Abstract
The in vitro inhibition of wild-type human immunodeficiency virus (HIV) by combinations of lopinavir and six other protease inhibitors over a range of two-drug combination ratios was evaluated. Combinations of lopinavir with indinavir, nelfinavir, amprenavir, tipranavir, and BMS-232632 generally displayed an additive relationship. In contrast, a consistent, statistically significant synergistic inhibition of HIV type 1 replication with combinations of lopinavir and saquinavir was observed. Analysis of the combination indices indicated that lopinavir with saquinavir was synergistic over the entire range of drug combination ratios tested and at all levels of inhibition in excess of 40%. Cellular toxicity was not observed at the highest drug concentrations tested. These results suggest that administration of combinations of the appropriate dose of lopinavir with other protease inhibitors in vivo may result in enhanced antiviral activity with no associated increase in cellular cytotoxicity. More importantly, the observed in vitro synergy between lopinavir and saquinavir provides a theoretical basis for the clinical exploration of a novel regimen of lopinavir-ritonavir and saquinavir.
Combination therapy using two protease inhibitors (PIs), either with or without accompanying therapy with nucleoside reverse transcriptase inhibitors (NRTIs), has been utilized in both therapy-naïve (2, 7, 17) and therapy-experienced patients. The rationale for dual PI therapy includes both the nonoverlapping resistance profiles of some PIs and, in particular, the pharmacokinetic enhancement of most PIs by coadministration with ritonavir (RTV) (10). Unlike combination regimens containing agents that act by inhibition of different viral enzymes, mechanism-based synergy of PI combinations is unlikely, based on the competitive nature of inhibition of human immunodeficiency virus (HIV) protease. Thus, the in vitro antiviral interactions of PIs have generally been found to be additive (3, 6, 14). However, pharmacologic mechanisms possibly leading to either synergy or antagonism between PIs theoretically exist (e.g., competitive absorption and/or egress from cells, competitive binding to serum proteins). For example, in one study, the in vitro interactions between indinavir (IDV) and saquinavir (SQV) and between IDV and nelfinavir (NFV) were found to be antagonistic (14, 16; D. J. Manion, D. P. Merrill, T. C. Chou, and M. S. Hirsch, Abstr. 36th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 11, p. 186, 1996). In contrast, antiviral synergy was observed between RTV and SQV and between RTV and tipranavir (TPV) in the presence or absence of human serum (3; A. Molla, S. Vasavanonda, T. Chernyavskiy, J. Praestgaard, A. Hsu, T. Lin, E. Sun, W. Kohlbrenner, and D. Kempf, Program Abstr. 2nd Int. Workshop HIV Drug Resist. Treat. Strategies, abstr. 39, p. 27, 1998).
Lopinavir (LPV; ABT-378) is a novel peptidomimetic HIV protease inhibitor with approximately 10-fold greater in vitro potency than RTV in the presence of human serum (15). Pharmacokinetic studies in animals have demonstrated low bioavailability of LPV when dosed alone. In contrast, coadministration of LPV with RTV achieved trough plasma concentrations of LPV >75-fold in excess of its in vitro 50% inhibitory concentration (IC50) against wild-type viruses in the presence of 50% human serum (13). These pharmacokinetic properties are attributable to the rapid CYP3A-mediated metabolism of LPV and its inhibition by RTV. Consequently, LPV-RTV (LPV/r) produced a significant decline in plasma HIV RNA in both treatment-naïve and PI-experienced patients when combined with NRTIs and/or nonnucleoside reverse transcriptase inhibitors (13; S. Deeks, S. Brun, Y. Xu, K. Real, C. Benson, H. Kessler, R. Murphy, D. Wheeler, C. Hicks, J. Eron, J. Feinberg, R. Gulick, P. Sax, R. Stryker, S. Riddler, M. Thompson, M. King, A. Potthoff, A. Hsu, R. Bertz, A. Molla, H. Mo, D. Kempf, A. Japou, and E. Sun, 7th Conf. Retrovir. Opportunistic Infect., abstr. 532, p. 176, 2000). At the licensed dose of LPV/r (400/100 mg twice a day [b.i.d.]), the plasma levels of LPV exceed those of RTV by 15- to 20-fold (Kaletra package insert). Combined with the difference in in vitro potency between the two inhibitors, the contribution of RTV to antiviral activity in vivo is likely to be negligible. Thus, the antiviral activity of LPV/r is due to LPV (Kaletra package insert).
