 8, and 18C capsular polysaccharide gene clusters. Infect. Immun. 69:1244-1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jiang, X.-M., B. Neal, F. Santiago, S. J. Lee, L. K. Romana, and P. R. Reeves. 1991. Structure and sequence of rfb (O antigen) gene cluster of Salmonella serovar typhimurium (strain LT2). Mol. Microbiol. 5:695-713. [DOI] [PubMed] [Google Scholar]
- 34.Johnson, J. G., and D. B. Wilson. 1977. Role of a sugar-lipid intermediate in colanic acid synthesis by Escherichia coli. J. Bacteriol. 129:225-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kolkman, M. A. B., D. Morrison, B. A. M. van der Zeijst, and P. J. M. Nuijten. 1996. The capsule polysaccharide synthesis locus of Streptococcus pneumoniae serotype 14: identification of the glycosyl transferase gene cps14E. J. Bacteriol. 178:3736-3741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kolkman, M. A. B., B. A. M. van der Zeijst, and P. J. M. Nuijten. 1997. Functional analysis of glycosyltransferases encoded by the capsular polysaccharide biosynthesis locus of Streptococcus pneumoniae serotype 14. J. Biol. Chem. 272:19502-19508. [DOI] [PubMed] [Google Scholar]
- 37.Kolkman, M. A. B., B. A. M. van der Zeijst, and P. J. M. Nuijten. 1998. Diversity of capsular polysaccharide synthesis gene clusters in Streptococcus pneumoniae. J. Biochem. (Tokyo) 123:937-945. [DOI] [PubMed] [Google Scholar]
- 38.Magee, A. D., and J. Yother. 2001. Requirement for capsule in colonization by Streptococcus pneumoniae. Infect. Immun. 69:3755-3761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mancuso, D. J., and T.-H. Chiu. 1982. Biosynthesis of glucosyl monophosphoryl undecaprenol and its role in lipoteichoic acid biosynthesis. J. Bacteriol. 152:616-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mengin-Lecreulx, D., L. Texier, M. Rousseau, and J. van Heijenoort. 1991. The murG gene of Escherichia coli codes for the UDP-N-acetylglucosamine: N-acetylmuramyl-(pentapeptide) pyrophosphoryl-undecaprenol N-acetylglucosamine transferase involved in the membrane steps of peptidoglycan synthesis. J. Bacteriol. 173:4625-4636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morona, J. K., R. Morona, and J. C. Paton. 1999. Analysis of the 5′ portion of the type 19A capsule locus identifies two classes of cpsC, cpsD, and cpsE genes in Streptococcus pneumoniae. J. Bacteriol. 181:3599-3605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Morona, J. K., R. Morona, and J. C. Paton. 1997. Characterization of the locus encoding the Streptococcus pneumoniae type 19F capsular polysaccharide biosynthetic pathway. Mol. Microbiol. 23:751-763. [DOI] [PubMed] [Google Scholar]
- 43.Morona, J. K., J. C. Paton, D. C. Miller, and R. Morona. 2000. Tyrosine phosphorylation of CpsD negatively regulates capsular polysaccharide biosynthesis in Streptococcus pneumoniae. Mol. Microbiol. 35:1431-1442. [DOI] [PubMed] [Google Scholar]
- 44.Osborn, M. J., and Y. Tze-Yuen. 1968. Biosynthesis of bacterial lipopolysaccharide. VII. Enzymatic formation of the first intermediate in biosynthesis of the O-antigen of Salmonella typhimurium. J. Biol. Chem. 243:5145-5152. [PubMed] [Google Scholar]
- 45.Pelosi, L., M. Boumedienne, N. Saksouk, J. Geiselmann, and R. A. Geremia. 2005. The glucosyl-1-phosphate transferase WchA (Cap8E) primes the capsular polysaccharide repeat unit biosynthesis of Streptococcus pneumoniae serotype 8. Biochem. Biophys. Res. Commun. 327:857-865. [DOI] [PubMed] [Google Scholar]
- 46.Reuber, T. L., and G. C. Walker. 1993. Biosynthesis of succinoglycan, a symbiotically important exopolysaccharide of Rhizobium meliloti. Cell 74:269-280. [DOI] [PubMed] [Google Scholar]
