Abstract
At least four broad-spectrum efflux pumps (Mex) are involved in elevated intrinsic antibiotic resistance as well as in acquired multidrug resistance in Pseudomonas aeruginosa. Substrate specificity of the Mex pumps has been shown to be determined by the cytoplasmic membrane component (MexB, MexD, MexF, and MexY) of the tripartite efflux pump system. Alignment of their amino acid sequences with those of the homologous AcrB and AcrD pump proteins of Escherichia coli showed conservation of five charged amino acid residues located in or next to transmembrane segments (TMS). These residues were mutated in the MexF gene by site-directed mutagenesis and replaced by residues of opposite or neutral charge. MexF proteins containing combined D410A and A411G substitutions located in TMS4 were completely inactive. Similarly, the substitutions E417K (next to TMS4) and K951E (TMS10) also caused loss of activity towards all tested antibiotics. The substitution E349K in TMS2 resulted in a MexF mutant protein which was unable to transport trimethoprim and quinolones but retained partial activity for the transport of chloramphenicol. All mutated MexF proteins were expressed at comparable levels when tested by Western blot analysis. It is concluded that charged residues located in or close to TMS are essential for proper function of MexF.
Drug efflux systems endow mammalian cells, yeast, fungi, and bacteria with the ability to become resistant to a variety of chemotherapeutic and antimicrobial agents. Based on their primary amino acid structure, bacterial drug efflux systems can be divided into four major groups: the major facilitator family (14), the staphylococcal (small) multidrug resistance pumps (21), the ATP-binding cassette transporters (3), and the resistance-nodulation-cell division (RND) family (25). Phylogenetically, the latter group can be further subdivided into three clusters which correlate with substrate specificity (26): one containing proteins responsible for heavy metal efflux (CzcA and CnrA), a second involved in secretion of nodulation factors (NolFGH), and the third cluster representing multidrug efflux pumps (Acr, Mex, and Mtr) from various gram-negative bacteria.
The Acr, Mex, and Mtr pumps described for Escherichia coli, Pseudomonas aeruginosa, and Neisseria spp., respectively, are composed of three individual proteins. The actual pump component is a 12-transmembrane-segment protein located in the cytoplasmic membrane (6, 7). It is an antiporter deriving the energy for the transport from the proton gradient. The second component is an outer membrane protein which is thought to be connected with the efflux pump by a third component, called a periplasmic link protein or membrane fusion protein (29). These three proteins form a channel which permits the extrusion of substrate molecules directly from the cytoplasm or the periplasm into the external medium (20). Several models have been proposed to explain the interactions between the three components of these efflux systems (10, 30).
The Mex pumps of P. aeruginosa are the best-studied RND-type pumps together with the AcrAB-TolC system of E. coli. The four different Mex systems differ in their substrate and expression profiles. MexAB-OprM is the only efflux pump that is constitutively expressed and therefore contributes to the natural resistance of P. aeruginosa to a variety of antimicrobial agents and organic solvents. The MexCD-OprJ (22) and MexEF-OprN (12) systems, which are not expressed under laboratory growth conditions, do not contribute to the natural resistance of this organism. These systems, once overexpressed due to mutational events, confer resistance to both charged and uncharged antibiotics (17). The recently described MexXY system (18, 24) is particular in that it pumps out, among other substrates, the hydrophilic aminoglycosides and is inducible by substrate antibiotics, such as tetracycline and gentamicin (16).
The basis for the diversity in substrate profile of RND pumps and in particular those of the Mex pumps remains unknown. Reports on the MexB efflux pump (8), on the major facilitator family members, such as Bmr from Bacillus subtilis and MdfA of E. coli (2), and on staphylococcal (small) multidrug resistance pump family members, such as EmrE from E. coli (28), highlight the importance of charged amino acids in transmembrane segments (TMS). In the present study, we describe the site-directed mutagenesis of conserved charged residues in three TMS of the MexF efflux pump protein of P. aeruginosa.
MATERIALS AND METHODS
Bacterial strains, media, and growth conditions.
