Abstract
Structure-function relationships in antimicrobial peptides have been extensively investigated in order to obtain improved analogs. Most of these studies have targeted either α-helical peptides or β-sheet peptides with multiple disulfide bridges. Tigerinins are short, nonhelical antimicrobial peptides with a single disulfide bridge. In this study, we have synthesized several analogs of tigerinin 1 with an aim to understand the structural basis of activity as well as improve its activity. The studies demonstrate that the loop structure of tigerinin 1 is essential for its optimal activity. However, linearization with increased cationic charges can compensate for loss of loop structure to some extent. Morphology of the cells after treatment with the active analogs shows extensive leakage of cytoplasmic contents. Tigerinin 1 and two of its analogs exhibit impressive activity against a variety of clinical bacterial isolates.
Antimicrobial peptides with broad-spectrum activity are widely distributed in nature and have been characterized from plants, insects, and amphibians as well as mammals, including humans (1, 3, 6-8, 21, 26, 31). Recent studies have confirmed their primacy in the innate immune system of organisms across the evolutionary scale (6, 12, 14). Although a majority of these peptides are believed to function by permeabilizing membranes, mechanisms involving inhibition of DNA, RNA, and/or protein biosynthesis have also been proposed (15, 22, 26, 33, 39). These peptides kill microorganisms rapidly compared to other antibiotics, and this class of peptides appears to be refractory to the development of resistance (13). All these attributes make them attractive candidates as next-generation therapeutic agents for treating multidrug-resistant bacterial infections. Structure-function studies on antimicrobial peptides have added significance in this direction, as there is a rapid increase in the emergence of microbes resistant to conventionally used antibiotics in recent years (10, 20, 36). However, a majority of such studies have been confined to linear peptides with a propensity for amphiphilic α-helical structure or amphiphilic β-sheet peptides stabilized by multiple disulfide bridges (9, 17, 18, 24, 30, 31, 35). In contrast to this, only a few nonhelical antimicrobial peptides with a single disulfide bridge, like thanatin and bactenecin, have been used in structure-function studies (11, 25, 37, 38). We have recently characterized a novel family of short, nonhelical antimicrobial peptides called tigerinins from the skin secretions of the Indian frog Rana tigerina (27). Tigerinins are a unique family of 11- to 12-residue peptides and are characterized by the presence of a stretch of predominantly hydrophobic amino acids with one cationic residue and an amidated C terminus. They also contain two cysteine residues linked by a disulfide bond to form a loop of nine amino acid residues. Tigerinins are thus distinctly different from any other known antimicrobial peptides, including the “rana box”-containing brevinin group of peptides from the genus Rana (16, 23, 29), and could provide novel lead molecules for design of antimicrobial peptides with possible therapeutic applications. In this study, we have chosen the most-active member of this family, tigerinin 1 (TGN-1), for structure-function analysis.
The sequences of the peptides used in the study are shown in Table 1. The synthesis and characterization of TGN-1 has been described earlier (27). Other peptides described in this study were synthesized manually on amide crowns (Chiron Technologies) by the solid-phase method using fluorenylmethoxy carbonyl chemistry (2). All amino acids were added as fluorenylmethoxy carbonyl hydroxy benzotriazole active esters. The peptides were cleaved from the resin by treatment with trifluoroacetic acid-thioanisole-phenol-water-ethanedithiol (16.5:1:1:1:0.5, vol/vol) overnight at room temperature. The peptides were checked for purity by high-performance liquid chromatography using a reverse-phase column (Water's μBondapak C18) using a solvent system of 0.1% aqueous trifluoroacetic acid and acetonitrile. The cysteines were deprotected with mercury(II) acetate in the ratio of 2 equivalents for each equivalent of cysteine. Mercuric sulfide salts formed were precipitated with 20 equivalents of β-mercaptoethanol. The peptides were then desalted and the disulfide bridges were formed by oxidation in 20% dimethyl sulfoxide (34). The disulfide-bridged peptides were checked for purity by high-performance liquid chromatography. The peptides were further characterized by matrix-assisted laser desorption ionization mass spectrometry.
TABLE 1.
