Abstract
Compounds from 16 classes of antimicrobial drugs were tested for their abilities to inhibit the in vitro multiplication of nanobacteria (NB), a newly discovered infectious agent found in human kidney stones and kidney cyst fluids from patients with polycystic kidney disease (PKD). Because NB form surface calcifications at physiologic levels of calcium and phosphate, they have been hypothesized to mediate the formation of tissue calcifications. We describe a modified microdilution inhibitory test that accommodates the unique growth conditions and long multiplication times of NB. This modified microdilution method included inoculation of 96-well plates and determination of inhibition by periodic measurement of the absorbance for 14 days in cell culture medium under cell culture conditions. Bactericidal or bacteriostatic drug effects were distinguished by subsequent subculture in drug-free media and monitoring for increasing absorbance. NB isolated from fetal bovine serum (FBS) were inhibited by tetracycline HCl, nitrofurantoin, trimethoprim, trimethoprim-sulfamethoxazole, and ampicillin at levels achievable in serum and urine; all drugs except ampicillin were cidal. Tetracycline also inhibited multiplication of isolates of NB from human kidney stones and kidney cyst fluids from patients with PKD. The other antibiotics tested against FBS-derived NB either had no effect or exhibited an inhibitory concentration above clinically achievable levels; the aminoglycosides and vancomycin were bacteriostatic. Antibiotic-induced morphological changes to NB were observed by electron microscopy. Bisphosphonates, aminocaproic acid, potassium citrate-citric acid solutions, and 5-fluorouracil also inhibited the multiplication of NB in a cidal manner. Insights into the nature of NB, the action(s) of these drugs, and the role of NB in calcifying diseases may be gained by exploiting this in vitro inhibition test system.
Are the soft tissue calcifications observed in patients with several important chronic diseases (e.g., atherosclerosis, Alzheimer's disease, and some cancers and dementias) caused by infectious agents (9)? There are hypotheses that microbes contribute to pathological tissue calcifications (29-32, 72) and, conversely, that microbes contribute to the fundamental pathogenesis of many chronic diseases, including those in which calcifications are evident (10). Biological apatites are present in the mineral phases of normal and pathological calcifications (63). The stimuli for calcium salt deposition in patients with these conditions are unclear, but nidi for precipitation and crystallization are needed even under supersaturation conditions (9). In this regard, antibiotics (27, 70, 74), bisphosphonates (5, 8, 20, 28, 38-40, 60), citrate (12), and other chemotherapeutics (5, 37) have been used with some success for the treatment of pathological calcification-related diseases. The inhibitory effects of antibiotics on the calcifications of surgically implanted artificial materials have also been shown (11). Are the effects of these drugs due to their commonly known primary mechanisms of antimicrobial action or to lesser known or even new modes of action, such as chelation or anti-inflammatory activities (10)?
We have recently isolated an infectious agent, nanobacteria (NB), that calcify (i.e., form apatite) under physiologic conditions (4, 46, 47-49, 53). NB cytotoxic to fibroblasts in vitro (15) were isolated from human (17) and bovine (51, 52) sera, kidney cyst fluids from patients with polycystic kidney disease (PKD) (45), demineralized kidney stone extracts (13, 48), and sclerotic carotid arteries and the aorta of a human at autopsy (L. Puskas, L.Tiszlavicz, L. Torday, and J. Papp, unpublished observations). In a small study, García Cuerpo et al. (34) found that translumbar, percutaneous intrarenal injection of NB into rats resulted in kidney stone formation. Others have reported NB by our methods in environmental waters (S. Burton and H. M. Lappin-Scott, Abstr. 100th Gen. Meet. Am. Soc. Microbiol., abstr. R19, 2000). Despite the duplication of our findings all or in part in several laboratories (6, 19, 34, 75), NB remain controversial entities due in part to their novel properties: small size (diameters, 0.1 to 0.5 μm), slow growth (replication times, 1 to 3 days), atypical morphology, apatite coat, and resistance to extraction of their component parts by conventional methods. For example, Cisar et al. (19) have reported that crystals formed under supersaturating conditions were capable of self-replication and concluded that NB probably do not exist. Unfortunately, they did not control for contamination with NB or the presence of microbial components in their reagents, nor did they seek control cultures of NB or the best available reagents specific for NB for use in their experiments. While nonbiogenic apatite is not immunogenic and thus is useful in support materials in orthopedics and dentistry, infection with NB (i.e., biogenic apatite) yields an immune response (E. O. Kajander and N. Çíftçíoglu, unpublished data). As discussed by Wainwright (80), NB are conceptually related to ultrasmall microbes in medicine and the environment.
The model for modern infectious disease research is to use parallel approaches to efficiently identify risks to human health. Thus, parallel studies of the biology of NB, the association of NB with diseased tissue, causality studies with animals, and responses to drugs are in keeping with this philosophy. In this work, our aim was to examine the effects, both by inhibition and by morphology analyses, of an array of antibiotics and other drugs on NB in vitro and thereby gain insights into the biology of NB and potential mechanisms of action in our test system. To accomplish this goal, we developed a first-generation inhibition test relevant to the slowly multiplying, calciferous nature of NB.
MATERIALS AND METHODS
Cultures of NB.
The test isolate of NB used in this study, designated Seralab 901045 (Seralab, Crawley Down, United Kingdom) was obtained by culturing pooled fetal bovine serum (FBS) in Dulbecco's modified Eagle's medium (DMEM; Life Technologies, Grand Island, N.Y.) containing FBS at a final concentration of 10% and 1 mmol of glutamine per liter, as described previously (15, 57). FBS-derived NB have been deposited in the German Collection of Microorganisms, Braunschweig, Germany, under accession nos. 5819 to 5821 (48). By negative staining, the sizes of the electron-dense particles of NB ranged from 0.2 to 0.3 μm up to 0.5 μm after 1 month in culture. They appear coccoid-coccobacillary in shape, either as single particles, in short chains, or predominantly as clusters. Needle-like apatite crystals are observed on their surfaces by transmission electron microscopy (TEM) (48). A biofilm surrounds NB, as evidenced by positive staining with safranin O by the method of Fessia and Griffin (32). Chemical analysis showed that by dry weight a pellet of NB consists of ∼25% calcium and ∼14% phosphate, compatible with hydroxyapatite by energy-dispersive X-ray microanalysis (48). NB are positive by the Limulus amoebocyte lysate assay (45).
Culturing was conducted by using strict aseptic techniques in a cell culture facility with incubation at 37°C in humidified 5% CO2-95% air. Subcultures were made by passing a small inoculum (1/10 of an old culture) into fresh DMEM supplemented with 10% of either the same serum or gamma-irradiated (30 kGy) FBS (Tamro, Ilomantsi, Finland; a similar product can be obtained from Life Technologies). The culture of NB used to test inhibitory activities was incubated for 3 weeks prior to use. The absence of classical microbes in cultures of NB was confirmed by subculturing 200-μl aliquots onto sheep blood agar and aerobic and anaerobic incubation at 37°C; samples were also examined by TEM. Culture material was stained with Hoechst 33258 fluorochrome DNA stain (Sigma Chemical Co., St. Louis, Mo.) as described earlier (58). Positive identification of NB included typical growth in cell culture medium with a doubling time of 1 to 3 days, characteristic morphology by scanning electron microscopy (SEM) or TEM and measurement of the absorbance at 650 nm (16, 18, 48), specific staining with Hoechst fluorochrome with the high (5-μg/ml) concentration (57), and positive immunofluorescence staining with specific monoclonal anti-NB antibodies 8/0 (porin protein epitope) and 5/2 (carbohydrate peptidoglycan epitope) (48). For selected inhibitory tests, two isolates of NB were obtained from human kidney stones (13) and one was obtained from the renal cyst fluid of a 23-year-old Finnish patient with advanced PKD (45).
Antimicrobials and drugs.