Bacterial strains and plasmids used in this work are listed in Table 1. The P. aeruginosa nfxC-type strain PT644 is a mexB::ΩHg derivative obtained by transduction of the Hg cassette from the mexB::ΩHg strain PAM1275 (kindly provided by O. Lomovskaya) into strain PT149 (nfxC) (formerly called PAO-7H) (12). Disruption of the mexF gene in strain PT644 resulted in the ΔmexF derivative PT644ΔF (see below). Escherichia coli DH10B was used for propagation of various plasmid constructs. E. coli and P. aeruginosa were grown in Luria-Bertani broth (LB) or on LB agar plates. When necessary, ampicillin (100 μg/ml), gentamicin (15 μg/ml), or tetracycline (15 μg/ml) for E. coli or gentamicin (15 μg/ml) or mercuric chloride (Hg) (12.5 μg/ml) for P. aeruginosa was added to growth medium. For trans complementation experiments of strain PT644ΔF with the mexF gene, 2 mM isopropyl-β-d-thiogalactopyranoside was added to growth medium. All bacterial cultures in LB were incubated at 37°C with shaking (200 rpm). Chemicals and antibiotics, if not specified, were from Sigma Chemicals.
TABLE 1.
Strains and plasmidsa
| Strain or plasmid | Description and/or genotype | Reference or source |
|---|---|---|
| Strains | ||
| P. aeruginosa | ||
| PAM1275 | PAO1, mexB::ΩHg | O. Lomovskaya |
| PT5 | PAO1 | Laboratory collection |
| PT149 | PT5nfxC (formerly PAO-7H) | 12 |
| PT640 | PT5, mexB::ΩHg, transduced from PAM1275, Hgr | This study |
| PT644 | PT149, mexB::ΩHg, transduced from PAM1275, Hgr | This study |
| PT644ΔF | PT644, ΔmexF, Hgr | This study |
| E. coli | ||
| DH10B | F−araD139 Δ(ara-leu) 7696 galU galK Δ(lac)X74 mcrΔ(mrr-hsdRMS-mcrBC) deoR Φ80dlacZΔM15 endA1 nupG recA1 rpsL | Laboratory collection |
| Plasmids | ||
| pIC20H | Multipurpose cloning vector, Apr | 15 |
| pME3087 | Mobilizable suicide vector for gene exchange in Pseudomonas spp., Tcr | D. Haas |
| pJQ200 | Low-copy-number suicide vector, Gmr | 23 |
| pMMB207 | Broad-host-range cloning/expression vector, Apr | 19 |
| pMMB207Gm | pMMB207 with a HindIII-XmnI fragment of pJQ200 replacing HindIII-DraI fragment, Gmr | This study |
| pSP858 | Source of Gmr-GFP cassette, Apr | 9 |
| pFLP2 | Plasmid carrying FLP recombinase, Apr | 9 |
| pOPNL4 | pUC119 with a 9-kbp fragment from cosmid pOPN4 containing mexE, mexF and oprN genes, Apr | 12 |
| pICN47 | pIC20H with 2-kbp Eco47III fragment from pOPNL4, Apr | This study |
| pICN64 | pICN47 with 1.7-kbp NcoI-BamHI fragment from pOPNL4; contains the mexF gene, Apr | This study |
| pMEBHN131 | pME3087 with 3.7-kbp BamHI-HindIII fragment from pICN64 carrying the mexF gene, Tcr | This study |
| pMEBHN15 | pMEBHN131 deleted of a 1-kbp PstI internal fragment of mexF and insertion of a 1.8-kbp PstI Gmr-GFP cassette from pSP858, Tcr Gmr | This study |
| pMMBN126 | pMMB207Gm with 3.7-kbp EcoRI-HindIII from pICN64 containing mexF gene, Gmr | This study |
| pL2EK | pMMBN126 carrying mexF gene with mutation E349K, Gmr | This study |
| pL4EK | pMMBN126 carrying mexF gene with mutation E417K, Gmr | This study |
| pL4DDAG | pMMBN126 carrying mexF gene with mutations D410A and D411G, Gmr | This study |
| pL10KE | pMMBN126 carrying mexF gene with mutation K951E, Gmr | This study |
Ap, ampicillin; Hg, mercuric chloride; Tc, tetracycline; Km, kanamycin; Gm, gentamicin.
Molecular biology techniques.