Sequences and activities of TGN-1 and its analogs
| Peptide | Sequencea | LC (μg/ml) of peptide ford:
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| E. coli W160-37 | S. aureus ATCC 8530 | Rabbit entero- pathogenic E. coli | Human entero- pathogenic E. coli | Vibrio cholerae | Pseudo- monas | Klebsiella pneumonia | Shigella flexneri | Proteus mirabilis | MRSAb | Salmonella enterica serovar Typhi H | ||
| TGN-1 | FCTMIPIPRCY-CONH2 | 10 | 10 | 35 | 25 | 120 | 150 | 50 | 70 | 150 | 50 | 45 |
| TGN-1-OH | FCTMIPIPRCY-COOH | 200 | 200 | NDc | ND | ND | ND | ND | ND | ND | ND | ND |
| TGN-1W | WCTMIPIPRCY-CONH2 | 6 | 6 | 15 | 10 | 30 | 100 | 40 | 30 | 100 | 40 | 30 |
| TGN-1WK | WCKMIPIPRCY-CONH2 | 4 | 4 | 15 | 10 | 30 | 30 | 20 | 15 | 80 | 15 | 20 |
| TGN-1L (lin) | WLTMIPIPRLY-CONH2 | 160 | 200 | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| TGN-1LK (lin) | WLKLIPIPKLY-CONH2 | 25 | 25 | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| TGN-1U (lin) | WUTMIPIPRUY-CONH2 | 120 | 120 | ND | ND | ND | ND | ND | ND | ND | ND | ND |
All cysteine containing peptides were linked by a disulfide bridge.
MRSA, methicillin-resistant S. aureus.
ND, not determined.
Strains mentioned in the last nine columns are clinical strains obtained from Postgraduate Institute of Basic Medical Sciences, Chennai, India.
Lethal concentrations (LCs) of the peptides were determined by growing the microorganism in nutrient broth (Bacto Difco nutrient broth) to mid-logarithmic phase and diluting to 106 CFU/ml in 10 mM sodium phosphate buffer (pH 7.4) (17, 32). Aliquots of 100 μl of culture so diluted were incubated with different concentrations of the peptides (20 to 200 μg/ml in multiples of 20 μg/ml) for 2 h at 37°C, at the end of which suitably diluted aliquots were plated on nutrient agar plates. The plates were incubated for 18 h at 37°C, and colonies formed were counted. Strains which were more sensitive were evaluated for growth at lower concentrations in multiples of either 2 or 5 μg/ml. The concentration of the peptides at which no viable colonies were formed was taken as the LC. The average of two independent experiments done in duplicate was taken for the calculation of LCs. The kinetics of killing was evaluated for Escherichia coli and Staphylococcus aureus by determining the viable cell counts as a function of time.
Samples were processed for electron microscopy in the following way. Liquid cultures of E. coli cells grown up to mid-logarithmic phase were washed well in sodium phosphate buffer (10 mM) and centrifuged for 3 min at 1,500 × g. The pellet formed was fixed in 2.5% glutaraldehyde in 0.1 M phosphate buffer for 3 h at 4°C. After fixation the pellet was washed with 0.1 M phosphate buffer three times. The samples were then postfixed on 1% osmium tetroxide in 0.1 M phosphate buffer for 2 h. Fixed samples were then thoroughly washed with phosphate buffer, following which they were dehydrated through a series of acetone gradients. Dehydrated samples were passed through propylene oxide and infiltrated with epoxy resin overnight. Samples were then embedded in pure resin (epoxy resin) and cured at 60°C for 72 h. Golden color sections were obtained using a Reichert Ultracut E microtome and were stained with 2% uranyl acetate and Reynold's lead citrate, and the sections were observed under a JEOL 100 CX electron microscope at 80 kV. Test samples were prepared by treating the E. coli cells with the peptides at their LCs (108 cells in 100 μl were treated with 70 μg of the peptides) for different lengths of time (5 to 30 min) and then fixed with glutaraldehyde and processed the same way as described above.