Antimicrobials were supplied as laboratory-grade powders of known potency, as follows: tetracycline HCl, doxycycline, rifampin, clarithromycin, trimethoprim, pyrazinamide, ampicillin, penicillin, and vancomycin were obtained from Orion (Espoo, Finland); ciprofloxacin was obtained from Bayer AG (Leverkusen, Germany); trimethoprim-sulfamethoxazole was obtained from Grunenthal (Stolberg, Germany); nitrofurantoin was obtained from Leiras (Helsinki, Finland); cephalothin was obtained from Eli Lilly (Copenhagen, Denmark); polymyxin B was obtained from Alcon Finland OY (Vantaa, Finland); clindamycin was obtained from Pharmacia-Upjohn (Vantaa, Finland); and commercial powders of spectinomycin, erythromycin, lincomycin, ethambutol, and chloramphenicol and sterile solutions of gentamicin, neomycin, kanamycin, and streptomycin were obtained from Sigma. Most antimicrobials were dissolved in DMEM to give a final concentration of 1 mg/ml and sterilized by membrane filtration (pore size, 0.2 μm; Millipore Corp., Bedford, Mass.). Erythromycin and clarithromycin were dissolved in 10% (vol/vol) aqueous ethanol and the solution was then made up to volume with DMEM before filter sterilization. Antimicrobial dilutions were prepared just prior to use in sterile plastic vials at double the required concentration in DMEM containing 10% gamma-irradiated FBS to allow 1:1 dilution of the inoculum of NB in MIC tests. The isolates of NB were tested against each antimicrobial at concentrations ranging from 500 to 0.48 μg/ml. Additionally, the following drugs were tested by using the same medium, supplements, and dilution series described above: two calcium inhibitors, the bisphosphonates dinatriumethidronate (etidronate; Roche Laboratories, Nutley, N.J.) and dinatriumclodronate (clodronate; Leiras, Helsinki, Finland); aspirin (Bayer); and p-aminosalicylic acid (PAS), lactic acid, pyruvate, acetic acid, formic acid, 6-aminocaproic acid, and 5-fluorouracil (5-FU) (all of which were supplied by Sigma). The potassium citrate-citric acid solution was prepared as described by Tanner (73). The American Type Culture Collection (ATCC) quality control strains used in standard susceptibility tests were not tested due to the lack of comparability of tests with those strains to the growth conditions used and the medium required for the testing of NB and the unique properties imparted by the calcium apatite coat of NB.
MIC and minimal bactericidal concentration (MBC) testing.
Inhibitory tests were performed in 96-well, flat-bottom cell culture plates by a modification of the method for mycoplasmas described by Hannan (41), which involved (i) a longer incubation period, (ii) drug dilutions from 500 to 0.48 μg/ml, and (iii) absorbance measurements at multiple time points. DMEM containing 10% gamma-irradiated FBS was used as the culture medium in the tests and for preparation of dilutions of NB and test compounds. After completion of the twofold serial dilutions for each compound, 100 μl of a suspension of the culture of NB with a turbidity equivalent to that of a 0.5 McFarland standard (optical density at 650 nm, 0.020) was added to each well in the microtiter plate. All compounds and each serial dilution were tested in triplicate; the duplicate experiments were run on different days. Each plate also contained four wells of uninoculated medium, which served as the sterility control; four inoculated wells of NB, devoid of any drug, were used as the growth controls. The plates were incubated at 37°C in a humidified atmosphere of 5% CO2-95% air for a maximum of 14 days. Growth was monitored on days 0, 4, 8, 12, and 14 by measuring the absorbance at 650 nm. Prior to absorbance measurement, the plate lid was warmed with a preheated metal plate to eliminate condensation on the lid. Growth curves with standard deviations were determined for each drug treatment. The growth of NB from positive growth control wells was confirmed by Hoechst staining and with anti-NB monoclonal antibodies as described earlier (15).
In an attempt to quantify indirectly the influences of the drugs on the slowly multiplying NB, an absorbance of 28 ± 3 for the positive growth control on day 4 was used as the reference point for establishing growth or no growth for each test compound. The National Committee for Clinical Laboratory Standards (NCCLS) has no information on susceptibility methods or recommendations for culture medium for the growth of NB (61). NB are positive by the Limulus amoebocyte lysate assay (45); thus, inhibition or noninhibition of the growth of NB was determined by using concentrations equivalent to the breakpoints established by the NCCLS for members of the family Enterobacteriaceae. None of the values have been validated for NB. Growth and inhibition curves for NB were determined for each drug treatment. Growth of NB from positive growth control wells was confirmed by Hoechst staining and with anti-NB monoclonal antibodies as described earlier (15).
The bactericidal or bacteriostatic effects of the drugs were determined. The drug-treated cultures of NB were subcultured (volume, 10 μl) into antibiotic-free medium (190 μl; 1/20 dilution) and incubated for 14 days. Each positive and negative control was handled in the same manner. When a subculture was negative for growth for any antibiotic or drug, the compound was classified as “NB-cidal”; if there was detectable growth as determined by a continuous increase in absorbance greater than that for the negative control, the compound was considered “NB-static.”
The effect of tetracycline HCl on the isolates of NB from two kidney stones and one cyst fluid sample from a patient with PKD (45) was determined by using the inhibitory test method described above. The growth was monitored weekly by measuring the absorbance at 650 nm for 2 weeks, on days 0, 7, and 14.
β-Lactamase testing.
Detection of β-lactamase was done by the chromogenic cephalosporin (Glaxo Pharmaceutical identification number 87/312) test developed by O'Callaghan et al. (62) by adding the solution directly into prepared cultures of NB or adding prepared culture material to a filter paper disk for evaluation by the diffusion technique (59). Reagents and test assays were those described by the manufacturer (Becton Dickinson Diagnostic Systems, Sparks, Md.) Two sample preparation methods were used. In the first one, 1 ml of a culture of NB was harvested after 30 days of incubation and centrifuged at 14,000 × g for 1 h, the supernatant was removed (and retained for later testing), and the pellet was washed three times with phosphate-buffered saline (PBS; pH 7.4) to remove the residual FBS in the culture medium. The pellet was resuspended in 100 μl of PBS. The second preparation method was performed as described above, but prior to testing, the cells were treated with a final concentration of 100 mM EGTA in PBS for 1 h in a 37°C water bath with vortexing at 15-min intervals. This was done to remove the calcium coat, as NB are not very labile to sonication. The positive controls were those recommended by the manufacturer. Testing of the positive control in a test solution containing EGTA was also done to determine any inhibition by the assay. The presence or absence of color production was recorded immediately, at 10 min, and after a maximum of 30 min.
Electron microscopy.
For SEM, NB cultured with and without antimicrobials or drugs were centrifuged at 14,000 × g for 15 min and the supernatant fraction was removed. The pellets were washed twice with PBS and fixed with 2% glutaraldehyde in PBS for 16 h. The fixed samples were washed twice with PBS, dehydrated with increasing ethanol concentrations, and dried with a critical point dryer. The samples were coated with a gold layer (thickness, 20 to 40 nm) prior to examination with a JSM-35 scanning electron microscope (JEOL, Tokyo, Japan) (15). For TEM, negative staining of NB before and after antibiotic treatment was performed by applying a 10-μl sample of a washed culture of NB in PBS onto Formvar- and carbon-supported copper grids (57, 67, 75) for 5 min, followed by removal of excess fluid with filter paper. The samples were then washed by dipping the grid in a series of 3 droplets of sterile water for injection, followed by submersion of the grid in a droplet of uranyl acetate for 1 to 2 s. The grids were air dried and examined by TEM.
RESULTS
Antibiotic inhibitory data.