The in vitro interactions between LPV and other PIs have not been characterized. To assess the potential combinations of this inhibitor with other PIs, the antiviral activities of LPV alone or in combination with IDV, SQV, amprenavir (APV), NFV, and the experimental PIs TPV and BMS-232632 over a range of two-drug combination ratios were evaluated for evidence of synergy, additivity, or antagonism. In vitro anti-HIV type 1 IIIB activities of LPV alone or in combination with other PIs were determined using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) dye reduction assay in MT4 cells in the presence of 10% fetal calf serum (FCS) as described previously (Molla et al., 2nd Int. Workshop HIV Drug Resist. Treat. Strategies). Percent cytotoxicity was calculated for drug alone and all combinations as the amount of formazan produced in wells containing drug-treated, uninfected cells. Assays were conducted in three separate experiments with triplicates. Therefore, nine data points for each combination were used for analysis of the combination index (CI).
CIs as defined by Chou (5) and Chou and Talalay (4) were calculated for each fixed ratio of LPV and another PI. Batch effect was ignored in the statistical analysis, as there did not appear to be differences associated with batches. For the modeling of dose response for each PI alone and for each fixed ratio of two PIs in combination, the following sigmoid Emax model was used: fa = Cγ/(Cγ +IC50γ), where fa is as defined earlier, C is total drug concentration, IC50 is the median-effect drug concentration, and γ is the shape factor of the sigmoidal curve (4). It was chosen over the median-effect equation {log[fa/(1 − fa)] as a linear function of log(C)} to accommodate observed fa values greater than 1. To achieve homogeneity in variances of the response variable across different drug concentrations, square root-transformed fa values were fit to the square root of the sigmoid Emax model. The procedure NLIN of SAS version 8.0 (SAS/STAT User's Guide; SAS Institute, Gary, N.C.) was used to perform the model fitting. Once IC50 and γ were estimated for a fixed ratio of two PIs in combinations and for each of the two PIs alone, the CI was calculated for the ratio of the two PIs in combinations for a selected fa value, assuming mutual exclusivity of the two PIs. Confidence intervals of CIs were calculated by simulation-of-parameter estimates as suggested by Belen'kii and Schinazi (1). At each fa value, for each PI alone and for each fixed ratio of two PIs in combination, IC50 and γ estimates were simulated 2,000 times from a bivariate normal distribution with means equal to the actual estimates and the variance-covariance matrix equal to the asymptotic variance-covariance matrix estimate. This led to 2,000 simulated CI estimates at each fa value for each fixed ratio of two PIs in combination. An approximate 95% two-sided confidence interval for CI was calculated as follows: actual CI estimate ± (1.96 × SD), where SD is the standard deviation of the simulated CI estimate. The IC50 values for each PI alone are shown in Table 1. Inhibition by combinations of PIs was assessed at five fixed molar concentration ratios, which were selected based on the relative IC50 values. No cellular toxicity was observed with LPV alone or in combination with any PI at the highest concentrations tested.
TABLE 1.