Initial evaluations of the antibacterial activities of the peptides were done on E. coli W 160-37 and S. aureus ATCC 8530 strains, and the results are shown in Table 1. TGN-1 was active against both these strains, with LCs in the range of 8 to 12 μg/ml. TGN-1 is amidated at the C terminus, and TGN-1 OH is its analog with a C-terminal free COOH group. This analog was almost inactive, with LCs of 200 μg/ml against both microorganisms, and this indicates that amidation at the C terminus is essential for its activity. The importance of positive charges on the peptide in its initial interaction with the negatively charged outer bacterial surface, which has a bearing on its antimicrobial activity, is well established (6, 31). Thus, the low activity of the analog with free C-terminal acid which would make it less cationic than TGN-1, is not unexpected. W is an amino acid frequently encountered in membrane active peptides and has been shown to influence the localization of these peptides into membrane interfaces (4, 5, 28, 32). Hence, the effect of replacing the amino terminal F of TGN-1 by W was evaluated. Replacing the N-terminal F by W generated TGN-1W. In TGN-1WK in addition to this F-to-W change, T3 is replaced by K. TGN-1W was marginally more active than TGN-1. Replacement of the polar amino acid T3 by cationic K in TGN-1W further increased its activity. In TGN-1L (lin) the two cysteine residues of TGN-1 are replaced by Ls, resulting in a linear analog. TGN-1U (lin) is another linear analog of TGN-1 with two alpha amino isobutyric acid residues in place of cysteines. TGN-1LK (lin) is an analog of TGN-1 with Ks replacing T3 and R. In contrast to the cyclic analogs, the linear analogs showed considerable decrease in their antibacterial activities; the most-active linear analog was TGN-1LK (lin). TGN-1 adopts a β-turn structure, and linearization could prevent the formation of a turn structure. Thus, the β-turn structure stabilized by the disulfide bridge appears to be essential for the activity of TGN-1. The residual activity of the linear analogs may stem from a fraction of molecules which could populate a turn structure. Increasing the cationicity of the linear analog [TGN-1 LK (lin)] appears to offset the need for the disulfide bridge. The in vitro efficacies of TGN-1 and its analogs in killing some clinical bacterial isolates were also investigated. The microorganisms tested and the LCs of the peptides against these strains are shown in Table 1. The microbes included highly virulent enteropathogenic strains of E. coli and methicillin-resistant S. aureus. It is evident that the majority of strains are susceptible to TGN-1 and its two analogs. TGN-1W and TGN-1WK are generally more potent than TGN-1. Strains of Proteus and Pseudomonas spp. were susceptible to relatively high concentrations of the peptides. It has been shown recently that a modified lipopolysaccharide structure in Proteus mirabilis strains make them less susceptible to antimicrobial peptides (19). The peptides did not exhibit hemolytic activity up to a concentration of 50 μg/ml (highest concentration tested).
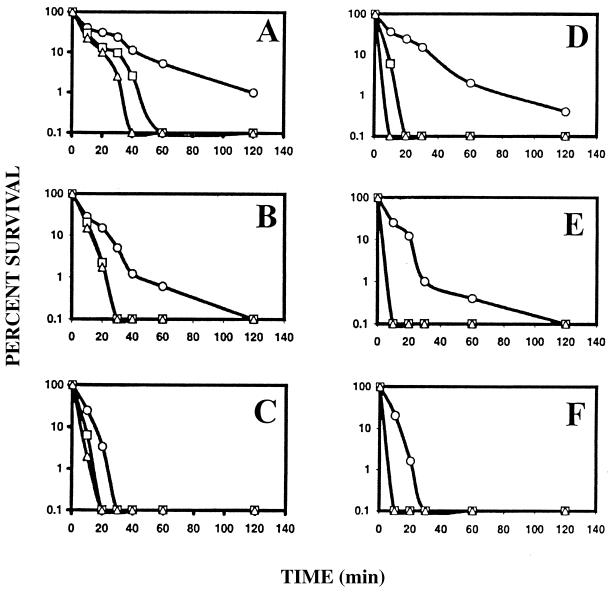
The kinetics of killing of E. coli and S. aureus by TGN-1 and its analogs were investigated. The results of the disulfide bridge-containing analogs are shown in Fig. 1. About 99% of cells were killed by TGN-1 after incubation of the cells with 6- μg/ml concentration of the peptide for 2 h (Fig. 1A and D). More-rapid killing was evident in the cases of TGN-1W and TGN-1WK. Complete killing was evident with these peptides at a concentration of 6 μg/ml after ∼60 and 40 min of incubation, respectively, in the case of E. coli (Fig. 1A). The corresponding incubation periods are ∼20 and 10 min in the case of S. aureus (Fig. 1D). At higher concentrations of these peptides, cells were killed in much shorter periods of incubation (Fig. 1B, C, E, and F). Thus, the analogs TGN-1W and TGN-1WK are more effective in killing bacteria than their parent peptide TGN-1. The kinetics of killing of E. coli and S. aureus cells by the linear analog TGN-1LK (lin) were also investigated. With this peptide at a concentration of 25 μg/ml, E. coli cells were killed after an incubation of ∼40 min and S. aureus cells were killed in ∼120 min (results not shown).
FIG. 1.