The FBS-derived NB isolate was susceptible to tetracycline HCl (1.95 μg/ml), nitrofurantoin (3.9 μg/ml), trimethoprim (3.9 μg/ml), trimethoprim-sulfamethoxazole (1.4 to 6.25 μg/ml), and ampicillin (7.8 μg/ml) at clinically achievable levels in serum or urine (Table 1) in the 14-day test. All antibiotics except ampicillin were NB-cidal; ampicillin was NB-static. The inhibitory concentration did not vary between incubation day 8 and incubation day 14 for any of the antibiotics tested except trimethoprim-sulfamethoxazole and ampicillin, for which fourfold and twofold increases, respectively, were observed. The data for all other antimicrobials tested are provided in Table 1. The aminoglycosides and vancomycin were also NB-static.
TABLE 1.
MICs and MBCs of selected antibiotics for NB at 14 days of incubation
| Antimicrobial | Mode of action | Chelation activitya | MIC and MBC (μg/ml) |
|---|---|---|---|
| Aminoglycosides | Protein synthesis (30S, initiation complex and misreading) | − | |
| Gentamicin | 250 | ||
| Kanamycin | 250 | ||
| Neomycin | 31.2 | ||
| Streptomycin | >500 | ||
| Aminocyclitol, spectinomycin | Protein synthesis | − | >500 |
| Tetracyclines | Protein synthesis (30S; aminoacyl tRNA) | + | |
| Tetracycline HCl | 1.95b | ||
| Doxycycline HCl | 62.5b | ||
| Chloramphenicol | Protein synthesis (peptidyltransferase) | − | >500 |
| Lincosamides | Protein synthesis | − | |
| Lincomysin | >500 | ||
| Clindamycin | >500 | ||
| Trimethoprim | Protein and DNA synthesis | − | 3.9b |
| Sulfonamide-trimethoprim | Protein and DNA synthesis | − | |
| Sulfamethoxazole | PABAc (dihydropteroate synthetase, dihydrofolic acid reductase) | 6.25/1.4b | |
| Trimethoprim | |||
| Urinary tract antiseptic, nitrofurantoin | Protein and DNA synthesis | − | 3.9b |
| β-Lactams: penicillins | Cell wall (transpeptidation reaction) | + | |
| Ampicillin | 7.8 | ||
| Penicillin | >500 | ||
| β-Lactams (cephalosporins), cephalothin | Cell wall (transpeptidation reaction) | >500 | |
| Macrolides | Protein synthesis (50S; aminoacyl translocation) | − | |
| Erythromycin | >500 | ||
| Clarithromycin | >500 | ||
| Fluoroquinolone, ciprofloxacin | DNA synthesis (DNA gyrase) | + | >500 |
| Glycopeptide, vancomycin | Cell wall mucopeptide synthesis (peptidoglycan synthetase) | − | 250 |
| Polymyxin, polymyxin B | Cell membrane (phosphatidylethanolamine) | − | >500 |
| Antituberculous agents | |||
| Pyrazinamide | − | >500 | |
| Rifampin | Initiation of transcription (RNA synthesis, DNA-dependent RNA polymerase) | − | >500 |
+, chelator; −, nonchelator or unknown.
The concentration of the antibiotic that was effective as both the MIC and the MBC.
PABA, p-aminobenzoic acid.
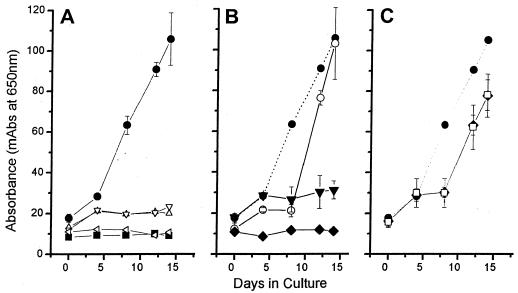
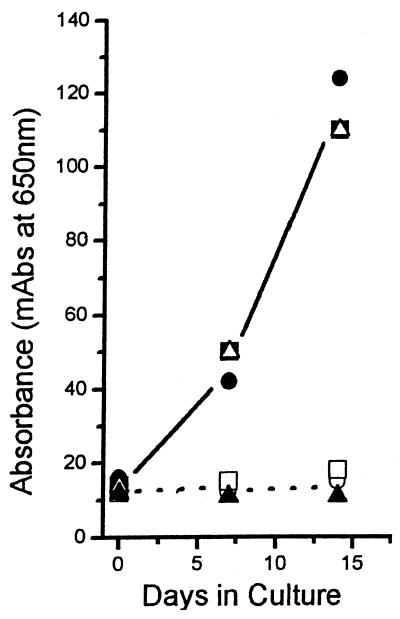
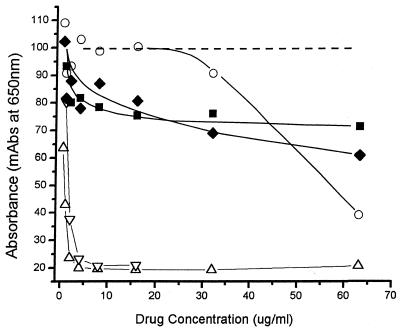
Growth curves for selected drugs against NB are depicted in Fig. 1. For those drugs with inhibitory activities against NB, no growth was observed throughout the test period, giving curves similar to that for the negative control. Tetracycline HCl, the only antibiotic tested against the human kidney stone and cyst fluid-derived isolates of NB, also inhibited the growth of NB and was NB-cidal (Fig. 2). For those drugs inactive against NB, the growth curves were either identical to the curve for the drug-free control or followed in parallel the curve for normal growth with a lag period of 1 to 2 generations (1.5 to 3 days). Inhibition curves for representative drugs active and inactive against NB are shown in Fig. 3.
FIG. 1.
Growth curves showing the activities of selected antibiotics and drugs (4 μg/ml) against NB. Each drug treatment was plotted against the positive (drug-free) growth control (•). (A) ▪, negative control (growth medium only); ▵, tetracycline; ▿, nitrofurantoin; ◃, etidronate. Note that the lines for the calcium inhibitor etidronate, tetracycline, and nitrofurantoin are almost identical to that for the negative control. (B) ▾, ampicillin; ○, doxycycline; ⧫, 5-FU. Note the marked difference in inhibition between tetracycline (A) and doxycycline (B). While some initial minimal growth was exhibited by NB exposed to ampicillin on day 4, there was no significant growth for the duration of the test period. The curve for 5-FU mimics that for the negative control in panel A. (C) □, gentamicin; ⧫, vancomycin. NB exposed to both gentamicin and vancomycin exhibited a 1- to 2-generation delay in growth compared to the growth control and then resumed growth in a pattern similar to that for the positive growth control. mAbs, milli-absorbance (10−3 A650).
FIG. 2.
Growth curves showing the activities of tetracycline against isolates of NB from two human kidney stones and one cyst fluid specimen from a patient with PKD. Data for the drug-treated isolates from humans were plotted against those for the positive (drug-free) growth control. Positive growth controls consisted of kidney stone isolates 1 (•) and 2 (▪) and an isolate from cyst fluid from a patient with PKD (▵). Curves for kidney stone isolates 1 (○) and 2 (□) and the cyst fluid isolate (▴) treated with 4 μg of tetracycline per ml for 14 days are also shown. The three human isolates of NB were inhibited by tetracycline at 4 μg/ml to the same degree that the test isolate of NB was (Fig 1A). In the absence of antibiotic, growth curves for these three human-derived isolates of NB resembled that for the positive growth control shown in Fig. 1A. mAbs, milli-absorbance (10−3 A650).
FIG. 3.
Dose-inhibition curves for selected antibiotics against NB. The dashed line represents the curve for positive growth control NB. ▵, is tetracycline; ▿, nitrofurantoin; ○, doxycycline; ▪, vancomycin; ⧫, gentamicin. The negative control is that represented in Fig. 1A. Both tetracycline and nitrofurantoin caused sharp declines in growth until the MIC was reached, with no further growth as the antibiotic concentrations increased. For doxycycline virtually no inhibition was observed until its MIC (62.5 μg/ml) was reached. Vancomycin and gentamicin showed an initial loss of absorbance versus concentration, but this was not sustained over a range of concentrations well above their achievable levels in serum. mAbs, milli-absorbance (10−3 A650).