Antiviral activity of PIs against wild-type virus determined in 10% FCS
| PI | IC50 ± SE (μM) |
|---|---|
| LPV | 0.033 ± 0.0006 |
| SQV | 0.023 ± 0.0011 |
| IDV | 0.046 ± 0.0016 |
| APV | 0.120 ± 0.0054 |
| NFV | 0.054 ± 0.0026 |
| TPV | 0.376 ± 0.0134 |
| BMS-232632 | 0.008 ± 0.0003 |
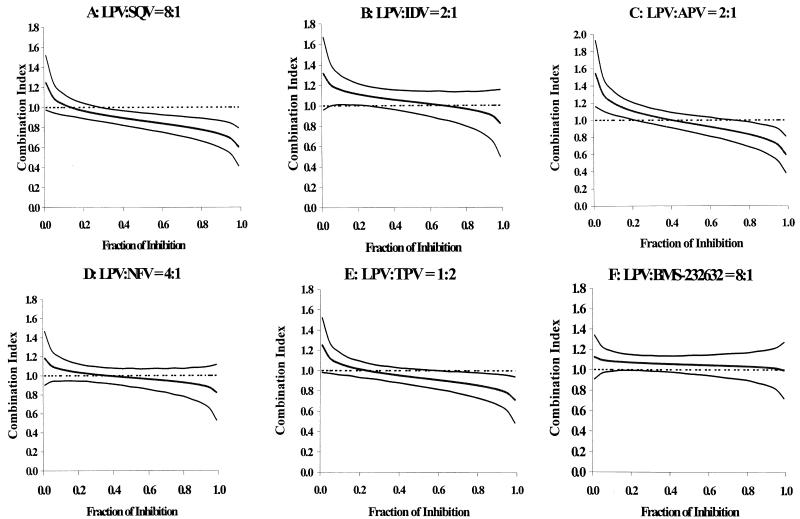
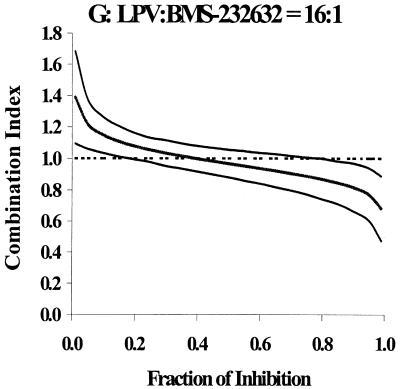
The concentration ratios for each PI combination are shown in Table 2, along with the ratios (LPV/other PI) of IC50 values determined for each PI alone in the present experiment. In each case, the range of concentration ratios chosen for the combination studies bracketed the actual IC50 ratio. The combination indices at 50, 75, 90, and 95% inhibition are provided in Table 2. The graphs of CI versus fraction of inhibition for combination ratios that most realistically reflect plasma concentration ratios that are likely to be observed clinically upon concomitant administration are provided in Fig. 1. If the 95% confidence interval overlapped a combination index of 1, the combined inhibition was judged to be additive. If the 95% confidence interval did not overlap a combination index of 1, the combined action was judged to be synergistic (upper bound of confidence interval, <1) or antagonistic (lower bound of confidence interval, >1). At one or more combination ratios, particularly at ≥75% inhibition, there was apparent synergy (upper 95% confidence interval, <1) between LPV and IDV, LPV and APV, LPV and TPV, and LPV and BMS-232632. However, for each of these four drug combinations, there were at least two concentration ratios for which synergy could not be demonstrated. Thus, the combination of LPV and IDV was not significantly different from additive at ratios of 2:1, 1:1, 1:3, and 1:5. Synergy was demonstrated at the 1:10 concentration ratio at 75, 90, and 95% inhibition levels. Similarly, a 2:1 ratio of LPV and APV produced a slightly synergistic effect at high inhibition levels, as did the combination of LPV with TPV (1:2 concentration ratio) and LPV with BMS-232632 (4:1 and 32:1 concentration ratios). Two other combination ratios of LPV and TPV were found to be synergistic at 50% inhibition or higher. Interestingly, the combination of LPV and NFV was found to be additive (4 concentration ratios) or antagonistic (1:1 concentration ratio). These results suggest that the virologic interaction between LPV and the above PIs is likely to be additive in vivo. However, pharmacokinetic interactions between LPV/r and the above PIs due to inhibition of metabolism by the RTV that is coformulated with LPV are likely to influence efficacy.
TABLE 2.