Kinetics of killing of bacteria by TGN-1 and its analogs. (A, B, and C) E. coli; (D, E, and F) S. aureus. Cells in the mid-logarithmic phase of growth (105 CFU) were incubated with different concentrations of tigerinins (6 μg/ml [A and D], 8 μg/ml [B and E], 10 μg/ml [C and F]), and aliquots were drawn out at different intervals after incubation and were placed on plated with nutrient broth. The number of colonies developed was determined after incubating the plates for 18 h at 37°C. Cells incubated in the absence of any peptide served as controls. Symbols: circle, TGN-1; square, TGN-1W; triangle, TGN-1WK.
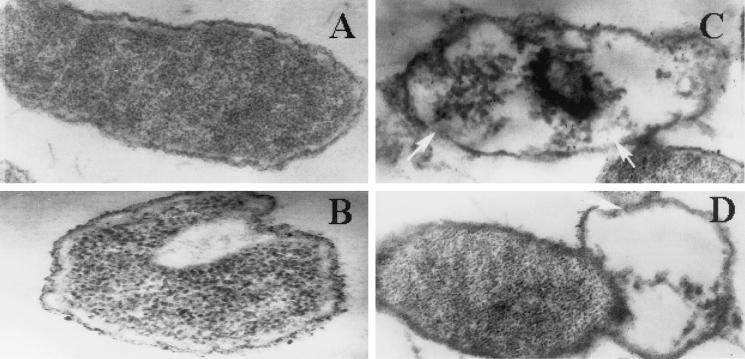
Tigerinins have earlier been shown to permeabilize bacterial membranes to N-phenyl-1-N-napthylamine and o-nitrophenyl-β-d-galactopyranoside (27). It can be reasonably assumed that the activities of the analogs of TGN-1 used in this study also are mediated by membrane permeabilization. The morphologies of E. coli cells treated with TGN-1 and analogs were examined by electron microscopy. It was necessary to use high concentrations of bacteria (∼109 CFU/ml) to prepare samples for transmission electron microscopy compared to ∼106 CFU/ml for determining the LCs. To ensure that the ratios of peptide to bacteria were similar in LC measurements and in preparing samples for transmission electron microscopy, 108 cells in 100 μl were treated with 70 μg of the peptides. Negative staining revealed that a longer duration of incubation (∼1 h) of E. coli cells with lytic concentrations of TGN-1 caused total lysis of cells, and very few cells were visible. Hence, the effect of incubation of cells for short durations was examined after fixing and sectioning. At 5 min of incubation (Fig. 2B) with LCs of TGN-1, thickening of the outer surface and blebs were discernible. The cytoplasmic membrane showed invagination, and the appearance of vacuoles in the cytoplasm was evident. After 15 min of incubation (Fig. 2C), membrane discontinuity and leakage of entire cytoplasmic contents were observed in many cells. The effect of TGN-1WK was very similar to that of TGN-1 (Fig. 2D), indicating that both the peptides exert their activities by similar mechanisms. These studies demonstrated that treatment of E. coli cells with TGN-1 and TGN-1WK leads to leakage of cytoplasmic contents, and the membrane permeabilization earlier observed may mediate this leakage. However, activation of autolytic mechanisms by the peptide cannot be ruled out.
FIG. 2.
Transmission electron micrographs of E. coli cells before treatment (A), 5 min after incubation with TGN-1 (B), 15 min after incubation with TGN-1 (C), and 15 min after incubation with TGN-1WK (D). Details are given in the materials and methods section. Arrows point out the membrane discontinuity. Magnification, ×25,000.
Thus, tigerinins are short peptides with a novel flexible structural motif, and the studies reported here demonstrate that it is possible to improve the activity of short nonhelical peptides by rational design of analogs and that these analogs hold promise as novel lead molecules for designing therapeutic molecules.
Acknowledgments
K.P.S. thanks the Council for Scientific and Industrial Research, New Delhi, India, for an award of Research Associateship.
We acknowledge the contribution of Usha Ananda Rao, Postgraduate Institute of Basic Medical Sciences, Chennai, India, in the collection of various clinical isolates used in the study and the experimental set. We thank M. Vairamani, Indian Institute of Chemical Technology, Hyderabad, India, for mass spectral analysis.
REFERENCES
- 1.Andreu, D., and L. Rivas. 1998. Animal antimicrobial peptides: an overview. Biopolymers 47:415-433. [DOI] [PubMed] [Google Scholar]
- 2.Atherton, E., and R. C. Sheppard. 1989. Solid phase peptide synthesis: a practical approach. IRL, Oxford, United Kingdom.