β-Lactamase testing.
β-Lactamase was not detected in any of the sample preparations under any of the test conditions, even after a maximum of 30 min. The control isolate was β-lactamase positive for all tests, including tests with the assay solution containing 100 mM EGTA.
Additional inhibitory data.
When 5-FU was present at 4.0 μg/ml, NB multiplication, as determined by measurement of the absorbance, was inhibited completely (Fig. 1B) and in an NB-cidal manner. An NB-cidal inhibition of multiplication of NB was also observed for the bisphosphonates, as shown for etidronate (Fig. 1A), citrate (data not shown) (45), and 6-aminocaproic acid (data not shown). Dose-dependent inhibition of NB multiplication was not observed for the other organic acids (PAS, lactic acid, pyruvate, acetic acid, formic acid, and aspirin) at the dilutions tested (500 to 0.48 μg/ml).
Morphological alterations.
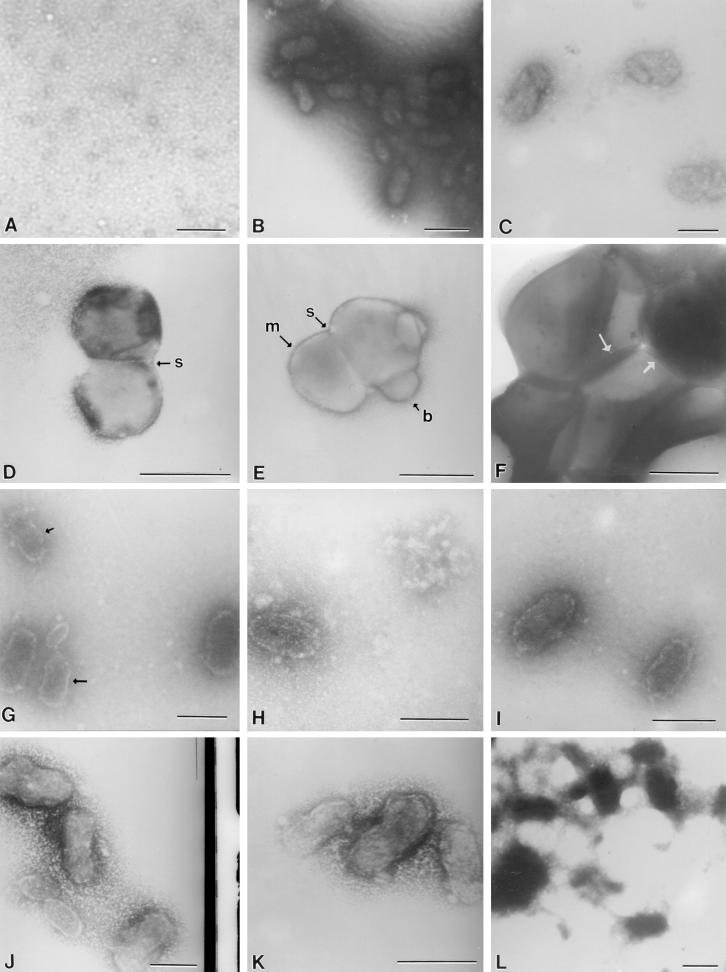
The negative control (Fig. 4A) contained only culture medium and confirmed the absence of common bacteria and NB under the test conditions used in the study. Typical electron-dense coccobacillus-shaped NB with defined borders ranged in size from approximately 150 to 250 nm (Fig. 4B). Neither the morphology nor the size of the NB varied during the 14-day incubation period. In contrast, NB treated with the bisphosphonate etidronate (1.0 μg/ml, 7 days) showed a marked loss of electron density and forms with irregular borders (Fig. 4C).
FIG. 4.
(A) Culture medium only; (B) electron-dense coccobacillary control NB; (C) etidronate-treated NB with a loss of electron density; (D and E) NB treated with EDTA revealing membrane (arrow labeled m) and septumlike structures (arrows labeled s); possible budding is noted (arrow labeled b); (F) S. aureus ATCC 25923 compared with treated and untreated NB; division planes are evident (arrows); (G and H) NB treated with tetracycline HCl at 0.5 and 4 μg/ml, respectively, illustrating the concentration-dependent loss of electron density; an increasing irregularity of NB borders was observed (arrows); (I and J) NB treated with nitrofurantoin (0.5 and 2.0 μg/ml, respectively) on day 1 demonstrating changes similar to those observed with tetracycline; the cells also enlarged as the concentration increased (J); (K and L) NB treated with ampicillin (15.6 μg/ml) on day 1 showing changes in morphology similar to those observed with the other drugs on day 7; (L) predominance of amorphous debris, as was also observed with tetracycline (data not shown). Bars, 200 nm.
NB treated with 10 mM EDTA, to diminish the mineral content, revealed coccoid forms virtually devoid of the apatite coat (Fig. 4D and E) in comparison to untreated NB (Fig. 4B). Evidence of cellular components was present, such as membranelike structures and a septum (Fig. 4D). Both a septumlike area, compatible with binary fission, and evidence of possible budding are also evident in Fig. 4E. Neither NB nor remnants were observed after 7 days of exposure to EDTA. For size and shape comparison, Staphylococcus aureus ATCC 25923 was negatively stained by the same procedure after 24 h of incubation in tryptic soy broth (Difco, Detroit, Mich.). Uniform cocci of typical size (∼500 nm) were observed, and division planes were visible (Fig. 4F).
After 1 day of exposure to tetracycline HCl at 0.5 and 4.0 μg/ml, a concentration-dependent loss of electron density and a progression in the irregularity of the borders of the NB were observed (Fig. 4G and H, respectively). The loss of electron density is consistent with a loss of calcium. At the higher concentration the NB appeared more ovoid and ghostlike (Fig. 4H). By day 7, there was also a marked reduction (∼90%) in the number of NB as the antibiotic concentration increased (data not shown). Similar morphological findings were recorded for NB exposed to nitrofurantoin, in which, as the concentration increased, the cells of the NB enlarged.
On day 1, the results of exposure of the NB to 15.6 μg of ampicillin per ml (Fig. 4K) were representative of the exposures to all concentrations examined from 0.48 through 15.6 μg/ml. NB exhibited various alterations ranging from almost a complete loss of electron density, a loss of defined borders, and clumping. On day 7 after exposure to ampicillin at 15.6 μg/ml (Fig. 4L), the NB appeared similar to the NB as they appeared on day 7 after they had been exposed to tetracycline, in which there was a predominance of amorphous material and conglomerates of fused NB devoid of the characteristic morphology. There was also an abundance of undefined background debris.
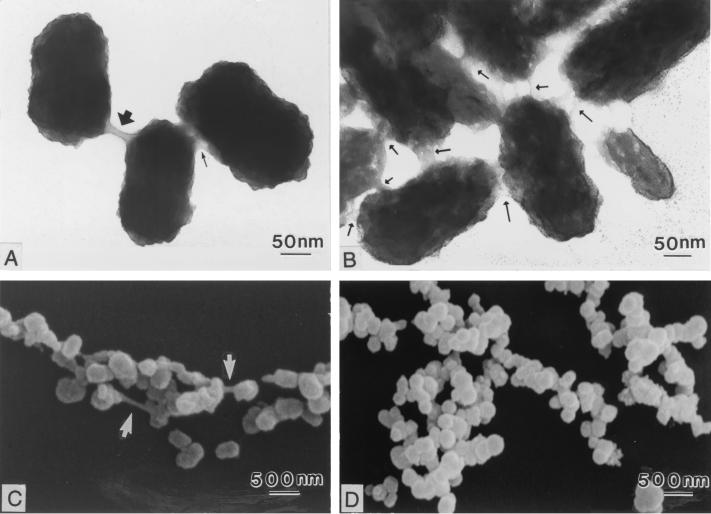
Figure 5 shows TEM micrographs of negatively stained NB and SEM micrographs of NB for NB exposed to gentamicin (500 μg/ml, day 14) and control NB. Although growth was ultimately not inhibited by gentamicin (Fig. 1C), a change in the putative biofilm of NB was observed. The control NB used for both TEM and SEM (Fig. 5B and D) were more clumped; negative staining for TEM revealed numerous slimelike bridges of various thicknesses between the NB (Fig. 5B). Gentamicin-treated NB were markedly less clumped, with fewer slimelike bridges than the control NB (Fig. 5A and C).