Plasmid DNA was routinely prepared by the alkaline lysis procedure (13) or by using a QIAGEN Plasmid Midi kit (Qiagen S.A.). Restriction endonucleases and T4 DNA ligase were from Stratagene Inc. Selected restriction fragments were isolated from electrophoresis agarose gels using a Nucleotrap (Macherey-Nagel GmbH & Co.) or a QIAquick (Qiagen S.A.) gel extraction kit as recommended by the manufacturer. Transformation of E. coli and P. aeruginosa with plasmid DNA was performed either by electroporation or by heat-shock treatment of competent cells. Mobilizable plasmids were transferred by triparental conjugation using pRK2013 as a helper plasmid (4). DNA sequencing was performed on double-stranded DNA templates using universal primers or custom primers (MWG S.A.). Nucleotide sequences were determined by the dideoxy-chain termination method (27) with a 377A DNA Sequencer (Perkin-Elmer Division Applied Biosystem) at the Medical Centre of the University of Geneva, Geneva, Switzerland. DNA alignments were obtained by using the BLASTN 2.02 program (1) provided by the National Center for Biotechnology Information.
Plasmid constructions.
The mexF gene (3.7-kbp fragment) was introduced into the multipurpose cloning vector pIC20H (15) by a two-step cloning approach. First, a 2-kbp Eco47III fragment from pOPNL4 (12) containing the 5′ end of the mexF gene was cloned into pIC20H digested by SmaI to obtain pICN47. Then, a 1.7-kbp NcoI-BamHI fragment carrying the 3′ portion of mexF was introduced into pICN47 to reconstitute the mexF gene, yielding plasmid pICN64. A 3.7-kbp EcoRI-HindIII fragment from pICN64 was cloned into pMMB207GM to yield plasmid pMMBN126. This last construction was transferred conjugally into P. aeruginosa strain PT644ΔF for trans complementation. The recombinant plasmids pL2EK, pL4EK, pL4DDAG, and pL10KE, resultant from point mutagenesis of the mexF gene, were obtained as described below.
Generation of an unmarked nonpolar mexF deletion strain.
An unmarked nonpolar deletion of the mexF gene in strain PT644 was generated according to the method described by Hoang et al. (9). First, the mexF gene carried as a 3.7-kbp BamHI-EcoRI fragment on plasmid pICN64 was cloned into the mobilizable suicide vector pME3087 (a gift from D. Haas) to obtain pMEBHN131. In this construct an internal 1.2-kbp PstI fragment of mexF was deleted and replaced by the Gm-GFP cassette excised by PstI digestion from plasmid pSP858 (9), yielding plasmid pMEBHN15. This construct was conjugally transferred into PT644 for replacement of the chromosomal mexF gene by homologous recombination. Tetracycline-sensitive (Tcs) and gentamicin-resistant (Gmr) colonies were tested by PCR for insertion of the Gm-GFP cassette into mexF. Plasmid pFLP2 (9), which contains the yeast Flp recombinase, was then mobilized into Tcs and Gmr colonies to excise the Gm-GFP cassette. All clones tested had lost the Gmr marker. Finally, plasmid pFLP2 was cured from Tcs and Gms clones by streaking onto LB agar plates supplemented with 5% sucrose. One carbenicillin-sensitive (Cbs) clone, called PT644ΔF, was confirmed by PCR to contain the expected deletion in mexF. The deletion caused a frame shift in the mexF open reading frame, creating a stop codon at position 1200 of the original mexF gene.
Site-specific mutagenesis.
Point mutations were introduced into the mexF gene carried by plasmid pICN64 using sense and antisense mutagenic primers in a one-step PCR procedure. Approximately 10 to 20 ng of plasmid DNA from E. coli served as a template for PCRs. The PCR mixture was composed of a 1 μM concentration of each primer, 8% dimethyl sulfoxide, each deoxynucleotide triphosphate at a concentration of 250 μM in 1× PCR buffer, and 1.25 U of Pfu DNA polymerase (Promega) in a final volume of 25 μl. The PCR program was 1 min at 95°C, followed by 20 cycles of 30 s at 94°C, 30 s at 45°C, 12 min at 68°C, and a 12-min final extension at 72°C. Then, the PCR product was treated with the DpnI restriction enzyme to digest template and hybrid DNA and to allow the enrichment of newly synthesized DNA containing the desired mutation.
Antimicrobial susceptibility assays.
The susceptibilities of strains to antimicrobial agents were determined by the broth microdilution method (11).
Production of polyclonal antiserum.