- 3.Bevins, C. L., and M. Zasloff. 1990. Peptides from frog skin. Annu. Rev. Biochem. 59:395-410. [DOI] [PubMed] [Google Scholar]
- 4.Blondelle, S. E., and R. A. Houghten. 1991. Hemolytic and antimicrobial activities of the twenty-four individual omission analogues of melittin. Biochemistry 30:4671-4678. [DOI] [PubMed] [Google Scholar]
- 5.Blondelle, S. E., and R. A. Houghten. 1991. Probing the relationships between the structure and hemolytic activity of melittin with a complete set of leucine substitution analogs. Peptide Res. 4:12-18. [PubMed] [Google Scholar]
- 6.Boman, H. C. 1995. Peptide antibiotics and their role in innate immunity. Annu. Rev. Immunol. 13:61-92. [DOI] [PubMed] [Google Scholar]
- 7.Broekaert, W. E. F., B. P. A. Cammue, M. F. C. De Bolle, K. Thevissen, G. W. De Samblanx, and R. W. Osborn. 1997. Antimicrobial peptides from plants. Crit. Rev. Plant Sci. 16:297-323. [Google Scholar]
- 8.Bulet, P., C. Hetru, J. L. Dimarcq, and D. Hoffmann. 1999. Antimicrobial peptides in insects; structure and function. Dev. Comp. Immunol. 23:329-344. [DOI] [PubMed] [Google Scholar]
- 9.Chen, J., T. J. Falla, H. Liu, M. A. Hurst, C. A. Fujii, D. A. Mosca, J. R. Embree, D. J. Loury, P. A. Radel, C. C. Chang, L. Gu, and J. C. Fiddes. 2000. Development of protegrins for the treatment and prevention of oral mucositis: structure activity relationships of synthetic protegrin analogues. Biopolymers 55:88-98. [DOI] [PubMed] [Google Scholar]
- 10.Chopra, I., J. Hodgson, B. Metcalf, and G. Poste. 1997. The search for antimicrobial agents effective against bacteria resistant to multiple antibiotics. Antimicrob. Agents Chemother. 41:497-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fehlbaum, P., P. Bulet, S. Chernysh, J. P. Briand, J. P. Roussel, L. Letellier, C. Hetru, and J. Hoffmann. 1996. Structure activity analysis of thanatin, a 21-residue inducible insect defense peptide with sequence homology to frog skin antimicrobial peptides. Proc. Natl. Acad. Sci. USA 93:1221-1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ganz, T. 1999. Defensins and host defense. Science 286:420-421. [DOI] [PubMed] [Google Scholar]
- 13.Hancock, R. E. 1997. Peptide antibiotics. Lancet 349:418-422. [DOI] [PubMed] [Google Scholar]
- 14.Hancock, R. E., and G. Diamond. 2000. The role of cationic antimicrobial peptides in innate host defences. Trends Microbiol. 8:402-410. [DOI] [PubMed] [Google Scholar]
- 15.Huang, H. W. 2000. Action of antimicrobial peptides: two-state model. Biochemistry 39:8347-8352. [DOI] [PubMed] [Google Scholar]
- 16.Kwon, M. Y., S. Y. Hong., and K. H. Lee. 1998. Structure-activity analysis of brevinin 1E amide, an antimicrobial peptide from Rana esulenta. Biochim. Biophys. Acta 1387:239-248. [DOI] [PubMed] [Google Scholar]
- 17.Lehrer, R. I., K. A. Barton, S. S. L. Harwig, T. Ganz, and M. E. Selsted. 1989. Interaction of human defensins with Escherichia coli. Mechanism of bactericidal activity. J. Clin. Investig. 137:167-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maloy, W. L., and U. P. Kari. 1995. Structure-activity studies on magainins and other host defense peptides. Biopolymers 37:105-122. [DOI] [PubMed] [Google Scholar]
- 19.McCoy, A. J., H. Liu., T. J. Falla, and J. S. Gunn. 2001. Identification of Proteus mirabilis mutants with increased sensitivity to antimicrobial peptides. Antimicrob. Agents Chemother. 45:2030-2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Neu, H. C. 1992. The crisis in antibiotic resistance. Science 257:1064-1073. [DOI] [PubMed] [Google Scholar]
- 21.Nicolos, P., and A. Mor. 1995. Peptides as weapons against microorganisms in the chemical defense system of vertebrates. Annu. Rev. Microbiol. 49:277-304. [DOI] [PubMed] [Google Scholar]