FIG. 5.
Gentamicin-treated NB. Negatively stained TEM micrographs of gentamicin-treated (500 μg/ml, day 14) NB (A) and control NB (B) are shown. Note the larger amount of putative biofilm in the control NB compared to that in the gentamicin-treated NB (arrows). There was a larger amount of clumping in the control NB (D), but there was less clumping in gentamicin-treated NB (C).
DISCUSSION
This report focuses on the effects of drugs on the growth and morphology of NB within the larger context of microbes as provocateurs of soft tissue calcifications, lesions that occur in a surprisingly wide array of important diseases (9). When drugs altered the morphology of NB there was a loss of (i) electron density, (ii) coccobacillary shape, and (iii) defined borders. Exceptions were enlarged NB observed with nitrofurantoin and the amorphous debris and paucity of residual NB observed with inhibitory concentrations of tetracycline and ampicillin. The relatedness, if any, of the findings for this test system for the detection of inhibition of NB to the reported drug-induced effects on the viability and morphology of classical bacteria and fungi is yet to be determined (25, 58, 82).
Regarding the test system for detection of the inhibition of NB described here, our earlier investigations showed that NB reach the log period of multiplication within a month if the A650 of the initial inoculum density was lower than 20 (turbidity equivalent to that of a 0.5 McFarland standard). The inoculum density of the NB used in this inhibitory test allowed us to obtain logarithmic growth over the 14-day test period. Previous work also demonstrated that the absorbance of NB grown in the presence of FBS is due to an increase in the number of NB and not an increase in the mass of each NB particle (16). In the present study, TEM of the negative control (Fig. 4B and 5B) showed no evidence of protein precipitation or classical crystal formation (56), thus discounting these factors as being responsible for the changes in absorbance. Furthermore, if protein precipitation had occurred, an increase in absorbance of the negative control (DMEM plus 10% gamma-irradiated FBS) should have occurred. Use of absorbance to monitor the growth of NB is preferred because NB can exhibit clumping, making particle counting by flow cytometry unreliable and SEM laborious.
In this study, NB (Seralab 901045) was inhibited in vitro at clinically achievable levels in serum and urine (36) by ampicillin, trimethoprim, trimethoprim-sulfamethoxazole, nitrofurantoin (a urinary antiseptic), and tetracycline HCl. It is commonly known that ampicillin inhibits bacterial cell wall synthesis, but like some other penicillins, it is also a calcium chelator (22, 64). The inhibition by ampicillin may also have been influenced by the lack of detectable β-lactamase in NB and the somewhat zwitterionic nature of ampicillin that enables it to penetrate the cell walls of gram-negative bacteria (55). Trimethoprim, trimethoprim-sulfamethoxazole, and nitrofurantoin are reported to inhibit protein and DNA syntheses; we did not find reports of calcium chelation activities for these drugs.
Tetracycline is reported to inhibit bacterial protein synthesis, chelate calcium, and inhibit metalloproteinases, a property of potential use in the treatment of osteoarthritis, periodontitis, and cancer (42). Tetracycline is already used in the treatment of some periodontal diseases and dental stone formation (69). NB have been isolated from human dental stones (14). There was a difference in the in vitro activity of tetracycline HCl (MIC, 1.95 μg/ml) and that of doxycycline (MIC, 62.5 μg/ml) against NB. Although doxycycline is more highly protein bound and approximately 10 times more lipophilic than tetracycline HCl (23), their activities against NB observed in vitro correlated with their comparative levels of calcium binding. The level of chelation of tetracycline to calcium (40%) is reported to be twice that for doxycycline (19%) (79).
The aminoglycosides are primarily known as inhibitors of protein synthesis, but more recently it has been recognized that they displace cell biofilm-associated calcium and magnesium that link polysaccharides of lipopolysaccharide molecules (65). Gentamicin, kanamycin, and neomycin did not block the multiplication of NB. However, gentamicin caused a reduction in the amount of putative biofilm surrounding NB (Fig. 5). NB are positive by the differential Limulus amoebocyte lysate assay (45), but the lipopolysaccharide of NB has not been sufficiently characterized to allow further speculation regarding the observed lack of activity of these antibiotics.
Tests of inhibition of other diverse organisms for up to 21 days have been reported (7, 26, 35, 42, 71). Because the drug levels in the growth media were not measured throughout the 14-day test period in this study, it was not possible to determine from the inhibition curves whether a postantibiotic effect or a sub-MIC (inhibitory) effect occurred (21, 83). The lag in growth associated with antibiotics inactive against NB (Fig. 1C) could have been due to drug deterioration, an alternate mode of initial inhibition, an effect of the slow metabolic rate of NB on the effect of the drug at the target site, poor diffusion across the apatite shell, development of a resistant mutant(s), or any combination thereof. There was some minimal initial growth of NB exposed to tetracycline HCl, nitrofurantoin, and ampicillin detectable only on day 4, but this was below or did not exceed the level of growth for the growth control; no further growth was detected for the remainder of the test period.
Etidronate and clodronate (bisphosphonates that lack N moieties), 5-FU, and aminocaproic acid (data not shown) completely inhibited the growth of NB, giving inhibition curves nearly identical to the NB-free negative control curve (Fig. 1A and C). We are unaware of any chelation activity for 5-FU, a drug known to inhibit DNA and RNA metabolism and processing, or aminocaproic acid, an antifibrinolytic drug that binds to lysine residues on plasmin, thereby blocking access to its substrate fibrin. As a drug class, bisphosphonates resemble a substituted pyrophosphate and chemisorb to calcium hydroxyapatite crystals; in so doing they block aggregation, growth, and mineralization of these crystals, leading to the prevention or retardation of heterotopic ossification (24, 56).
Importantly, bisphosphonates exhibit additional mechanisms of action beyond chelation which are of potential relevance to inhibition of growth of mammalian and microbial cells. In osteoclasts N-substituted bisphosphonates also inhibit the addition of long-chain lipids to GTP binding proteins, leading to apoptosis, decreased numbers of osteoclasts, and reductions in the levels of bone loss (33, 68). The accumulation of bisphosphonates in bone may also decrease the levels of binding of disseminated cancer cells to bone and increase drug concentrations to levels able to kill cancer cells adjacent to bone (44). Bisphosphonates may kill cancer cells, in part, via inhibition of metalloproteinases (43), enzymes also found in microbes. Price et al. (66) have challenged the idea that chelation is solely responsible for the inhibition of both artery calcification and bone resorption by alendronate. Interestingly, NB have been found in calcified human carotid arteries and aorta but not healthy vessels (Puskas et al., unpublished).
It is not clear that inhibition of the growth of NB requires chelation. Calcium binding may contribute to the effects against NB of the potassium citrate and citric acid mixture (45) and some of the antimicrobics and other drugs tested. However, the nonchelators nitrofurantoin, 5-FU, and aminocaproic acid were active against NB. Conversely, ciprofloxacin, a known chelator, was not active. The classical mechanisms of action of these drugs against NB would be consistent with reports that NB contain protein, DNA, apatite, and muramic acid (48, 53) and are positive for endotoxin by the differential Limulus amoebocyte lysate assay and by immunoblotting (45). Of course, drugs effective against NB in vitro may act via nonclassical mechanisms. New mechanisms of antibiotic action are increasingly appearing in the literature, such as (i) gentamicin's effect on ribosomes to correct the enzyme deficiency that causes Hurler's syndrome in cultured fibroblasts from patients (54) and (ii) the discovery that a genetically engineered live-attenuated human immunodeficiency virus will reproduce only in the presence of doxycycline (77).
