Abstract
The effects of a series of dihydroethano- and ethenoanthracene derivatives on chloroquine (CQ) accumulation in CQ-susceptible strain 3D7 and CQ-resistant clone W2 were assessed. The levels of CQ accumulation increased little or none in CQ-susceptible strain 3D7 and generally increased markedly in CQ-resistant strain W2. At 10 μM, 28 compounds yielded cellular accumulation ratios (CARs) greater than that observed with CQ alone in W2. At 10 μM, in strain W2, 21 of 31 compounds had CQ CARs two or more times higher than that of CQ alone, 15 of 31 compounds had CQ CARs three or more times higher than that of CQ alone, 13 of 31 compounds had CQ CARs four or more times higher than that of CQ alone, and 9 of 31 compounds had CQ CARs five or more times higher than that of CQ alone. At 1 μM, 17 of 31 compounds had CQ CARs two or more times higher than that of CQ alone, 12 of 31 compounds had CQ CARs three or more times higher than that of CQ alone, 6 of 31 compounds had CQ CARs four or more times higher than that of CQ alone, and 3 of 31 compounds had CQ CARs five or more times higher than that of CQ alone. At 1 μM, 17 of 31 compounds were more potent inducers of CQ accumulation than verapamil and 12 of 31 compounds were more potent inducers of CQ accumulation than promethazine. The nature of the basic group seems to be associated with increases in the levels of CQ accumulation. At 1 and 10 μM, 10 of 14 and 13 of 14 compounds with amino group (amines and diamines), respectively, had CARs ≥3, while at 1 and 10 μM, only 1 of the 13 derivatives with amido groups had CARs ≥3. Among 12 of the 31 compounds which were more active inducers of CQ accumulation than promethazine at 1 μM, 10 had amino groups and 1 had an amido group.
Chloroquine (CQ) has been one of the most successful antimalarial drugs ever developed. During the past 20 years, strains of Plasmodium falciparum have become resistant to CQ and other antimalarial drugs (49, 57). The universal success of CQ before the development of resistance has led to investigations of the mode of action of CQ and its mechanisms of resistance. CQ reaches the parasite food vacuole, where it accumulates due to the local acid pH and the weak base properties of the drug (59). The activity of CQ depends on a high level of accumulation of the drug within the parasite, and drug resistance is indicated by reduced levels of drug accumulation.
Some interesting observations suggest that the mechanism of malarial CQ resistance may be similar to the mechanism of mammalian multidrug resistance (MDR). In cancer cell lines, the resistance-reversing actions of chemosensitizing drugs have been fairly well characterized. MDR is defined as the MDR conferred on cell lines by increased levels of expression of the human MDR-1 protein (P-glycoprotein) or closely related animal equivalents (41). Resistant parasites actively expel CQ (35), probably by means of a transporter encoded by an MDR gene (19). Two genes, pfmdr1 and pfmdr2, that are homologues of the mammalian MDR genes were cloned from P. falciparum (27, 60). Pgh-1, the product of pfmdr1, was found to be localized in the food vacuole membrane (19), suggesting that it could be involved in drug transport across this membrane (33). It was initially suggested that Pgh-1 pumps CQ out of the food vacuole and is expressed in CQ-resistant parasites (35). Nevertheless, the level of resistance does not correlate with the level of Pgh-1 expression (58). In addition, selection of CQ resistance is associated with a decrease in the number of copies of pfmdr1 (4). Another possibility could be that Pgh-1 pumps CQ into the food vacuole. This notion would agree with the idea that drug resistance may be due to mutations in pfmdr1. It is incompatible with the demonstration that the gene for the CQ resistance phenotype does not segregate with pfmdr1 in a genetic cross of CQ-susceptible and CQ-resistant strains (56). The presence of a mutation in the tyrosine-86 allele was considered to be associated with CQ resistance (31). However, epidemiological studies conducted with a larger number of samples to provide a meaningful analysis found no correlation between a mutation in the tyrosine-86 allele and CQ resistance (7). Several studies failed to show any association between a mutation in Pgh-1 and CQ resistance (12). Although Pgh-1 may act as a drug pump, it does not seem to be involved in resistance to CQ. Pgh-1 might act as a chloride channel or as a modulator of such a channel (52). As a chloride channel, Pgh-1 may be constitutively expressed in the vacuolar membrane to allow the maximal conversion of the proton motive force of the vacuolar H+ pump to achieve an acid pH. The pH of the food vacuole affects the level of drug accumulation by proton trapping, as well as hemoglobin digestion and the crystallization of ferriprotoporphyrin into hemozoin, both of which are pH-dependent activities (45). A lower pH would lead to enhanced CQ accumulation and drug susceptibility, and drug resistance could therefore result from mutations in Pgh-1.
It has recently been shown that CQ resistance in a P. falciparum cross maps to a 36-kb segment of chromosome 7 (46). This segment accommodates cg2, a gene that encodes a unique protein which has been detected in the parasite cytosol, the parasitophorous space, and the food vacuole in association with hemozoin. This Cg2 molecule could therefore be implicated in CQ transport and in the inhibition of ferriprotoporphyrin IX polymerization. Polymorphisms in cg2 were highly associated with CQ resistance (8), but allelic modification experiments have ruled out a role for this gene in CQ resistance (25).