A 500-bp NcoI-BglII DNA fragment from plasmid pICN64, encoding part of the periplasmic loop between transmembrane segments 1 and 2 of MexF, was cloned into the His tag expression vector pQE60 (Qiagen S.A.), yielding plasmid pQEN65. An overnight culture of JM109 (pQEN65) diluted 20- to 50-fold was incubated at 37°C with ampicillin (100 μg/ml) until the cell density reached an optical density at 600 nm of approximately 0.5. Subsequent induction with 1 mM isopropyl-β-d-galactopyranoside and analysis of total cell extracts by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) revealed the presence of a 17-kDa polypeptide. This His-tagged protein was purified under denaturing conditions (8 M urea) on a nickel-nitrilotriacetic acid (Ni-NTA) resin column as described in the QIAexpressionist handbook (Qiagen S.A.). The purified protein was injected into a rabbit followed by two further boosts (Eurogentec, Seraing, Belgium).
Isolation of total membranes, protein electrophoresis, and Western blot analyses.
Cells grown in LB were harvested, resuspended in 50 mM Tris-HCl (pH 7.5), and broken by sonication in the presence of 100 μg of lysozyme/ml. Unbroken cells were removed by centrifugation at 10,000 × g for 10 min. Supernatants containing total membranes were concentrated 50-fold on a minicon column (Amicon). The concentrated extracts were resuspended in 2× SDS loading buffer. Samples were heated at 100°C for 5 min, subjected to electrophoresis in SDS-10% PAGE gels, and stained with 0.1% Coomassie blue solution. Alternatively the proteins were transferred to nitrocellulose membranes. The blots were incubated with a 1:1,000 dilution of the primary anti-MexF antibody in TBS (10 μM Tris [pH 7.4], 150 mM NaCl) plus 0.1% Tween 20 and subsequently revealed with alkaline phosphate-conjugated goat anti-rabbit immunoglobulin G (Bio-Rad) using the Lumi-Light Western blotting substrate kit (Roche).
RESULTS AND DISCUSSION
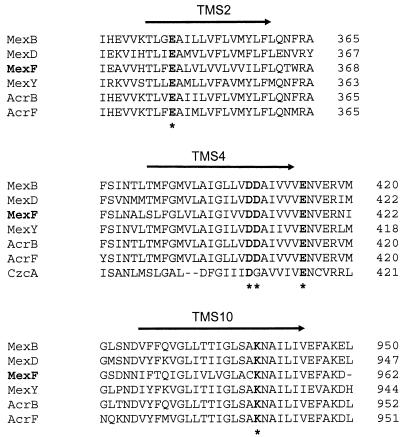
Among the four efflux pumps described for P. aeruginosa, MexEF-OprN displays the most restricted antibiotic substrate profile, consisting of chloramphenicol, trimethoprim, and all fluoroquinolones. Sequence alignment of MexF with the other Mex pump proteins of P. aeruginosa and the Acr homologues of E. coli revealed the existence of three conserved negatively charged residues and a unique positive residue, which are located in MexF in TMS2 (Glu349), TMS4 (Asp410 and Asp411), and TMS10 (Lys951), respectively (Fig. 1). The negatively charged residue Glu417, located just outside of TMS4, is also conserved and has been described as part of a DDXXXXXE motif (where X is any amino acid) suspected to be involved in proton translocation activity in bacterial antiporters (5).
FIG. 1.
Conserved amino acid residues in TMS 2, 4, and 10 of the RND proteins. Alignment of three TMS of the Acr (B and F) (E. coli), Mex (B, D, F, and Y) (P. aeruginosa), and CzcA (Ralstonia spp.) efflux pump proteins are presented. Conserved charged residues are shown in bold letters, and amino acid residues mutated in MexF are shown by asterisks. The arrow indicates the TMS and its orientation in the membrane (periplasm → cytoplasm).
Site-directed mutagenesis of charged amino acid residues in MexF.
In order to investigate the role of these five conserved charged residues, we used site-directed mutagenesis to replace the glutamate residues by positively charged lysine residues (E349K and E417K), the combined two aspartate residues by neutral residues (D410A and D411G), and the lysine residue by a negatively charged glutamate (K951E). The resistance levels conferred by each of the mutant proteins was assessed in the ΔmexF strain PT644ΔF by MIC determinations.
Mutations in TMS4.