Microbes, their toxins, or their shed components may contribute to pathological calcifications in several ways. They may (i) damage cellular membranes, resulting in exposure of tissue components capable of forming crystallizing nidi (2, 81), or (ii) alter local levels of calcium and phosphate in tissue to saturating concentrations that in turn promote crystal formation on available nidi; (iii) the microbe may be calcified directly (29-31, 72, 76, 78); or (iv) microbial components may interact with tissue components to form complexes that are hybrid nidi. NB or its fragments may be nidi, but they are not necessarily the only nidi for the formation of pathological calcifications (9, 13-15, 34). As microbial components are known to bind to apatite (3), NB may also contribute directly to the primary pathogenesis of disease by acting as a system for the delivery of microbial and other toxins to tissues (1, 50), a process that would require endocytosis (15). Future research is required to determine the classical and potentially novel mechanism(s) by which drugs inhibit the growth of NB, alter the morphology of NB, and affect the genesis of diverse types of microbial and tissue calcifications.
ADDENDUM IN PROOF
Ciprofloxacin, although a chelator, has been reported not to exhibit chelation activity in a bacterial test system (B. Lindner, A. Wiese, K. Brandenburg, U. Seydel, and A. Dalhoff, Antimicrob. Agents Chemother. 46:1568-1570, 2002).
Acknowledgments
We thank all those who supplied antibiotics, which enabled this study to be carried out; Orion for the samples of tetracycline HCl, rifampin, clarithromycin, trimethoprim, pyrazinamide, doxycycline, ampicillin, and vancomycin; and Kuopion Yliopiston Apteekki for additional antibiotics. We also thank Alpo Pelttari and Robert Caughey for expertise in electron microscopy and Larry Crosset for TEM prints.
Financial support was obtained from the Finnish Academy and TEKES and through a grant from the Children's Miracle Network Research Fund—Peoria.
REFERENCES
- 1.Åkerman, K. K., J. T. Kuikka, N. Çiftçioglu, J. Parkkinen, K. A. Bergström, I. Kuronen, and E. O. Kajander. 1997. Radiolabeling and in vivo distribution of nanobacteria in rabbit. Proc. SPIE Int. Soc. Opt. Eng. 3111:436-442. [Google Scholar]
- 2.Anderson, H. C. 1988. Mechanisms of pathologic calcification. Rheum. Dis. Clin. N. Am. 14:303-319. [PubMed] [Google Scholar]
- 3.Barry, E. D., and G. R. Siragusa. 1997. Hydroxyapatite adherence as a means to concentrate bacteria. Appl. Environ. Microbiol. 63:4069-4074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Björklund, M., N. Çiftçioglu, and E. O. Kajander. 1998. Extraordinary survival of nanobacteria under extreme conditions. Proc. SPIE Int. Soc. Opt. Eng. 3441:123-129. [Google Scholar]
- 5.Bone, H. G., J. E. Zerwekh, F. Britton, and C. Y. Pak. 1979. Treatment of calcium urolithiasis with diphosphonate: efficacy and hazards. J. Urol. 121:568-571. [DOI] [PubMed] [Google Scholar]
- 6.Breitschwerdt, E. B., S. Sontakke, A. Cannedy, S. I. Hancock, and J. M. Bradley. 2001. Infection with Bartonella weissii and detection of nanobacterium antigens in a North Carolina beef herd. J. Clin. Microbiol. 39:879-882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Broughton, E. S., and L. E. Flack. 1986. The susceptibility of a strain of Leptospira interrogans serogroup ichterohaemorrhagiae to amoxycillin, erythromycin, lincomycin, tetracycline, oxytetracycline and minocycline. Zentbl. Bacteriol. Microbiol. Hyg. 261:425-431. [DOI] [PubMed] [Google Scholar]
- 8.Bushinsky, D. A., K. J. Neumann, J. Asplin, and N. S. Krieger. 1999. Alendronate decreases urine calcium and supersaturation in genetic hypercalciuric rats. Kidney Int. 55:234-243. [DOI] [PubMed] [Google Scholar]
- 9.Carson, D. A. 1998. An infectious origin of extraskeletal calcification. Proc. Natl. Acad. Sci. USA 95:7846-7847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cassell, G. H. 1998. Infectious causes of chronic inflammatory diseases and cancer. Emerg. Infect. Dis. 4:475-487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chandy, T., S. C. Vasudev, and C. P. Sharma. 1996. Changes in polyurethane calcification due to antibiotics. Artif. Organs 20:752-760. [DOI] [PubMed] [Google Scholar]
- 12.Cicerello, E., F. Merlo, G. Gambaro, l. Maccatrozzo, A. Fandella, B. Baggio, and G. Anselmo. 1994. Effect of alkaline citrate therapy on clearance of residual renal stone fragments after extracorporeal shock wave lithotripsy in sterile calcium and infection nephrolithiasis. J. Urol. 151:5-9. [DOI] [PubMed] [Google Scholar]
- 13.Çiftçioglu, N., M. Björklund, K. Kuorikoski, K. Bergström, and E. O. Kajander. 1999. Nanobacteria: an infectious cause for kidney stone formation. Kidney Int. 56:1893-1898. [DOI] [PubMed] [Google Scholar]
- 14.Çiftçioglu, N., V. Çiftçioglu, H. Vali, E. Turcott, and E. O. Kajander. 1998. Sedimentary rocks in our mouth: dental pulp stones made by nanobacteria. Proc. SPIE Int. Soc. Opt. Eng. 3441:130-135. [Google Scholar]
- 15.Çiftçioglu, N., and E. O. Kajander. 1998. Interaction of nanobacteria with cultured mammalian cells. Pathophysiology 4:259-270. [Google Scholar]
- 16.Çiftçioglu, N., and E. O. Kajander. 1999. Growth factors for nanobacteria. Proc. SPIE Int. Soc. Opt. Eng. 3755:113-119. [Google Scholar]
- 17.Çiftçioglu, N., I. Kuronen, K. Åkerman, E. Hiltunen, J. Laukkanen, and E. O. Kajander. 1997. A new potential threat in antigen and antibody products: nanobacteria, p. 99-103. In F. Brown, D. Burton, P. Doherty, J. Mekalanos, and E. Norrby (ed.), Vaccines 97. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 18.Çiftçioglu, N., A. Pelttari, and E. O. Kajander. 1997. Extraordinary growth phases of nanobacteria isolated from mammalian blood. Proc. SPIE Int. Soc. Opt. Eng. 3111:429-435. [Google Scholar]
- 19.Cisar, J. O., D.-Q. Xu, J. Thompson, W. Swaim, L. Hu, and D. J. Kopecko. 2000. An alternative explanation of nanobacteria-induced biomineralization. Proc. Natl. Acad. Sci. USA 97:11511-11515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cohen, H., V. Solomon, I. S. Alferiev, E. Breuer, A. Ornoy, N. Patlas, N. Eidelman, G. H'agele, and G. Golomb. 1998. Bisphosphonates and tetracycline: experimental models for their evaluation in calcium-related disorders. Pharm. Res. 15:606-613. [DOI] [PubMed] [Google Scholar]
- 21.Craig, W. 1998. Pharmacokinetic/phamacodynamic parameters: rational for antibacterial dosing of mice and men. Clin. Infect. Dis. 26:1-12. [DOI] [PubMed] [Google Scholar]
- 22.Crossland, J. 1970. Lewis' pharmacology, 4th ed., p. 1124-1125. Livingstone, London, United Kingdom.