Recently, pfcrt, a gene with 13 exons, was identified near cg2 on chromosome 7 (26). This transmembrane protein localizes to the parasite digestive vacuole, the site of CQ action, where increased acidification of the compartment is associated with a point mutation in pfcrt (26). One mutation at position 76 was present in all resistant isolates and was absent from all susceptible isolates. pfcrt genotypes are strongly linked with in vitro and in vivo CQ resistance (3, 9, 18, 20, 22). The mutation in the threonine-76 allele of the protein encoded by pfcrt can be used as a marker in surveillance for CQ-resistant falciparum malaria. Mutations in Pfcrt appear to be associated with changes in vacuolar pH (26). Models of CQ resistance that incorporate the effects of mutations in pfcrt in different ways can be envisaged: the decrease in the vacuolar pH associated with mutations in pfcrt reduces the drug-heme interaction responsible for toxicity, and drug flux across the membrane of the digestive vacuole is directly altered by a structural change in the Pfcrt molecule itself or by an effect of Pfcrt on the functions of other molecules involved in the physiology of the digestive vacuole. Changes in pH are reported to have a strong effect on the conversion of soluble heme to insoluble aggregates (21, 23). The midpoint pH of this conversion is close to the vacuolar pH values of CQ-resistant parasites (50). The formation of insoluble heme is much more efficient at the more acid vacuolar pH values of CQ-resistant parasites. Acidification of the digestive vacuole would leave significantly less free heme available for the formation of toxic complexes with CQ.
The reversal of CQ resistance by compounds with little intrinsic antimalarial activities may become a chemotherapeutic alternative (38). Several compounds like verapamil (1, 35, 39, 43), desipramine (5, 6, 13, 17), and antihistaminic drugs (10, 40, 42) have been demonstrated in the past decade to have promising capabilities to reverse the CQ resistance in parasite isolates in vitro and in animal models (36, 40, 47). The reversal of CQ resistance by verapamil was proposed to occur by modulating the activity of the parasitic Na+-H+ exchanger via the calcium- and calmodulin-dependent pathway (44). Nevertheless, Bray et al. (16) provided definitive evidence that CQ uptake is determined by the binding of CQ to ferriprotoporphyrin IX. Recently, it has been shown that the digestive vacuole of a CQ-resistant strain is more acidic than the digestive vacuoles of CQ-susceptible parasites (23). Verapamil normalizes the vacuolar pH of the CQ-resistant parasites to a value near that measured for CQ-susceptible parasites (by increasing the pH) and has no effect on CQ-susceptible parasites (51). In fact, it seems that acidification of the digestive vacuole contributes to drug resistance via the effects that the pH has on the solubility of unpolymerized heme found in the vacuole (50). Reversers of CQ resistance increase the pH of acid vesicles where ferriprotoporphyrin IX is generated, and this increased pH therefore increases the affinity of CQ-ferriprotoporphyrin IX binding.
We previously synthesized and evaluated three dihydroethano- and ethenoanthracene compounds which showed synergistic activities when they were used in combination with CQ against CQ-resistant parasites (2). The aim of this study was to assess the capacities of these 3 compounds and 28 other analogue derivatives to increase the level of accumulation of CQ in CQ-resistant P. falciparum parasites.
MATERIALS AND METHODS
Strains of P. falciparum.
CQ-resistant clone W2 (from Indochina) and CQ-susceptible strain 3D7 (from Africa) were maintained in culture. When required for the drug assays, the cultures were synchronized by sorbitol lysis (37).
Drugs.
The synthesis of some of the dihydroanthracene derivatives used in this study was described previously (2, 28, 34).
Verapamil and promethazine were obtained from Sigma Chemical (St. Louis, Mo.). The drugs used for the reversal of resistance (referred to as reversal drugs; 1 and 10 μM) were prepared in RPMI 1640 medium (Life Technologies, Paisley, United Kingdom) buffered with 25 mM HEPES and 25 mM NaHCO3 containing 3 nM labeled CQ. Silicon oil 550 was purchased from Dow Corning (BDH Laboratory Supplies, Poole, United Kingdom). Radiolabeled CQ diphosphate ([3H]CQ; 26 Ci/mmol) was purchased from Amersham Pharmacia Biotech (Little Chalfont, United Kingdom).
The lipophilicities of the drugs tested were expressed by calculation of the log P value (determined with Pallas software [version 2.0; CompuDrug Chemistry Ltd.]).
Measurement of [3H]CQ uptake by parasitized erythrocytes.
The level of accumulation of [3H]CQ was measured essentially by the protocol of Bray et al. (16). Infected erythrocytes with a level of parasitemia of 2% and a hematocrit of 2% were suspended in RPMI 1640 medium buffered with 25 mM HEPES and 25 mM NaHCO3. Eppendorf microcentrifuge tubes were loaded with 400 μl of silicon oil 550, 1 ml of reaction buffer containing [3H]CQ and reversal drugs on top of the oil, and then 25 μl of an appropriately concentrated cell suspension with a hematocrit of 75%. The cell suspension was mixed with the reaction buffer, and the mixture was incubated for 1 h at 37°C in an atmosphere of 10% O2-6% CO2-84% N2 with 95% relative humidity. After 1 min of centrifugation at 13,000 × g, 100 μl of the buffer was processed for scintillation counting. The infected erythrocyte pellets were washed with distilled water, lysed with quaternary hydroxide-glacial acetic acid-hydrogen peroxide (5:5:2), and left in an oven at 50°C for 2 h. They were then processed for scintillation counting. The level of CQ accumulation is expressed as the cellular accumulation ratio (CAR), which is the ratio of the amount of radiolabeled CQ in parasites (amount of [3H]CQ in parasitized erythrocytes − amount of [3H]CQ in uninfected erythrocytes) to the amount of [3H]CQ in a similar volume of buffer after incubation (14). The same procedure was applied to uninfected erythrocytes from the same source as the erythrocytes used in the P. falciparum culture.