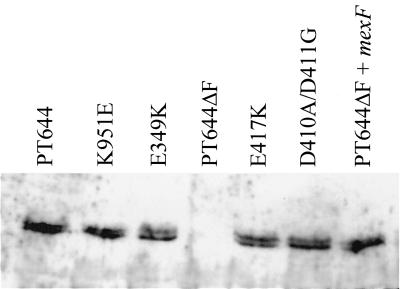
Simultaneous substitution of both D410A and D411G residues in TMS4 completely inactivated the MexF protein (pL4DDAG) (Table 2) without affecting its expression levels, as shown by Western blot analysis (Fig. 2). These data are in agreement with those reported by Guan et al. (8), who found that the equivalent residues D407 and D408 of the MexB protein are essential for MexB function. In contrast to Guan's study, we also mutated the conserved E417 residue (corresponding to E414 in MexB). The E417K substitution yielded a normally expressed MexF protein (Fig. 2) but was unable to transport any of the MexF substrate molecules (pL4EK) (Table 2). Hence, this residue, located next to TMS4 in the cytoplasmic side of MexF, is also essential for MexF function. E417 is part of the conserved DDXXXXXE motif predicted to be involved in proton translocation in the CzcA heavy metal transporter of Ralstonia spp. (5). Indeed, substitution of the residue D408Q in CzcA (corresponding to D410 in MexF) resulted in a protein able to promote passive diffusion of metal cations across proteoliposomes but unable to catalyze proton/cation antiport in vivo (5). Since the drug-transporting pumps Acr and Mex are able to extrude uncharged hydrophobic molecules like organic solvents and chloramphenicol, it was proposed that a charge relay system was not involved in drug/proton translocation (5, 31). Our results with MexF proteins mutated in residues D410/D411 and E417, however, demonstrate that the DDXXXXXE motif is essential even for the transport of uncharged hydrophobic drugs, such as chloramphenicol.
TABLE 2.
Effect of amino acid substitutions in MexF on MIC profiles
| Strain | MIC (μg/ml)a
|
||||
|---|---|---|---|---|---|
| TMP | CHL | CIP | NAL | ATM | |
| PT5 | 128 | 32-64 | 0.125 | 128 | 4 |
| PT644 | 1,024 | 1,024 | 1 | >256 | 1 |
| PT644ΔF | 16 | 4 | 0.03 | 16 | 0.5 |
| PT644ΔF(pMMBN126) | 128 | 256-512 | 0.125 | 64 | 0.5 |
| PT644ΔF(pL4DDAG) | 16 | 4 | 0.03 | 16 | 0.5 |
| PT644ΔF(pL4EK) | 16 | 4 | 0.03 | 16 | 0.5 |
| PT644ΔF(pL10KE) | 16 | 4 | 0.03 | 16 | 0.5 |
| PT644ΔF(pL2EK) | 16 | 16 | 0.03 | 16 | 0.5 |
TMP, trimethoprim; CHL, chloramphenicol; CIP, ciprofloxacin; NAL, nalidixic acid; ATM, aztreonam.
FIG. 2.
Western blot analysis of MexF mutants. Total protein extracts of PT644ΔF harboring the different plasmid constructions corresponding to MexF E349K, D410A/D411G, E417K and K951E mutants are presented. The nfxC strain PT644 served as a control. Samples were analyzed by SDS-PAGE followed by Western blot analysis using polyclonal antibodies against MexF. Each lane contained 5 μg of total protein.
Mutation in TMS10.
The only conserved positively charged residue among RND pumps was K951, located in TMS10. Substitution of this residue (K951E) also eliminated MexF activity for all of the tested antibiotics (pL10KE) (Table 2), without affecting its expression (Fig. 2). Hence, residue K359 is also essential for pump activity.
Mutation in TMS2.
The substitution E349K in TMS2 resulted in a MexF mutant protein unable to transport trimethoprim and quinolones but retaining partial activity towards chloramphenicol (pL2EK) (Table 2), while expression of the mutated MexF protein was unaffected (Fig. 2). This could mean either that E349 is involved in substrate selectivity or, alternatively, that the activity of the mutant protein is generally decreased such that the preferred substrate molecule chloramphenicol (MIC ratio of 256 for the strain couple PT644/PT644ΔF) is still transported, while ciprofloxacin and trimethoprim (MIC ratios of 32 and 64, respectively) are not. The latter possibility would be in agreement with results from the MexB analysis, where the E346K substitution, equivalent to E349K in MexF, also resulted in a mutant MexB protein which showed reduced transport activity of its antibiotic substrates (8). Involvement of the residue E349 in substrate selectivity is supported by studies of the multidrug transporter MdfA of E. coli., where a single membrane-embedded negatively charged residue (E26) is critical for recognition of positively charged drugs but not for the uncharged chloramphenicol (2).