- 23.Cunha, B. A., C. M. Sibley, and A. M. Ristuccia. 1982. Doxycycline. Ther. Drug Monit. 4:115-135. [DOI] [PubMed] [Google Scholar]
- 24.D'Aoust, P., C. A. McCulloch, H. C. Tenenbaum, and P. C. Lekic. 2000. Etidronate (HEBP) promotes osteoblast differentiation and wound closure in rat calvaria. Cell Tissue Res. 302:353-363. [DOI] [PubMed] [Google Scholar]
- 25.Davis, K. J., P. Vogel, D. L. Fritz, K. E. Steele, M. L. Pitt, S. L. Welkos, A. M. Friedlander, and W. R. Byrne. 1997. Bacterial filamentation of Yersinia pestis by beta-lactam antibiotics in experimentally infected mice. Arch. Pathol. Lab. Med. 121:865-868. [PubMed] [Google Scholar]
- 26.DeCross, A., B. Marshall, R. McCallum, S. Hoffman, L. Barrett, and R. Guerrant. 1993. Metronidazole susceptibility testing for Helicobacter pylori: comparison of disk, broth, and agar dilution methods and their clinical relevance. J. Clin. Microbiol. 31:1971-1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dubey, N. K., H. B. Tavadia, and M. Hehir. 1988. Malacoplakia: a case involving epididymis and a case involving bladder complicated by calculi. J. Urol. 139:359-361. [DOI] [PubMed] [Google Scholar]
- 28.Ebisuno, S., Y. Kohjimoto, T. Nishikawa, M. Nishihata, T. Inagaki, T. Komura, and T. Ohkawa. 1998. Effects of etidronate disodium on crystallizations in synthetic urine and calcium oxalate crystal adhesion to Madin-Darby canine kidney (MDCK) cells. Int. J. Urol. 5:582-587. [DOI] [PubMed] [Google Scholar]
- 29.Ennever, J., and H. Creamer. 1967. Microbiological calcification: bone mineral and bacteria. Calcium Tissue Res. 1:87-93. [DOI] [PubMed] [Google Scholar]
- 30.Ennever, J., and F. E. Summers. 1975. Calcification by Candida albicans. J. Bacteriol. 122:1391-1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ennever, J., J. J. Vogel, and J. L. Streckfuss. 1974. Calcification by Escherichia coli. J. Bacteriol. 119:1061-1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fessia, S. L., and M. J. Griffin. 1991. A method for assaying biofilm capacity on polyurethane-coated slides. Peritoneal Dialysis Int. 11:144-146. [PubMed] [Google Scholar]
- 33.Fisher, J. E., M. J. Rogers, J. M. Halasy, S. P. Luckman, D. E. Hughes, P. J. Masarachia, G. Wesolowski, R. G. G. Russell, G. A. Rodan, and A. A. Reszka. 1999. Alendronate mechanism of action: geranylgeraniol, an intermediate in the mevalonate pathway, prevents inhibition of osteoclast formation, bone resorption, and kinase activation in vitro. Proc. Natl. Acad. Sci. USA 96:133-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.García Cuerpo, E., E. O. Kajander, N. Çiftçioglu, F. L. Castellano, C. Correa, J. Gonzales, F. Mampaso, F. Liano, E. G. De Gabiola, and Y. A. E. Berrilero. 2000. Nanobacteria: un modelo de neo-litogenesis experimental. Arch. Esp. Urol. 53:291-303. [PubMed] [Google Scholar]
- 35.Garcia-Rodriguez, J., J. Garcia Sanches, M. Garcia Garcia, E. Garcia Sanches, and J. Munoz Bellido. 1989. In vitro activities of new oral β-lactams and macrolides against Campylobacter pylori. Antimicrob. Agents Chemother. 33:1650-1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Garrison, J. 2000. Optimal properties of agents used to treat acute, uncomplicated urinary tract infections. Drug Ther. Topics 29:23-26. [Google Scholar]
- 37.Gocmen, A., M. F. Toppare, N. Kiper, and N. Buyukpamukcu. 1992. Treatment of pulmonary alveolar microlithiasis with a diphosphonate—preliminary results of a case. Respiration 59:250-252. [DOI] [PubMed] [Google Scholar]
- 38.Goldstein, M. R. 2000. Bisphosphonate therapy and vascular calcification. JAMA 283:1424-1425. [PubMed] [Google Scholar]
- 39.Goodman, W. G., and I. B. Salusky. 2001. Non-invasive assessments of cardiovascular disease in patients with renal failure. Curr. Opin. Nephrol. Hypertens. 10:365-369. [DOI] [PubMed] [Google Scholar]
- 40.Gupta, M., O.-L. Tuncay, E. Valderrama, and A. D. Smith. 1997. Inhibition of calcium oxalate urolithiasis in a rat model of lithogenesis using bisphosphonates. J. Endourol. 11:1-4. [DOI] [PubMed] [Google Scholar]
- 41.Hannan, P. C. T., G. D. Windsor, A. deJong, N. Schmer, and M. Stegemann. 1997. Comparative susceptibilities of various animal-pathogenic mycoplasmas to fluoroquinolones. Antimicrob. Agents Chemother. 41:2037-2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hartvig Hartzen, S., L. Percival Andersen, A. Bremmelgaard, H. Colding, M. Arpi, J. Kristiansen, T. Justesen, F. Espersen, N. Frimodt-Moller, and O. Bonnevie. 1997. Antimicrobial susceptibility of 230 Helicobacter pylori strains: importance of medium, inoculum, and incubation time. Antimicrob. Agents Chemother. 41:2634-2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hidalgo, M., and S. G. Eckhardt. 2001. Development of matrix metalloproteinase inhibitors in cancer therapy. J. Natl. Cancer Inst. 93:178-193. [DOI] [PubMed] [Google Scholar]
- 44.Hiraga, T., P. J. Williams, G. R. Mundy, and T. Yoneda. 2001. The bisphosphonate ibandronate promotes apoptosis in MDA-MB-231 human breast cancer cells in bone metastases. Cancer Res. 61:4418-4424. [PubMed] [Google Scholar]
- 45.Hjelle, J. T., M. A. Miller-Hjelle, I. R. Poxton, O. Kajander, N. Çiftçioglu, M. L. Jones, R. C. Caughey, R. Brown, P. D. Millikin, and F. S. Darras. 2000. Endotoxin and nanobacteria in polycystic kidney disease. Kidney Int. 57:2360-2374. [DOI] [PubMed] [Google Scholar]
- 46.Kajander, E. O. 4August1992. Culture and detection method for sterile-filterable autonomously replicating biological particles. U.S. patent 5,135,851.
- 47.Kajander, E. O., M. Björklund, and N. Çiftçioglu. 1999. Nanobacteria and man, p. 195-204. In J. Seckcbach (ed.), Enigmatic microorganisms and life in extreme environments. Kluwer Academic Publishers, Dordrecht, The Netherlands.
- 48.Kajander, E. O., and N. Çiftçioglu. 1998. Nanobacteria: an alternative mechanism for pathogenic intra- and extracellular calcification and stone formation. Proc. Natl. Acad. Sci. USA 95:8274-8279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kajander, E. O., and N. Çiftçioglu. 1999. Nanobacteria as extremophiles. Proc. SPIE Int. Soc. Opt. Eng. 3755:106-112. [Google Scholar]
- 50.Kajander, E. O., N. Çiftçioglu, M. A. Miller-Hjelle, and J. T. Hjelle. 2001. Nanobacteria: controversial pathogens in nephrolithiasis and polycystic kidney disease. Curr. Opin. Nephrol. Hypertens. 10:445-452. [DOI] [PubMed] [Google Scholar]
- 51.Kajander, E. O., T. Heinonen, I. Kuronen, K. Luoto, and N. Çiftçioglu. 1999. Nanobacteria, mycoplasma and bacterial L-forms; problems for sterile filtration, p. 279-292. In G. Wirtanen, S. Salo, and A. Mikkola (ed.), 30th R3 Nordic Contamination Control Symposium. VTT Technical Research Center of Finland, Espoo, Finland.