RESULTS
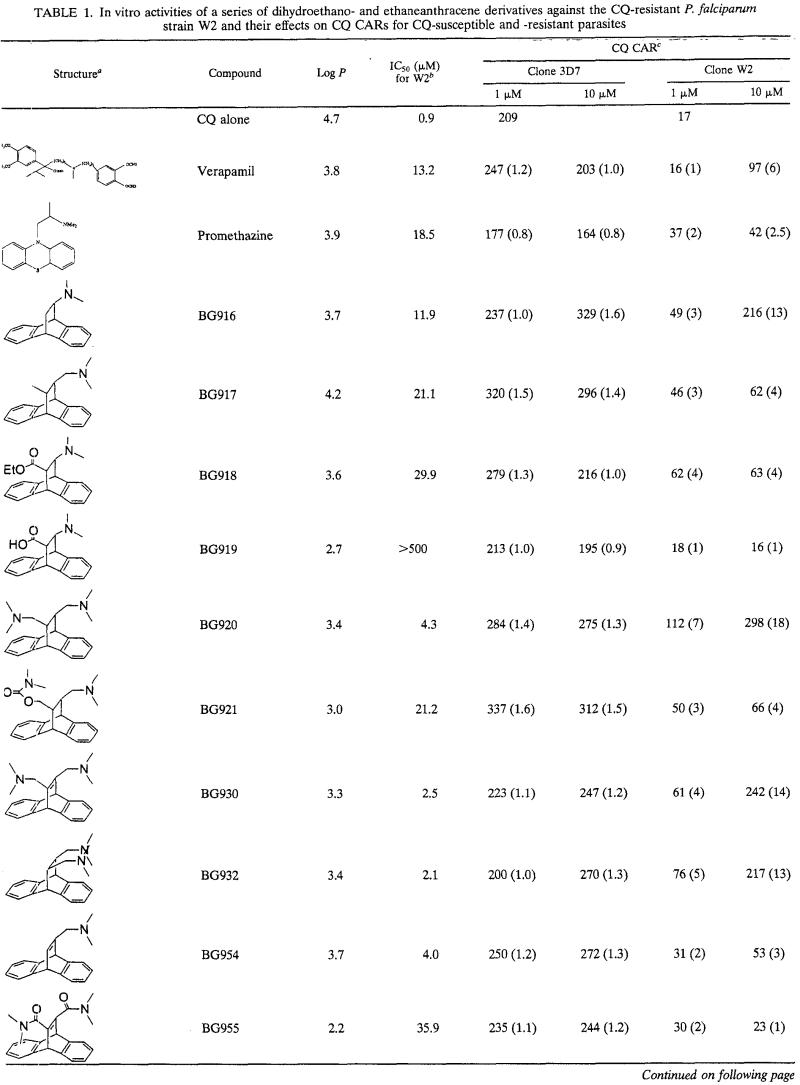
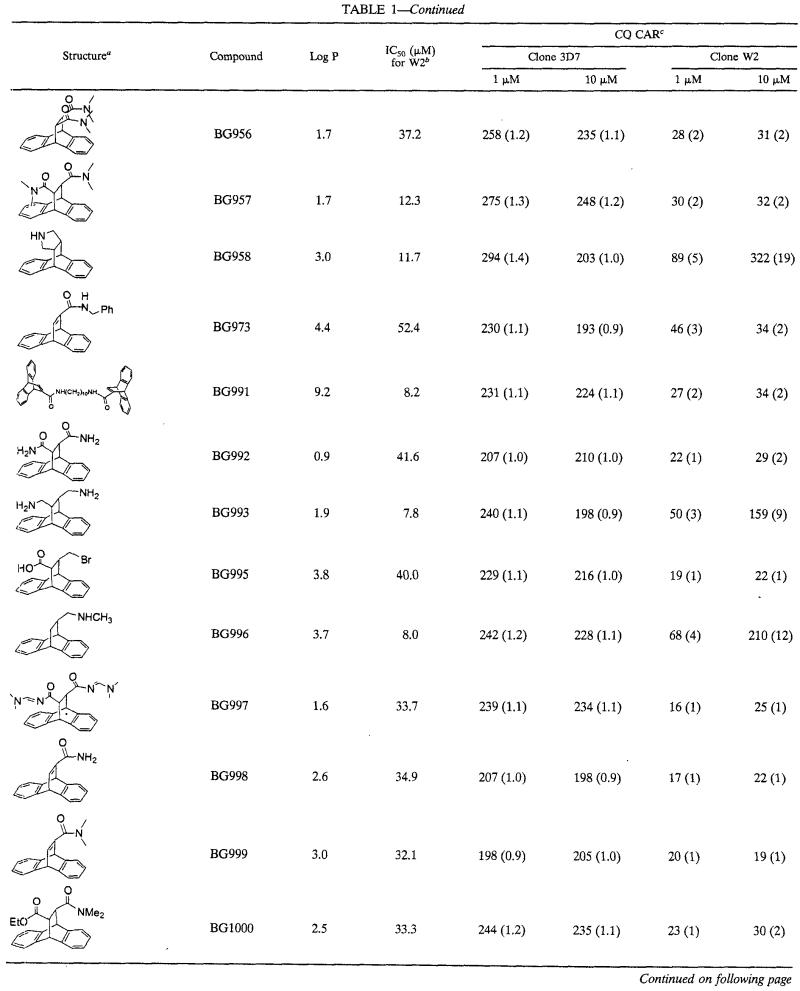
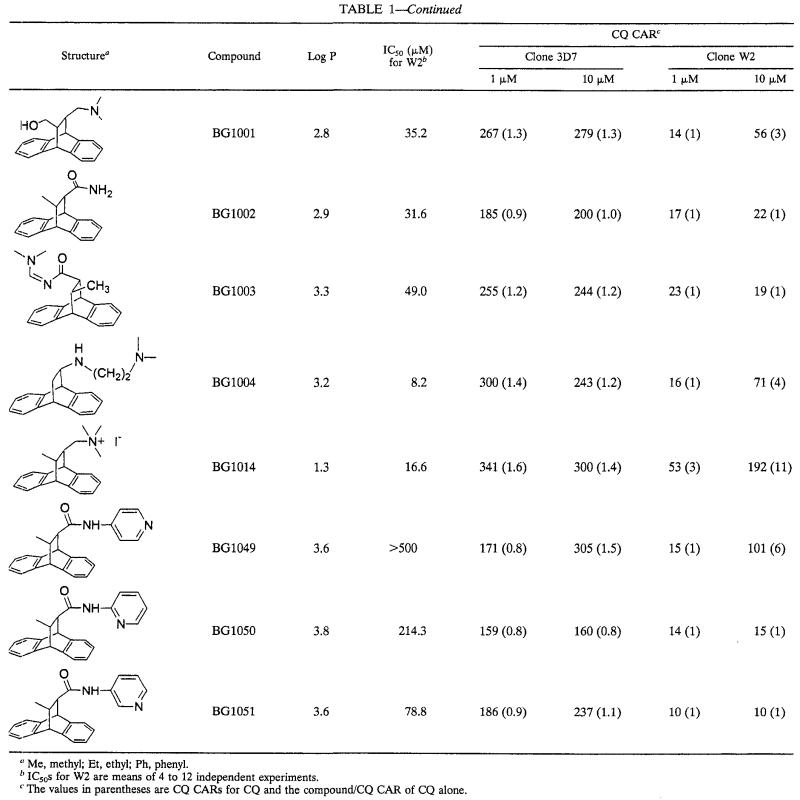
The series of dihydroanthracene derivatives had intrinsic antimalarial activities in vitro at concentrations that ranged from 2 to >500 μM. The CARs of CQ are summarized in Table 1. The levels of CQ accumulation increased little or none in CQ-susceptible strain 3D7 and generally increased markedly in CQ-resistant strain W2. At 10 μM, 28 compounds yielded CARs greater than those observed with CQ alone in W2. For statistical significance, it was considered that only compounds with CARs two or more times higher than that of CQ increased the level of accumulation of CQ. At 10 μM, in W2, 21 of 31 compounds had CQ CARs two or more times higher than that of CQ alone, 15 of 31 compounds had CQ CARs three or more times higher than that of CQ alone, 13 of 31 compounds had CQ CARs four or more times higher than that of CQ alone, and 9 of 31 compounds had CQ CARs five or more times higher than that of CQ alone (Table 1). At 1 μM, 17 of 31 compounds had CQ CARs two or more times higher than that of CQ alone, 12 of 31 compounds had CQ CARs three or more times higher than that of CQ alone, 6 of 31 compounds had CQ CARs four or more times higher than that of CQ alone, and 3 of 31 compounds had CQ CARs five or more times higher than that of CQ alone. At 1 μM, 17 of 31 compounds were more potent inducers of CQ accumulation than verapamil and 12 of 31 compounds were more potent inducers of CQ accumulation than promethazine. The nature of the basic group seems to be associated with increases in the levels of CQ accumulation. At 1 and 10 μM, 10 of 14 and 13 of 14 compounds with amino group (amines and diamines), respectively, had CARs ≥3 (Table 2). At 1 and 10 μM, only 1 of 13 derivatives with amido groups had CARs ≥3. Among 17 of the 31 compounds which were more potent inducers of CQ accumulation than verapamil at 1 μM, 11 had amino groups and 5 had amido groups. Among 12 of 31 compounds which were more active inducers of CQ accumulation than promethazine at 1 μM, 10 had amino groups and 1 had an amido group. The CARs obtained at concentrations of 1 and 10 μM are not correlated with lipophilicity (r = 0.093 and 0.011, respectively).