Taken together, our results on MexF and those of Guan and Nakae (8) on the MexB protein suggest that the five conserved charged residues located in TMS 2, 4, and 10 are involved in properties common both to efflux pump proteins and probably also to other RND members. They could be responsible for the spatial arrangement of TMS in the membrane determined by ionic interactions between these charged residues or for the translocation of protons which is common to all proton motif force-driven efflux pump proteins. Further investigations at the atomic level are required to analyze the topology of these important efflux pump proteins.
Acknowledgments
We thank L. Kocjancic-Curty and Catherine Cherbuliez for excellent technical assistance. We are grateful to D. Haas for gifts of plasmids.
This work was supported by grant no. 3100-055961 from the Swiss National Science Foundation and by grant no. C00.0048 from the COST action B16 to T.K.
REFERENCES
- 1.Altschul, S. F., T. L. Madden, A. A. Schaffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids. Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Edgar, R., and E. Bibi. 1999. A single membrane-embedded negative charge is critical for recognizing positively charged drugs by the Escherichia coli multidrug resistance protein MdfA. EMBO J. 18:822-832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fath, M. J., and R. Kolter. 1993. ABC transporters: bacterial exporters. Microbiol. Rev. 57:995-1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Figurski, D. H., and D. R. Helinski. 1979. Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc. Natl. Acad. Sci. USA 76:1648-1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goldberg, M., T. Pribyl, S. Juhnke, and D. H. Nies. 1999. Energetics and topology of CzcA, a cation/proton antiporter of the resistance-nodulation-cell division protein family. J. Biol. Chem. 274:26065-26070. [DOI] [PubMed] [Google Scholar]
- 6.Gotoh, N., T. Kusumi, H. Tsujimoto, T. Wada, and T. Nishino. 1999. Topological analysis of an RND family transporter, MexD of Pseudomonas aeruginosa. FEBS Lett. 458:32-36. [DOI] [PubMed] [Google Scholar]
- 7.Guan, L., M. Ehrmann, H. Yoneyama, and T. Nakae. 1999. Membrane topology of the xenobiotic-exporting subunit, MexB, of the MexA, B-OprM extrusion pump in Pseudomonas aeruginosa. J. Biol. Chem. 274:10517-10522. [DOI] [PubMed] [Google Scholar]
- 8.Guan, L., and T. Nakae. 2001. Identification of essential charged residues in transmembrane segments of the multidrug transporter MexB of Pseudomonas aeruginosa. J. Bacteriol. 183:1734-1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoang, T. T., R. R. Karkhoff-Schweizer, A. J. Kutchma, and H. P. Schweizer. 1998. A broad-host-range Flp-FRT recombination system for site-specific excision of chromosomally located DNA sequences: application for isolation of unmarked Pseudomonas aeruginosa mutants. Gene 212:77-86. [DOI] [PubMed] [Google Scholar]
- 10.Johnson, J. M., and G. M. Church. 1999. Alignment and structure prediction of divergent protein families: periplasmic and outer membrane proteins of bacterial efflux pumps. J. Mol. Biol. 287:695-715. [DOI] [PubMed] [Google Scholar]
- 11.Jorgensen, J. H., M. J. Ferraro, W. A. Craig, G. V. Doern, S. M. Finegold, J. Fung-Tomc, S. L. Hansen, J. Hindler, L. B. Reller, J. M. Swenson, F. C. Tenover, R. T. Testa, and M. A. Wikler. 1997. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. National Committee for Clinical Laboratory Standards, Villanova, Pa.