In vitro CIs for LPV and other PIs
| PI | Ratio | CI (95% confidence interval) for % inhibition ofa:
|
|||
|---|---|---|---|---|---|
| 50 | 75 | 90 | 95 | ||
| SQV | 0.5 | 0.86 (0.76, 0.97) | 0.79 (0.64, 0.94) | 0.72 (0.53, 0.92) | 0.68 (0.46, 0.91) |
| 1 | 0.88 (0.79, 0.98) | 0.82 (0.69, 0.95) | 0.76 (0.60, 0.93) | 0.72 (0.53, 0.91) | |
| 2 | 0.81 (0.73, 0.90) | 0.77 (0.64, 0.90) | 0.73 (0.57, 0.89) | 0.70 (0.52, 0.89) | |
| 4 | 0.85 (0.77, 0.94) | 0.77 (0.66, 0.88) | 0.70 (0.56, 0.84) | 0.66 (0.49, 0.82) | |
| 8 | 0.87 (0.79, 0.94) | 0.79 (0.69, 0.90) | 0.73 (0.59, 0.87) | 0.69 (0.53, 0.84) | |
| IDV | 0.1 | 0.94 (0.85, 1.03) | 0.83 (0.71, 0.94) | 0.73 (0.58, 0.88) | 0.67 (0.50, 0.83) |
| 0.2 | 0.99 (0.90, 1.08) | 0.94 (0.81, 1.07) | 0.89 (0.72, 1.07) | 0.86 (0.66, 1.07) | |
| 0.4 | 1.04 (0.94, 1.14) | 1.03 (0.88, 1.17) | 1.02 (0.83, 1.21) | 1.01 (0.77, 1.25) | |
| 0.8 | 1.01 (0.92, 1.10) | 0.97 (0.84, 1.10) | 0.93 (0.75, 1.10) | 0.90 (0.70, 1.10) | |
| 1.6 | 1.04 (0.93, 1.14) | 0.98 (0.83, 1.13) | 0.93 (0.72, 1.14) | 0.90 (0.64, 1.15) | |
| APV | 0.1 | 0.96 (0.86, 1.06) | 0.90 (0.76, 1.04) | 0.84 (0.66, 1.03) | 0.81 (0.59, 1.02) |
| 0.2 | 0.98 (0.88, 1.08) | 0.94 (0.81, 1.08) | 0.91 (0.72, 1.10) | 0.88 (0.67, 1.10) | |
| 0.4 | 1.00 (0.91, 1.09) | 1.04 (0.90, 1.18) | 1.08 (0.88, 1.28) | 1.10 (0.84, 1.36) | |
| 0.8 | 1.01 (0.91, 1.12) | 0.94 (0.80, 1.08) | 0.87 (0.69, 1.05) | 0.82 (0.62, 1.03) | |
| 1.6 | 0.96 (0.86, 1.06) | 0.86 (0.72, 0.99) | 0.77 (0.60, 0.93) | 0.71 (0.52, 0.90) | |
| NFV | 0.25 | 1.09 (0.97, 1.22) | 1.06 (0.88, 1.25) | 1.03 (0.77, 1.29) | 1.01 (0.71, 1.32) |
| 0.5 | 0.95 (0.86, 1.04) | 0.92 (0.79, 1.05) | 0.90 (0.72, 1.07) | 0.87 (0.67, 1.08) | |
| 1 | 1.11 (1.01, 1.21) | 1.20 (1.04, 1.36) | 1.30 (1.05, 1.56) | 1.38 (1.05, 1.71) | |
| 2 | 0.99 (0.90, 1.09) | 0.98 (0.84, 1.11) | 0.96 (0.77, 1.15) | 0.95 (0.72, 1.18) | |
| 4 | 0.98 (0.88, 1.08) | 0.94 (0.80, 1.08) | 0.90 (0.71, 1.09) | 0.87 (0.65, 1.10) | |
| TPV | 0.032 | 0.85 (0.79, 0.92) | 0.77 (0.69, 0.86) | 0.70 (0.59, 0.81) | 0.66 (0.54, 0.78) |
| 0.063 | 0.92 (0.84, 0.99) | 0.86 (0.77, 0.96) | 0.82 (0.69, 0.95) | 0.79 (0.63, 0.94) | |
| 0.125 | 0.99 (0.91, 1.07) | 0.99 (0.88, 1.10) | 0.99 (0.84, 1.15) | 1.00 (0.81, 1.19) | |
| 0.25 | 0.99 (0.90, 1.07) | 0.95 (0.84, 1.07) | 0.93 (0.77, 1.08) | 0.91 (0.72, 1.09) | |
| 0.50 | 0.93 (0.85, 1.01) | 0.87 (0.76, 0.99) | 0.82 (0.67, 0.97) | 0.78 (0.61, 0.96) | |
| BMS | 2 | 1.06 (0.97, 1.15) | 1.03 (0.90, 1.15) | 0.99 (0.82, 1.16) | 0.97 (0.76, 1.17) |
| 4 | 0.93 (0.86, 1.01) | 0.86 (0.76, 0.96) | 0.79 (0.66, 0.92) | 0.75 (0.60, 0.90) | |
| 8 | 1.05 (0.96, 1.14) | 1.04 (0.91, 1.16) | 1.02 (0.85, 1.20) | 1.01 (0.81, 1.22) | |
| 16 | 0.97 (0.88, 1.06) | 0.89 (0.77, 1.01) | 0.82 (0.66, 0.97) | 0.77 (0.60, 0.94) | |
| 32 | 0.91 (0.83, 1.00) | 0.82 (0.70, 0.93) | 0.73 (0.59, 0.87) | 0.68 (0.52, 0.84) | |
Bold text indicates those combinations tested for which the 95% confidence intervals did not bracket a combination index of 1.