TABLE 1.
In vitro activities of a series of dihydroethano- and ethaneanthracene derivatives against the CQ-resistant P. falciparum strain W2 and their effects on CQ CARs for CQ-susceptible and -resistant parasites
Me, methyl; Et, ethyl; Ph, phenyl.
IC50s for W2 are means of 4 to 12 independent experiments.
The values in parentheses are CQ CARs for CQ and the compound/CQ CAR of CQ alone.
TABLE 2.
Effects at 1 and 10 μM of dihydroethano- and ethaneanthracene derivatives with substituted groups on CARs of CQ
| Substituted group | Drug | No. of compounds with CARs ≥3/total no.
|
|
|---|---|---|---|
| 1 μM | 10 μM | ||
| Secondary amine | BG958, BG996 | 2/2 | 2/2 |
| Tertiary amine | BG916, BG917, BG954 | 2/3 | 3/3 |
| Tertiary amine and alcohol | BG1001 | 0/1 | 1/1 |
| Tertiary amine and carboxylic acid | BG919 | 0/1 | 0/1 |
| Tertiary amine and ester | BG918 | 1/1 | 1/1 |
| Tertiary amine and carbamate | BG921 | 1/1 | 1/1 |
| Diamine | BG1004 | 0/1 | 1/1 |
| Primary diamine | BG993 | 1/1 | 1/1 |
| Tertiary diamine | BG920, BG930, BG932 | 3/3 | 3/3 |
| Primary amide | BG998, BG1002 | 0/2 | 0/2 |
| Secondary amide | BG973, BG1049, BG1050, BG1051 | 1/4 | 1/4 |
| Tertiary amide | BG999 | 0/1 | 0/1 |
| Tertiary amide and ester | BG1000 | 0/1 | 0/1 |
| Primary diamide | BG992 | 0/1 | 0/1 |
| Secondary diamine | BG991 | 0/1 | 0/1 |
| Tertiary diamide | BG955, BG956, BG957 | 0/3 | 0/3 |
| Quaternary ammonium | BG1014 | 1/1 | 1/1 |
| Carbamidine | BG1003 | 0/1 | 0/1 |
| Dicarbamidine | BG997 | 0/1 | 0/1 |
| Bromide and carboxylic acid | BG995 | 0/1 | 0/1 |
DISCUSSION
The activity of CQ depends on a high level of accumulation of the drug within the parasite, and drug resistance stems from reduced levels of drug accumulation. Differentials in uptake versus efflux of CQ by CQ-susceptible and CQ-resistant parasites have both been proposed as explanations for the lower levels of accumulation of CQ by CQ-resistant parasites, but the debate has not yet been settled (11, 15, 30, 54). Some investigators have concluded that drug resistance can be accounted for by a decrease in the level of CQ uptake by CQ-resistant parasites (15, 24, 54), while others believe that CQ-resistant parasites appear to counter the drug by expelling it rapidly via a pump-mediated mechanism (35).