- 47.Rick, P. D., K. Barr, K. Sankaran, J. Kajimura, J. S. Rush, and C. J. Waechter. 2003. Evidence that the wzxE gene of Escherichia coli K-12 encodes a protein involved in the transbilayer movement of a trisaccharide-lipid intermediate in the assembly of enterobacterial common antigen. J. Biol. Chem. 278:16534-16542. [DOI] [PubMed] [Google Scholar]
- 48.Rick, P. D., G. L. Hubbard, M. Kitaoka, H. Nagaki, T. Kinoshita, S. Dowd, V. Simplaceanu, and C. Ho. 1998. Characterization of the lipid-carrier involved in the synthesis of enterobacterial common antigen (ECA) and identification of a novel phosphoglyceride in a mutant of Salmonella typhimurium defective in ECA synthesis. Glycobiology 8:557-567. [DOI] [PubMed] [Google Scholar]
- 49.Rubens, C. E., L. M. Heggen, R. F. Haft, and M. R. Wessels. 1993. Identification of cpsD, a gene essential for type III capsule expression in group B streptococci. Mol. Microbiol. 8:843-855. [DOI] [PubMed] [Google Scholar]
- 50.Sorensen, U. B. S., J. Henrichsen, H. C. Chen, and S. C. Szu. 1990. Covalent linkage between the capsular polysaccharide and the cell wall peptidoglycan of Streptococcus pneumoniae revealed by immunochemical methods. Microb. Pathog. 8:325-334. [DOI] [PubMed] [Google Scholar]
- 51.Umbreit, J. N., and J. L. Strominger. 1972. Isolation of the lipid intermediate in peptidoglycan biosynthesis from Escherichia coli. J. Bacteriol. 112: 1306-1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.van Dam, J. E., A. Fleer, and H. Snippe. 1990. Immunogenicity and immunochemistry of Streptococcus pneumoniae capsular polysaccharides. Antonie Leeuwenhoek 58:1-47. [DOI] [PubMed] [Google Scholar]
- 53.van Heijenoort, J. 1996. Murein synthesis, p. 1025-1034. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology. ASM Press, Washington, D.C.
- 54.van Selm, S., M. A. B. Kolkman, B. A. M. van der Zeijst, K. A. Zwaagstra, W. Gaastra, and J. P. M. van Putten. 2002. Organization and characterization of the capsule biosynthesis locus of Streptococcus pneumoniae serotype 9V. Microbiology 148:1747-1755. [DOI] [PubMed] [Google Scholar]
- 55.Watkinson, R. J., H. Hussey, and J. Baddiley. 1971. Shared lipid phosphate carrier in the biosynthesis of teichoic acid and peptidoglycan. Nat. New Biol. 229:57-59. [DOI] [PubMed] [Google Scholar]
- 56.Whitfield, C., and I. S. Roberts. 1999. Structure, assembly and regulation of expression of capsules in Escherichia coli. Mol. Microbiol. 31:1307-1319. [DOI] [PubMed] [Google Scholar]
- 57.Winkelstein, J. A. 1981. The role of complement in host's defense against Streptococcus pneumoniae. Rev. Infect. Dis. 3:289-298. [DOI] [PubMed] [Google Scholar]
- 58.Wood, W. B., and M. R. Smith. 1949. Inhibition of surface phagocytosis by capsular “slime layer” of pneumococcus type III. J. Exp. Med. 90:85-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wright, A. 1971. Mechanism of conversion of the salmonella O antigen by bacteriophage ɛ34. J. Bacteriol. 105:927-936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wright, A., M. Dankert, P. Fennessey, and P. W. Robbins. 1967. Characterization of a polyisoprenoid compound functional in O-antigen biosynthesis. Proc. Natl. Acad. Sci. USA 57:1798-1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yother, J., G. L. Handsome, and D. E. Briles. 1992. Truncated forms of PspA that are secreted from Streptococcus pneumoniae and their use in functional studies and cloning of the pspA gene. J. Bacteriol. 174:610-618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yother, J., and J. White. 1994. Novel surface attachment mechanism of the Streptococcus pneumoniae protein PspA. J. Bacteriol. 176:2976-2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yuasa, R., M. Levinthal, and H. Nikaido. 1969. Biosynthesis of cell wall lipopolysaccharide in mutants of Salmonella. V. A mutant of Salmonella typhimurium defective in the synthesis of cytidine diphosphoabequase. J. Bacteriol. 100:433-444. [DOI] [PMC free article] [PubMed] [Google Scholar]