FIG. 1.
Plots of combination indices (middle lines) ± 95% confidence intervals (upper and lower lines) versus fraction of inhibition for combinations of LPV and other PIs.
In sharp contrast to the other combinations, the in vitro interaction of LPV and SQV was statistically significantly synergistic at all five combination ratios tested, with 95% confidence intervals of <1 at combination concentrations producing 40% inhibition or higher (Table 2). CIs ranged from 0.66 to 0.87. Importantly, cellular toxicity was not observed at the highest concentrations of LPV and SQV tested, either alone or in combination. Thus, the observed antiviral synergy was not associated with any adverse effect on cell proliferation caused by the LPV-SQV combination. The mechanism of synergy involving LPV-SQV is not clear. Since both are competitive inhibitors of HIV protease and since HIV protease has only a single active site, synergy at the level of protease inhibition is highly unlikely. However, in vitro assays of antiviral activity are far more complex than enzyme inhibition assays, and it is possible that interactions that affect cellular penetration and/or cellular egress might be responsible for the synergy observed in the present experiments. To this end, SQV has been shown to be a substrate for the drug transporters Pgp and MRP1, and intracellular levels of SQV are increased in CD4 cells overexpressing the above proteins upon addition of specific inhibitors (9). The interaction of LPV with MRP1 has not been studied; however, LPV appears to be an inhibitor of Pgp in CACO-2 cells (E. Everitt, personal communication). Increased cellular concentrations of SQV in the presence of LPV might therefore account for the observed synergy. Pgp activity has been observed in primary CD4 T lymphocytes (8), although to our knowledge the levels of cellular egress pumps present in MT4 cells, the cell line used for this study, have not been characterized.
Antiviral synergy has previously been observed between RTV and TPV against RTV-resistant virus, whereas an additive to moderately synergistic antiviral effect was observed against RTV-sensitive virus (3). The combinations of NFV with RTV and NFV with SQV produced additive effects, whereas NFV with IDV displayed a slightly antagonistic interaction (14). Additive interactions were also observed when combining BMS-186,318 with either SQV or IDV (6), and antagonistic interactions were observed when combining NFV or SQV with IDV (14, 16; Manion et al., 36th ICAAC). We previously observed synergy between RTV and SQV in vitro (Molla et al., 2nd Int. Workshop HIV Drug Resist. Treat. Strategies).