We detected a 12-fold difference in the level of CQ accumulation between CQ-susceptible strain 3D7 and CQ-resistant W2 strain, which is consistent with previous findings (16, 39, 48). As expected, with verapamil and promethazine, no significant alteration in the levels of CQ uptake was observed in CQ-susceptible strain 3D7 (16, 39, 53, 55). The level of CQ accumulation increased little in strain 3D7 in assays with the 31 compounds, as expected. Our results are consistent with those of other reports demonstrating increased levels of CQ accumulation in the presence of verapamil and a single low extracellular concentration of CQ. Verapamil at 10 μM is able to increase the level of CQ uptake sixfold over that for the parasite control for CQ-resistant parasites, while at concentrations ranging from 2 to 10 μM, it increases the level of CQ accumulation two- to fivefold, as determined in previous works (14, 16, 32, 39, 55). Large increases in the levels of CQ accumulation in CQ-resistant parasites were detected for 15 compounds tested at 10 μM and 12 compounds tested at 1 μM, with CARs being ≥3, indicating that these compounds are more potent inducers of CQ accumulation than verapamil and promethazine. Lipophilicity does not seems to be important for the increase in the level of CQ accumulation in parasites. While most chemosensitizers bear little structural similarity to one another, several essential components are believed to play a role in the reversal of resistance. These include the presence of benzene groups, a secondary or tertiary nitrogen, and a cationic charge (29, 48). Most of our compounds have these characteristics. However, the presence of a secondary or a tertiary nitrogen is not enough to increase the level of CQ accumulation. The nature of the basic group seems to be associated with increases in the levels of CQ accumulation. At 1 and 10 μM, 10 of 14 and 13 of 14 compounds with amino group (amines and diamines), respectively, induced increases in the levels of CQ accumulation (CARs, ≥3), while only 1 of 13 of the derivatives with an amido group gave the same result. Among the 12 of 31 compounds which were more active inducers of CQ accumulation than promethazine at 1 μM, 10 had amino groups and 1 had an amido group. The presence of a secondary or a tertiary nitrogen is required to increase the level of CQ accumulation in CQ-resistant parasites, and the presence of one or two amino groups is especially required.
Some of these compounds, such as BG917, BG954, BG958 or BG920, and BG996, have previously been shown to have synergistic effects in combination with CQ (2; B. Pradines et al., submitted for publication). BG958, BG920, and BG996 were the most potent inducers of increases in CQ activity against CQ-resistant parasites. These three amino derivatives had CARs ≥4 when they were tested at 1 μM and CARs ≥12 when they were tested at 10 μM. BG917, whose ability to potentiate CQ activity is better than that of verapamil, had CARs between 3 and 4 when it was tested at 1 and 10 μM. BG954, which is a less potent inducer of CQ activity than verapamil, had CARs ≤3 when it was tested at 1 and 10 μM. The time has come to systematically relate all of the CQ CARs to the pharmacodynamic interaction between CQ and the compounds tested and to assess whether there is a correlation between the synergistic effects of the compounds and CQ on the activity of CQ and their capacities to increase the level of CQ accumulation.
These compounds, which possess a protonatable nitrogen at physiological pH, could act by ionic interactions with three potential targets: Pgh-1, Cg2, and Pfcrt. Our compounds with the ability to reverse CQ resistance are structural analogues of CQ. Our first hypothesis was to suppose that these derivatives might enhance the level of CQ accumulation and improve the ability of CQ to access ferriprotoporphyrin IX by binding in a competitive way to a CQ transmembrane transporter such as Pgh-1 or Pfcrt. This increased level of accumulation of CQ could be the result of the higher affinities of the dihydroethano- and ethenoanthracene compounds for the export transporter. The reversal mechanism would be assumed to result from competition between our derivatives and CQ for efflux translocation sites, thus causing an increase in the steady-state level of accumulation of CQ and hence a return to susceptibility. However, our data do not directly support the conclusion that dihydroethano- and ethenoanthracene compounds interact with proteins such as Pgh-1 or Pfcrt, and it is not yet known if our compounds directly compete for a CQ-binding site on drug transporters involved in P. falciparum resistance.
Assessment of the effects of the compounds tested on the level of CQ accumulation allowed the rapid screening of compounds which could show synergistic effects in combination with CQ. Some of these dihydroethano- and ethenoanthracene derivatives hold much promise as effective antimalarial agents for use in combination with CQ. It is therefore important to identify the target of these compounds and to assess their in vitro activities in combination with CQ, their in vitro toxicities, and their efficacies in animal models.
Acknowledgments
We thank S. A. Ward and P. G. Bray (Department of Pharmacology and Therapeutics, University of Liverpool, Liverpool, United Kingdom) for fruitful discussions.
This work was supported by the Délégation Générale pour l'Armement (contrat d'objectif 9810060). This work was carried out as a part of the COST B16 European Program.