- 12.Köhler, T., M. Michea-Hamzehpour, U. Henze, N. Gotoh, L. Kocjancic-Curty, and J. C. Pechère. 1997. Characterization of MexE-MexF-OprN, a positively regulated multidrug efflux system of Pseudomonas aeruginosa. Mol. Microbiol. 23:345-354. [DOI] [PubMed] [Google Scholar]
- 13.Maniatis, T., E. F. Fritsch, and J. Sambrook. 1982. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 14.Marger, M. D., and M. H. J. Saier. 1993. A major superfamily of transmembrane facilitators that catalyse uniport, symport and antiport. Trends. Biochem. Sci. 18:13-20. [DOI] [PubMed] [Google Scholar]
- 15.Marsh, J. L., M. Erfle, and E. J. Wykes. 1984. The pIC plasmid and phage vectors with versatile cloning sites for recombinant selection by insertional inactivation. Gene 32:481-485. [DOI] [PubMed] [Google Scholar]
- 16.Masuda, N., E. Sakagawa, S. Ohya, N. Gotoh, H. Tsujimoto, and T. Nishino. 2000. Contribution of the MexX-MexY-oprM efflux system to intrinsic resistance in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 44:2242-2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Masuda, N., E. Sakagawa, S. Ohya, N. Gotoh, H. Tsujimoto, and T. Nishino. 2000. Substrate specificities of MexAB-OprM, MexCD-OprJ, and MexXY-oprM efflux pumps in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 44:3322-3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mine, T., Y. Morita, A. Kataoka, T. Mizushima, and T. Tsuchiya. 1999. Expression in Escherichia coli of a new multidrug efflux pump, MexXY, from Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 43:415-417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morales, V. M., A. Bäckman, and M. Bagdasarian. 1991. A series of wide-host-range low-copy-number vectors that allow direct screening for recombinants. Gene 97:39-47. [DOI] [PubMed] [Google Scholar]
- 20.Nikaido, H. 1996. Multidrug efflux pumps of gram-negative bacteria. J. Bacteriol. 178:5853-5859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paulsen, I. T., M. H. Brown, and R. A. Skurray. 1996. Proton-dependent multidrug efflux systems. Microbiol. Rev. 60:575-608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Poole, K., N. Gotoh, H. Tsujimoto, Q. Zhao, A. Wada, T. Yamasaki, S. Neshat, J. Yamagishi, X. Z. Li, and T. Nishino. 1996. Overexpression of the mexC-mexD-oprJ efflux operon in nfxB-type multidrug-resistant strains of Pseudomonas aeruginosa. Mol. Microbiol. 21:713-724. [DOI] [PubMed] [Google Scholar]
- 23.Quandt, J., and M. F. Hynes. 1993. Versatile suicide vectors which allow direct selection for gene replacement in Gram-negative bacteria. Gene 127:15-21. [DOI] [PubMed] [Google Scholar]
- 24.Ramos-Aires, J., T. Köhler, H. Nikaido, and P. Plésiat. 1999. Involvement of an active efflux system in the natural resistance of Pseudomonas aeruginosa to aminoglycosides. Antimicrob. Agents Chemother. 43:2624-2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saier, M. H., R. Tam, A. Reizer, and J. Reizer. 1994. Two novel families of bacterial membrane proteins concerned with nodulation, cell division and transport. Mol. Microbiol. 11:841-847. [DOI] [PubMed] [Google Scholar]
- 26.Saier, M. H. J., I. T. Paulsen, M. K. Sliwinski, S. S. Pao, R. A. Skurray, and H. Nikaido. 1998. Evolutionary origins of multidrug and drug-specific efflux pumps in bacteria. FASEB J. 12:265-274. [DOI] [PubMed] [Google Scholar]
- 27.Sanger, F., S. Nicklen, and A. S. R. Coulson. 1977. DNA sequencing with chain terminating inhibitors. Proc. Natl. Acad. Sci. USA 74:5463-5467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yerushalmi, H., and S. Schuldiner. 2000. An essential glutamyl residue in EmrE, a multidrug antiporter from Escherichia coli. J. Biol. Chem. 275:5264-5269. [DOI] [PubMed] [Google Scholar]
- 29.Zgurskaya, H. I., and H. Nikaido. 1999. AcrA is a highly asymmetric protein capable of spanning the periplasm. J. Mol. Biol. 285:409-420. [DOI] [PubMed] [Google Scholar]
- 30.Zgurskaya, H. I., and H. Nikaido. 1999. Bypassing the periplasm: reconstitution of the AcrAB multidrug efflux pump of Escherichia coli. Proc. Natl. Acad. Sci. USA 96:7190-7195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zgurskaya, H. I., and H. Nikaido. 2000. Multidrug resistance mechanisms: drug efflux across two membranes. Mol. Microbiol. 37:219-225. [DOI] [PubMed] [Google Scholar]