- 52.Kajander, E. O., I. Kuronen, K. Åkerman, A. Pelttari, and N. Çiftçioglu. 1997. Nanobacteria from blood, the smallest culturable autonomously replicating agent on earth. Proc. SPIE Int. Soc. Opt. Eng. 3111:420-428. [Google Scholar]
- 53.Kajander, E. O., E. Tahvanien, I. Kuronen, and N. Çiftçioglu. 1994. Comparison of staphylococci and novel bacteria-like particles from blood. Zentbl. Bakteriol. Parasitenkd. Infektkrankh. Hyg. Abt. 1 Orig. (Suppl.) 26:147-149. [Google Scholar]
- 54.Lewis, R. 2001. Antibiotic corrects genetic glitch. The Scientist 15:16. [Google Scholar]
- 55.Livermore, D., and J. D. Williams. 1996. β-Lactams: mode of action and mechanisms of bacterial resistance, p. 517. In V. Lorian (ed.), Antibiotics in laboratory medicine. The William & Wilkins Co., Baltimore, Md.
- 56.Miller, P. D. 1998. Efficacy and safety of cyclical etidronate therapy in the long term treatment of osteoporosis. Rev. Contemp. Pharmacother. 9:255-260. [Google Scholar]
- 57.Miller-Hjelle, M. A., J. T. Hjelle, N. Çiftçioglu, and E. Olavi Kajander. Nanobacteria. Methods for growth and identification of this recently discovered calciferous agent. In W. Olson (ed.). Rapid microbiological methods for the 21st century, in press. Davis Horwood International Publishers, Ltd., Raleigh, N.C.
- 58.Mintz, K. P., and P. M. Fives-Taylot. 2000. impA, a gene coding for an inner membrane protein, influences colonial morphology of Actinobacillus actinomycetemcomitans. Infect. Immun. 68:6580-6586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Montgomery, K., L. Raymundo, and W. L. Drew. 1979. Chromogenic cephalosporin spot test to detect beta-lactamase in clinically significant bacteria. J. Clin. Microbiol. 9:205-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mundy, G. R. 1999. Bisphosphonates as anticancer drugs. Expert Opin. Investig. Drugs 8:2009-2015. [DOI] [PubMed] [Google Scholar]
- 61.National Committee for Clinical Laboratory Standards. 2000. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 5th ed., vol. 17, no. 2. Approved standard M7-A4. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 62.O'Callaghan, C. H., A. Morris, S. Kirby, and A. Shingler. 1972. A novel method for detection of beta-lactamase b using a chromogenic cephalosporin substrate. Antimicrob. Agents Chemother. 1:283-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Okazaki, M., and R. Z. LeGeros. 1996. Properties of heterogeneous apatites containing magnesium, fluoride, and carbonate. Adv. Dent. Res. 10:252-259. [DOI] [PubMed] [Google Scholar]
- 64.Organic Materials Review Institute. 2001. TAP reviews compiled by Organic Materials Review Institute for the National Organic Standards Board, p. 1-11. [Online.] http://www.omri.org/hydroxyquinoline_sulfate.pdf.
- 65.Peterson, A., R. E. W. Hancock, and E. McGroaty. 1985. Binding of polycationic antibiotics and polyamines to lipopolysaccharide of Pseudomonas aeruginosa. J. Bacteriol. 164:1256-1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Price, P. A., S. A. Faux, and M. K. Williamson. 2001. Bisphosphonates alendronate and ibandronate inhibit artery calcification at doses comparable to those that inhibit bone resorption. Arterioscler. Thromb. Vasc. Biol. 21:817-824. [DOI] [PubMed] [Google Scholar]
- 67.Robards, A. W., and A. J. Wilson (ed.). 1993. Basic biological preparation techniques for TEM, p. 84. In Procedures in electron microscopy. John Wiley & Sons, Chichester, United Kingdom.
- 68.Rodan, G. A., and J. T. Martin. 2000. Therapeutic approaches to bone diseases. Science 289:1508-1514. [DOI] [PubMed] [Google Scholar]
- 69.Ryan, M. E., S. Ramamurthy, and L. M. Golub. 1996. Matrix metalloproteinases and their inhibition in periodontal treatment. Curr. Opin. Periodontol. 3:85-96. [PubMed] [Google Scholar]
- 70.Selikowitz, S. M., and C. A. Olsson. 1976. Effect of tetracycline on calcium oxalate calculi: in vivo and in vitro studies. Investig. Urol. 14:124-127. [PubMed] [Google Scholar]
- 71.Simor, A., S. Ferro, and D. Low. 1989. Comparative in vitro activities of six new fluoroquinolones and other oral antimicrobial agents against Campylobacter pylori. Antimicrob. Agents Chemother. 37:473-481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Streckfuss, J. L., W. N. Smith, L. R. Brown, and M. M. Campbell. 1974. Calcification of selected strains of Streptococcus mutans and Streptococcus sanguis. J. Bacteriol. 120:502-506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tanner, G. A. 1998. Potassium citrate/citric acid intake improves renal function in rats with polycystic kidney disease. J. Am. Soc. Nephrol. 9:1242-1248. [DOI] [PubMed] [Google Scholar]
- 74.Vaessen, C., T. Roumeguere, J. Simon, and C. Schulman. 1997. Medical treatment of ureteral calculi. Acta Urol. Belg. 65:19-22. [PubMed] [Google Scholar]
- 75.Vali, H., M. D. McKee, N. Çiftçioglu, S. K. Sears, F. L. Plows, E. Chevet, P. Ghiasi, M. Plavsic, E. O. Kajander, and R. N. Zare. 2001. Nanoforms: a new type of protein-associated mineralization. Geochim. Cosmochim. Acta 65:63-74. [Google Scholar]
- 76.Van Dijk, S., D. D. Dean, Y. Zhao, J. M. Cirgwin, Z. Schwartz, and B. D. Boyan. 1998. Purification, amino acid sequence, and cDNA sequence of novel calcium-precipitating proteolipids involved in calcification of Corynebacterium matruchotii. Calcif. Tissue Int. 62:350-358. [DOI] [PubMed] [Google Scholar]
- 77.Verhoef, K., G. Marzio, W. Hillen, H. Bujard, and B. Berkhout. 2001. Strict control of human immunodeficiency virus type 1 replication by a genetic switch: tet for tat. J. Virol. 75:979-987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Vogel, J. J., and W. N. Smith. 1976. Calcification of membranes isolated from Bacterionema matruchotii. J. Dent. Res. 55:1080-1083. [DOI] [PubMed] [Google Scholar]
- 79.von Wittenau, M. S. 1968. Some pharmacokinetic aspects of doxycycline metabolism in man. Chemother. Suppl. 13:41-50. [DOI] [PubMed] [Google Scholar]
- 80.Wainwright, M. 1999. Nanobacteria and associated ‘elementary bodies’ in human disease and cancer. Microbiology 145:2623-2624. [DOI] [PubMed] [Google Scholar]
- 81.Wiessner, J. H., A. T. Hasegawa, L. Y. Hung, G. S. Mandel, and N. S. Mandel. 2001. Mechanisms of calcium oxalate crystal attachment to injured renal collecting duct cells. Kidney Int. 59:637-644. [DOI] [PubMed] [Google Scholar]
- 82.Yokochi, T., K. Narita, A. Morikawa, K. Takahashi, Y. Kato, T. Sugiyama, N. Koide, M. Kawai, M. Fukada, and T. Yoshida. 2000. Morphological change in Pseudomonas aeruginosa following antibiotic treatment of experimental infection in mice and its relation to susceptibility to phagocytosis and to release of endotoxin. Antimicrob. Agents Chemother. 44:205-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhanel, G. G., D. J. Hoban, and G. K. M. Harding. 1992. Subinhibitory antibiotic concentrations: a review of the in vitro and in vivo data. Can. J. Infect. Dis. 3:193-201. [DOI] [PMC free article] [PubMed] [Google Scholar]