REFERENCES
- 1.Adovelande, J., J. Deleze, and J. Schrevel. 1998. Synergy between two calcium channel blockers, verapamil and fantofarone (SR33557), in reversing chloroquine resistance in Plasmodium falciparum. Biochem. Pharmacol. 55:433-440. [DOI] [PubMed] [Google Scholar]
- 2.Alibert-Franco, S., C. Santelli-Rouvier, J. Barbe, B. Pradines, C. Houdouin, and D. Parzy. 1999. 9,10-(3′,4′-Pyrrolidino)-9,10-dihydroanthracene and structurally related compounds as synergistic antimalarial drugs. Heterocyclic Commun. 5:235-240. [Google Scholar]
- 3.Babiker, H. A., S. J. Pringle, A. Abdel-Muhsin, M. Mackinnon, P. Hunt, and D. Walliker. 2001. High-level chloroquine resistance in Sudanese isolates of Plasmodium falciparum is associated with mutations in the chloroquine resistance transporter gene pfcrt and the multidrug resistance gene pfmdr1. J. Infect. Dis. 183:1535-1538. [DOI] [PubMed] [Google Scholar]
- 4.Barnes, D. A., S. J. Foote, D. Galatis, D. J. Kemp, and A. F. Cowman. 1992. Selection for high level chloroquine resistance results in deamplification of pfmdr1 gene and increased sensitivity to mefloquine in Plasmodium falciparum. EMBO J. 11:3067-3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Basco, L. K., and J. Le Bras. 1990. Desipramine or cyproheptadine for reversing chloroquine resistance. Lancet 335:422. [DOI] [PubMed] [Google Scholar]
- 6.Basco, L. K., and J. Le Bras. 1990. Reversal of chloroquine resistance with desipramine in isolates of Plasmodium falciparum from Central and West Africa. Trans. R. Soc. Trop. Med. Hyg. 84:479-481. [DOI] [PubMed] [Google Scholar]
- 7.Basco, L. K., and P. Ringwald. 1998. Molecular epidemiology of malaria in Yaounde, Cameroon. III. Analysis of chloroquine resistance and point mutations in the multidrug resistance 1 (pfmdr1) gene of Plasmodium falciparum. Am. J. Trop. Med. Hyg. 59:577-581. [DOI] [PubMed] [Google Scholar]
- 8.Basco, L. K., and P. Ringwald. 1999. Molecular epidemiology of malaria in Yaounde, Cameroon. V. Analysis of the omega repetitive region of the Plasmodium falciparum CG2 gene and chloroquine resistance. Am. J. Trop. Med. Hyg. 61:807-813. [DOI] [PubMed] [Google Scholar]
- 9.Basco, L. K., and P. Ringwald. 2001. Analysis of the key pfcrt point mutation and in vitro and in vivo response to chloroquine, Yaoude, Cameroon. J. Infect. Dis. 183:1828-1831. [DOI] [PubMed] [Google Scholar]
- 10.Basco, L. K., P. Ringwald, and J. Le Bras. 1991. Chloroquine potentiating action of antihistaminics in Plasmodium falciparum in vitro. Ann. Trop. Med. Parasitol. 85:223-228. [DOI] [PubMed] [Google Scholar]
- 11.Bayoumi, R. A. L., H. A. Babiker, and D. E. Arnot. 1994. Uptake and efflux of chloroquine by chloroquine resistant Plasmodium falciparum clones recently isolated in Africa. Acta Trop. 58:141-149. [DOI] [PubMed] [Google Scholar]
- 12.Bhattacharya, P. R., S. Biswas, and L. Kabilan. 1997. Alleles of the Plasmodium falciparum pfmdr I gene appear not to be associated with chloroquine resistance in India. Trans. R. Soc. Trop. Med. Hyg. 91:454-455. [DOI] [PubMed] [Google Scholar]
- 13.Bitonti, A. J., A. Sjoerdsma, P. P. McCann, D. E. Kyle, A. M. J. Oduola, R. N. Rossan, W. K. Milhous, and D. E. Davidson. 1988. Reversal of chloroquine resistance in malaria parasite Plasmodium falciparum by desipramine. Science 242:1301-1303. [DOI] [PubMed] [Google Scholar]
- 14.Bray, P. G., M. K. Boulter, G. Y. Ritchie, R. E. Howells, and S. A. Ward. 1994. Relationship of global chloroquine transport and reversal of resistance in Plasmodium falciparum. Mol. Biochem. Parasitol. 63:87-94. [DOI] [PubMed] [Google Scholar]
- 15.Bray, P. G., R. E. Howells, G. Y. Pitchie, and S. A. Ward. 1992. Rapid chloroquine efflux phenotype in both chloroquine-sensitive and chloroquine-resistant Plasmodium falciparum. A correlation of chloroquine sensitivity with energy-dependent drug accumulation. Biochem. Pharmacol. 44:1317-1324. [DOI] [PubMed] [Google Scholar]
- 16.Bray, P. G., O. Janneh, K. J. Raynes, M. Munghthin, H. Ginsburgh, and S. A. Ward. 1999. Cellular uptake of chloroquine is dependent on binding to ferriprotoporphyrin IX and is independent of NHE activity in Plasmodium falciparum. J. Cell Biol. 145:363-376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carosi, G., S. Caligaris, G. Fadat, F. Castelli, A. Matteelli, D. Komka-Bemba, and G. Roscigno. 1991. Reversal of chloroquine resistance of wild isolates of Plasmodium falciparum by desipramine. Trans. R. Soc. Trop. Med. Hyg. 85:723-724. [DOI] [PubMed] [Google Scholar]
- 18.Cooper, R. A., M. T. Ferdig, X. Z. Su, L. M. Ursos, J. Mu, T. Nomura, H. Fujioka, D. A. Fidock, P. D. Roepe, and T. E. Wellems. 2002. Alternative mutations at position 76 of the vacuolar transmembrane protein Pfcrt are associated with chloroquine resistance and unique stereospecific quinine and quinidine responses in Plasmodium falciparum. Mol. Pharmacol. 61:35-42. [DOI] [PubMed] [Google Scholar]