This study has several limitations. First, the interaction studies were conducted in a single transformed cell line (MT4) that, although lymphocyte derived, may have different cellular pharmacological characteristics than primary peripheral blood mononuclear cells, the major target of HIV infection in vivo. Second, the interaction studies described here were conducted for a single wild-type laboratory strain of HIV and the generality of the results to wild-type or resistant clinical strains is unknown. Despite the above limitations, the observation of the different degree of interaction between LPV and SQV, compared to that between LPV and the other PIs studied, suggests that the in vivo combination of LPV/r and SQV is unlikely to be antagonistic, and thus it should be studied. A third limitation to the present study is that the interactions were studied in the presence of 10% FCS but no human serum. The addition of 50% human serum has been shown to markedly attenuate the in vitro activity of several PIs, including SQV and, to a lesser extent, LPV (12). Nonetheless, if the mechanism for the observed synergy between LPV and SQV is intracellular, protein binding by FCS and human serum, which are extracellular, should influence the observed potency of the combined PIs but not the degree of interaction. Synergy might be observed as a consequence of competitive binding to FCS by LPV and SQV in combination, producing higher free concentrations of one or both inhibitors. However, the LPV is approximately 96% bound to 10% FCS (D. Hickman, S. Vasavanonda, G. Nequist, C. Sanneman, J. Schmidt, R. Bertz, H. Mo, A. Molla, K. Marsh, S. Roberts, R. Granneman, D. Kempf, and A. Hsu, Abstr. 41st Intersci. Conf. Antimicrob. Agents Chemother., abstr. I-1740, 2001), and saturation of binding to FCS by either LPV or SQV at the relatively low inhibitor concentrations used in these experiments is unlikely.
A positive interaction between LPV and SQV in vivo might be manifested in several ways. Administration of coformulated LPV/r (Kaletra) at 400/100 mg b.i.d. with a single dose of SQV (Fortovase) at 800 mg b.i.d. produced a positive pharmacokinetic interaction and elevated mean trough plasma concentrations of SQV by ca. 3.5-fold to 0.32 μg/ml (A. Hsu, R. Bertz, E. Ashbrenner, W. Lam, S. Schweitzer, K. Rynkiewicz, K. Erdman, P. Chen, R. Brooks, Q. Ji, P. Bryan, L. Williams, S. Dennis, A. Japour, B. Bernstein, G. R. Granneman, and E. Sun, First Int. Workshop Clin. Pharmacol. HIV Ther., abstr. 2.4, 2000). Preliminary results suggest that viral isolates from patients who previously failed therapy with another PI and then subsequently failed LPV/r therapy remain susceptible to SQV (A. Molla, S. Brun, K. Garren, H. Mo, B. Richards, T. Marsh, J. Sylte, M. King, L. Han, E. Sun, and D. Kempf, 5th Int. Workshop HIV Drug Resist. Treat. Strategies, abstr. 64, 2001). These results, combined with apparently dissimilar patterns of phenotypic resistance to LPV and SQV among isolates from patients failing multiple PI therapy (11), suggest that the resistance pathways of LPV and SQV are substantially nonoverlapping. These observations, combined with the in vitro synergy between LPV and SQV observed in this study, provide a theoretical basis for the clinical exploration of a novel regimen of LPV/r and SQV.
REFERENCES
- 1.Belen'kii, M. S., and R. F. Schinazi. 1994. Multiple drug effect analysis with confidence interval. Antivir. Res. 25:1-11. [DOI] [PubMed] [Google Scholar]
- 2.Cameron, D. W., A. J. Japour, Y. Xu, A. Hsu, J. Mellors, C. Farthing, C. Cohen, D. Poretz, M. Markowitz, S. Follansbee, J. B. Angel, D. McMahon, D. Ho, V. Devanarayan, R. Rode, M. Salgo, D. J. Kempf, G. R. Granneman, J. Leonard, and E. Sun. 1999. Ritonavir and saquinavir combination therapy for the treatment of HIV infection. AIDS 13:213-224. [DOI] [PubMed] [Google Scholar]
- 3.Chong, K. T., and P. J. Pagano. 1997. In vitro combination of PNU-140690, a human immunodeficiency virus type 1 protease inhibitor, with ritonavir against ritonavir-sensitive and -resistant clinical isolates. Antimicrob. Agents Chemother. 41:2367-2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chou, T. C., and P. Talalay. 1984. Quantitative analysis of dose effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv. Enzyme Regul. 22:27-55. [DOI] [PubMed] [Google Scholar]
- 5.Chou, T. C. 1991. The median-effect principle and the combination index for quantitation of synergism, p. 65-69. In T. C. Chou and D. C. Rideout (ed.), Synergism and antagonism in chemotherapy. Academic Press, San Diego, Calif.