- 22.Oren, Z., and Shai. 1999. Mode of action of amphipathic α-helical antimicrobial peptides. Biopolymers. 47:451-463. [DOI] [PubMed] [Google Scholar]
- 23.Ponti, D., G. Hignoma, L. Hangoni, D. de Biase, H. Simmaco, and D. Barra. 1999. Expression and activity of cyclic and linear analogues of esculentin-1, an antimicrobial peptide from amphibian skin. Eur. J. Biochem. 263:921-927. [DOI] [PubMed] [Google Scholar]
- 24.Rao, A. G. 1999. Conformation and antimicrobial activity of linear derivatives of tachyplesin lacking disulfide bonds. Arch. Biochem. Biophys. 361:127-134. [DOI] [PubMed] [Google Scholar]
- 25.Romeo, D., B. Skerlavaj., M. Bolognesi, and R. Gennaro. 1998. Structure and bactericidal activity of an antibiotic dodecapeptide purified from bovine neutrophils. J. Biol. Chem. 263:9573-9575. [PubMed] [Google Scholar]
- 26.Saberwal, G., and R. Nagaraj. 1994. Cell lytic and antibacterial peptides that act by perturbing the barrier function of membranes: facets of their conformational features, structure-function correlations and membrane-perturbing abilities. Biochim. Biophys. Acta 1197:109-131. [DOI] [PubMed] [Google Scholar]
- 27.Sai, K. P., M. V. Jagannadham, M. Vairamani, N. P. Raju, A. S. Devi, R. Nagaraj, and N. Sitaram. 2001. Tigerinins: novel antimicrobial peptides from the Indian frog Rana tigerina. J. Biol. Chem. 276:2701-2707. [DOI] [PubMed] [Google Scholar]
- 28.Schiffer, M., C. H. Chang, and F. J. Stevens. 1992. The functions of tryptophan residues in membrane proteins. Protein. Eng. 5:213-214. [DOI] [PubMed] [Google Scholar]
- 29.Simmaco, M., G. Mignogna, and D. Barra. 1999. Antimicrobial peptides from amphibian skin: what do they tell us? Biopolymers 47:435-450. [DOI] [PubMed] [Google Scholar]
- 30.Sitaram, N., and R. Nagaraj. 1990. A synthetic 13-residue peptide corresponding to the hydrophobic region of bovine seminalplasmin has antibacterial activity and also causes lysis of red blood cells. J. Biol. Chem. 265:10438-10442. [PubMed] [Google Scholar]
- 31.Sitaram, N., and R. Nagaraj. 1999. Interaction of antimicrobial peptides with biological and model membranes: structural and charge requirements for activity. Biochim. Biophys. Acta 1462:29-54. [DOI] [PubMed] [Google Scholar]
- 32.Subbalakshmi, C., V. Krishnakumari, R. Nagaraj, and N. Sitaram. 1996. Requirements for antibacterial and hemolytic activities in the bovine neutrophil derived 13-residue peptide indolicidin. FEBS Lett. 395:48-52. [DOI] [PubMed] [Google Scholar]
- 33.Subbalakshmi, C., and N. Sitaram. 1998. Mechanism of antimicrobial action of indolicidin. FEMS Microbiol. Lett. 160:91-96. [DOI] [PubMed] [Google Scholar]
- 34.Tam, J., P., C. R. Wu, W. Liu, and J. W. Zhang. 1991. Disulfide bond formation in peptides by dimethyl sulfoxide. Scope and applications. J. Am. Chem. Soc. 113:6657-6662. [Google Scholar]
- 35.Tossi, A., L. Sandri, and A. Giangaspero. 2000. Amphipathic, alpha-helical antimicrobial peptides. Biopolymers 55:4-30. [DOI] [PubMed] [Google Scholar]
- 36.Walsh, C. 2000. Molecular mechanisms that confer antibacterial drug resistance. Nature 406:775-787. [DOI] [PubMed] [Google Scholar]
- 37.Wu, M., and R. E. Hancock. 1999. Interaction of the cyclic antimicrobial peptide bactenecin with the outer and cytoplasmic membrane. J. Biol. Chem. 274:29-35. [DOI] [PubMed] [Google Scholar]
- 38.Wu, M., and R. E. Hancock. 1999. Improved derivatives of bactenecin, a cyclic dodecameric cationic peptide. Antimicrob. Agents Chemother. 43:1274-1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang, L., A. Rozek, and R. E. Hancock. 2001. Interaction of cationic antimicrobial peptides with model membranes. J. Biol. Chem. 276:35174-35722. [DOI] [PubMed] [Google Scholar]