- 19.Cowman, A. F., S. Karze, D. Galatis, and J. G. Culvenor. 1991. A P-glycoprotein homologue of Plasmodium falciparum is isolated on the digestive vacuole. J. Cell Biol. 113:1033-1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Djimdé, A., O. K. Doumbo, J. F. Cortese, K. Kayentao, S. Doumbo, Y. Diourté, A. Dicko, X. Su, T. Nomura, D. A. Fidock, T. E. Wellems, and C. V. Plowe. 2001. A molecular marker for chloroquine-resistant falciparum malaria. N. Engl. J. Med. 344:257-263. [DOI] [PubMed] [Google Scholar]
- 21.Dorn, A., S. R. Vippagunta, H. Matile, C. Jaquet, J. L. Vennerstrom, and R. G. Ridley. 1998. An assessment of drug-haematin binding as a mechanism for inhibition of haematin polymerization by quinoline antimalarials. Biochem. Pharmacol. 55:727-736. [DOI] [PubMed] [Google Scholar]
- 22.Durand, R., S. Jafari, J. Vauzelle, J. F. Delabre, Z. Jesic, and J. Le Bras. 2001. Analysis of pfcrt point mutations and chloroquine susceptibility in isolates of Plasmodium falciparum. Mol. Biochem. Parasitol. 114:95-102. [DOI] [PubMed] [Google Scholar]
- 23.Dzekunov, S. M., L. M. B. Ursos, and P. D. Roepe. 2000. Digestive vacuolar pH of intact intraerythrocytic P. falciparum either sensitive or resistant to chloroquine. Mol. Biochem. Parasitol. 110:107-124. [DOI] [PubMed] [Google Scholar]
- 24.Ferrari, V., and D. J. Cutler. 1991. Simulation of kinetic data on the influx and efflux of chloroquine by erythrocytes infected with P. falciparum. Evidence for a drug-importer in chloroquine sensitive strains. Biochem. Pharmacol. 42:67-79. [DOI] [PubMed] [Google Scholar]
- 25.Fidock, D. A., T. Nomura, R. A. Cooper, X. Su, A. K. Talley, and T. E. Wellems. 2000. Allelic modification of cg2 and cg1 genes do not alter the chloroquine response of drug-resistant Plasmodium falciparum. Mol. Biochem. Parasitol. 110:1-10. [DOI] [PubMed] [Google Scholar]
- 26.Fidock, D. A., T. Nomura, A. K. Talley, R. A. Cooper, S. M. Dzekunov, M. T. Ferdig, L. M. B. Ursos, A. bir Singh Sidhu, B. Naudé, K. W. Deitsch, X. Su, J. C. Wooton, P. D. Roepe, and T. E. Wellems. 2000. Mutations in the P. falciparum digestive vacuole transmembrane protein PfCRT and evidence for their role in chloroquine resistance. Mol. Cell 6:861-871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Foote, S. J., J. K. Thompson, A. F. Cowman, and D. J. Kemp. 1989. Amplification of the multidrug resistance gene in some chloroquine resistant isolates of P. falciparum. Cell 57:921-930. [DOI] [PubMed] [Google Scholar]
- 28.Fruzinski, A., J. Karolak-Wojciechowska, S. Alibert-Franco, C. Santelli-Rouvier, and J. Barbe. 1999. Synthesis and molecular structures of 11-benzylamido-9,10-dihydro-9,10-ethenoanthracenes. J. Chem. Crystallogr. 29:1201-1204. [Google Scholar]
- 29.Gerena, L., G. T. S. Bass, D. E. Kyle, A. M. Oduola, W. K. Milhous, and R. K. Martin. 1992. Fluoxetine hydrochloride enhances in vitro susceptibility to chloroquine in resistant Plasmodium falciparum. Antimicrob. Agents Chemother. 36:2761-2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ginsburg, H., and M. Krugliak. 1992. Quinoline-containing antimalarials--mode of action, drug resistance and its reversal. An update with unresolved puzzles. Biochem. Pharmacol. 43:63-70. [DOI] [PubMed] [Google Scholar]
- 31.Grobusch, M. P., I. S. Adagu, P. G. Kremsner, and D. C. Warhurst. 1998. Plasmodium falciparum: in vitro chloroquine susceptibility and allele-specific PCR detection of pfmdr1 (Asn)86(Tyr) polymorphism in Lamberene, Gabon. Parasitology 116:211-217. [DOI] [PubMed] [Google Scholar]
- 32.Haruki, K., P. G. Bray, M. Ono, and S. A. Ward. 2000. Potent enhancement of the sensitivity of Plasmodium falciparum to chloroquine by the bisbenzylisoquinoline alkaloid cepharanthin. Antimicrob. Agents Chemother. 44:2706-2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Karcz, S. R., D. Galatis, and A. F. Cowman. 1993. Nucleotide binding properties of a P-glycoprotein homologue from Plasmodium falciparum. Mol. Biochem. Parasitol. 58:269-276. [DOI] [PubMed] [Google Scholar]
- 34.Karolak-Wojciechowska, J., H. B. Trzezwinska, S. Alibert-Franco, C. Santelli-Rouvier, and J. Barbe. 1998. The crystal and molecular structures of 9,10-dihydro-9,10-ethano and ethenoanthracenes. J. Chem. Crystallogr. 28:905-911. [Google Scholar]
- 35.Krogstad, D. J., I. Y. Gluzman, D. E. Kyle, A. M. J. Oduola, S. K. Martin, W. K. Milhous, and P. H. Schlesinger. 1987. Efflux of chloroquine from Plasmodium falciparum: mechanism of chloroquine resistance. Science 238:1283-1285. [DOI] [PubMed] [Google Scholar]
- 36.Kyle, D. E., W. K. Milhous, and R. N. Rossan. 1993. Reversal of Plasmodium falciparum resistance to chloroquine in Panamanian Aotus monkeys. Am. J. Trop. Med. Hyg. 48:126-133. [DOI] [PubMed] [Google Scholar]
- 37.Lambros, C., and J. P. Vanderberg. 1979. Synchronization of Plasmodium falciparum erythrocytic stages in culture. J. Parasitol. 55:418-420. [PubMed] [Google Scholar]
- 38.Martin, S. K., A. M. J. Oduola, and W. K. Milhous. 1987. Reversal of chloroquine resistance in Plasmodium falciparum by verapamil. Science 235:899-901. [DOI] [PubMed] [Google Scholar]
- 39.Martiney, J. A., A. Cerami, and A. F. G. Slater. 1995. Verapamil reversal of chloroquine resistance in the malaria parasites Plasmodium falciparum is specific for resistant parasites and independent of weak base effect. J. Biol. Chem. 270:22393-22398. [DOI] [PubMed] [Google Scholar]
- 40.Oduola, A. M. J., A. Sowunmi, W. K. Milhous, T. G. Brewer, D. E. Kyle, L. Gerena, R. N. Rossan, L. A. Salako, and B. G. Schuster. 1998. In vitro and in vivo reversal of chloroquine resistance in Plasmodium falciparum with promethazine. Am. J. Trop. Med. Hyg. 58:625-629. [DOI] [PubMed] [Google Scholar]
- 41.Pedersen, P. L. 1995. Multidrug-resistance: a fascinating, clinically relevant problem in bioenergetics. J. Bioenerg. Biomembr. 27:3-5. [DOI] [PubMed] [Google Scholar]
- 42.Peters, W., R. Ekong, B. L. Robinson, and D. C. Warhurst. 1990. The chemotherapy of rodent malaria. XLV. Reversal of chloroquine resistance in rodent and human Plasmodium by antihistaminic agents. Ann. Trop. Med. Parasitol. 84:541-551. [DOI] [PubMed] [Google Scholar]
- 43.Ryall, J. C. 1987. Reversal of chloroquine resistance in falciparum malaria. Parasitol. Today 3:256. [DOI] [PubMed] [Google Scholar]
- 44.Sanchez, C. P., S. Wünsch, and M. Lanzer. 1997. Identification of a chloroquine importer in Plasmodium falciparum: differences in import kinetics are genetically linked with the chloroquine resistant phenotype. J. Biol. Chem. 272:2652-2658. [DOI] [PubMed] [Google Scholar]
- 45.Slater, A. F. G., and A. Cerami. 1992. Inhibition by chloroquine of a novel haem polymerase enzyme activity in malaria trophozoites. Nature 355:167-169. [DOI] [PubMed] [Google Scholar]
- 46.Su, X., L. A. Kirkman, H. Fujioka, and T. E. Wellems. 1997. Complex polymorphisms in a approximately 330 kDa protein are linked to chloroquine resistant Plasmodium falciparum in Southeast Asia and Africa. Cell 91:593-603. [DOI] [PubMed] [Google Scholar]
- 47.Tanabe, K., M. Kato, A. Izumo, A. Hagiwara, and S. Doi. 1990. Plasmodium chabaudi: in vivo effects of Ca2+ antagonists on chloroquine resistant and chloroquine sensitive parasites. Exp. Parasitol. 70:419-426. [DOI] [PubMed] [Google Scholar]
- 48.Taylor, D., J. C. Walden, A. H. Robins, and P. J. Smith. 2000. Role of the neurotransmitter reuptake-blocking activity of antidepressants in reversing chloroquine resistance in vitro in Plasmodium falciparum. Antimicrob. Agents Chemother. 44:2689-2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Trape, J. F., G. Pison, M. P. Preziosi, C. Enel, A. Desgrées du Loû, V. Delauney, B. Samb, E. Lagarde, J. F. Molez, and F. Simondon. 1998. Impact of chloroquine resistance on malaria mortality. C. R. Acad. Sci. 321:689-697. [DOI] [PubMed] [Google Scholar]
- 50.Ursos, L. M. B., K. F. Dubay, and P. D. Roepe. 2001. Antimalarial drugs influence the pH dependent solubility of heme via apparent nucleation phenomena. Mol. Biochem. Parasitol. 112:11-17. [DOI] [PubMed] [Google Scholar]
- 51.Ursos, L. M. B., S. M. Dzekunov, and P. D. Roepe. 2000. The effects of chloroquine and verapamil on digestive vacuolar pH of P. falciparum either sensitive or resistant to chloroquine. Mol. Biochem. Parasitol. 110:125-134. [DOI] [PubMed] [Google Scholar]
- 52.Van Es, H. H. G., H. Renkema, H. Aerts, and E. Schurr. 1994. Enhanced lysosomal acidification leads to increased chloroquine accumulation in CHO cells expressing the pfmdr1 gene. Mol. Biochem. Parasitol. 68:209-219. [DOI] [PubMed] [Google Scholar]
- 53.Van Schalkwyk, D. A., J. C. Walden, and P. J. Smith. 2001. Reversal of chloroquine resistance in Plasmodium falciparum using combinations of chemosensitizers. Antimicrob. Agents Chemother. 45:3171-3174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Verdier, F., J. Le Bras, F. Clavier, I. Hatin, and M. Blayo. 1985. Chloroquine uptake by P. falciparum infected human erythrocytes during in vitro cultures and its relationship to chloroquine resistance. Antimicrob. Agents Chemother. 27:561-564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Walter, R. D., M. Seth, and A. P. Bhaduri. 1993. Reversal of chloroquine resistance in Plasmodium falciparum by CDR 87/209 and analogues. Trop. Med. Parasitol. 44:5-8. [PubMed] [Google Scholar]
- 56.Wellems, T. E., L. J. Panton, I. Y. Gluzman, I. Y., V. E. do Rosario, R. W. Gwadz, A. Walker-Jonah, and D. J. Krogstad. 1990. Chloroquine resistance not linked to MDR-like genes in a Plasmodium falciparum cross. Nature 354:253-255. [DOI] [PubMed] [Google Scholar]
- 57.Wernsdorfer, W. H. 1991. The development and spread of drug-resistant malaria. Parasitol. Today 7:297-303. [DOI] [PubMed] [Google Scholar]
- 58.Wilson, C. M., S. K. Volkman, S. Thaithong, R. K. Martin, D. E. Kyle, W. K. Milhous, and D. F. Wirth. 1993. Amplification of pfmdr1 associated with mefloquine and halofantrine resistance in Plasmodium falciparum from Thailand. Mol. Biochem. Parasitol. 57:151-160. [DOI] [PubMed] [Google Scholar]
- 59.Yayon, A., Z. I. Cabantchik, and H. Ginsburg. 1985. Susceptibility of human malaria parasites to chloroquine is pH dependent. Proc. Natl. Acad. Sci. USA 82:2784-2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zalis, M. G., C. M. Wilson, Y. Zhang, and D. F. Wirth. 1993. Characterization of the pfmdr2 gene for P. falciparum. Mol. Biochem. Parasitol. 62:83-92. [DOI] [PubMed] [Google Scholar]