- 6.Deminie, C. A., C. M. Bechtold, D. Stock, M. Alam, F. Diang, A. H. Balch, T.-C. Chou, M. Prichard, R. Colonno, and P.-F. Lin. 1996. Evaluation of reverse transcriptase and protease inhibitors in two-drug combinations against human immunodeficiency virus replication. Antimicrob. Agents Chemother. 40:1346-1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gisolf, E. H., S. Jurriaans, J. Pelgrom, F. van Wanzeele, M. E. van der Ende, M. J. Borst, F. de Wolf, A. J. Japour, and A. S. Danner. 2000. The effect of treatment intensification in HIV infection: a study comparing treatment with ritonavir/saquinavir and ritonavir/saquinavir/stavudine. AIDS 14:405-413. [DOI] [PubMed] [Google Scholar]
- 8.Huisman, M. T., J. W. Smit, and A. H. Schinkel. 2000. Significance of P-glycoprotein for the pharmacology and clinical use of protease inhibitors. AIDS 14:237-242. [DOI] [PubMed] [Google Scholar]
- 9.Jones, K., P. G. Bray, S. H. Khoo, R. A. Davey, E. R. Meaden, S. A. Ward, and D. J. Back. 2001. P-glycoprotein and transporter MRP1 reduce HIV protease inhibitor uptake in CD4 cells: potential for accelerated viral drug resistance. AIDS 15:1353-1358. [DOI] [PubMed] [Google Scholar]
- 10.Kempf, D. J., K. C. Marsh, G. Kumar, A. D. Rodrigues, J. F. Denissen, E. McDonald, M. J. Kukulka, A. Hsu, G. R. Granneman, P. A. Baroldi, E. Sun, D. Pizzuti, J. J. Plattner, D. W. Norbeck, and J. M. Leonard. 1997. Pharmacokinetic enhancement of inhibitors of the human immunodeficiency virus protease by coadministration with ritonavir. Antimicrob. Agents Chemother. 41:654-660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kempf, D. J., J. D. Isaacson, M. S. King, S. C. Brun, Y. Xu, K. Real, B. M. Bernstein, A. J. Japour, E. Sun, and R. A. Rode. 2001. Identification of genotypic changes in human immunodeficiency virus protease that correlate with reduced susceptibility to the protease inhibitor lopinavir among viral isolates from protease inhibitor-experienced patients. J. Virol. 75:7462-7469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Molla, A., S. Vasavanonda, G. Kumar, H. L. Sham, M. Johnson, B. Grabowski, J. F. Denissen, W. Kohlbrenner, J. J. Plattner, J. M. Leonard, D. W. Norbeck, and D. J. Kempf. 1998. Human serum attenuates the activity of protease inhibitors toward wild-type and mutant human immunodeficiency virus. Virology 250:255-262. [DOI] [PubMed] [Google Scholar]
- 13.Murphy, R. L., S. Brun, C. Hicks, J. J. Eron, R. Gulick, M. King, A. C. White, C. Benson, M. Thompson, H. A. Kessler, S. Hammer, R. Bertz, A. Hsu, A. Japour, and E. Sun. 2001. ABT-378/ritonavir plus stavudine and lamivudine for the treatment of antiretroviral-naïve adults with HIV infection: 48-week results. AIDS 15:F1-F9. [DOI] [PubMed] [Google Scholar]
- 14.Patick, A., K. Boritzki, and L. A. Bloom. 1997. Activities of the human immunodeficiency virus type 1 (HIV-1) protease inhibitor nelfinavir mesylate in combination with reverse transcriptase and protease inhibitors against acute HIV-1 infection in vitro. Antimicrob. Agents Chemother. 41:2159-2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sham, H. L., D. J. Kempf, A. Molla, K. C. Marsh, C. M. Chen, W. Kati, K. Stewart, R. Lal, A. Hsu, D. Detebenner, M. Korneyeva, S. Vasavanonda, E. McDonald, A. Saldivar, N. Wideburg, X. Chen, P. Niu, C. Park, V. Jayanti, B. Grabowski, G. R. Granneman, E. Sun, A. J. Japour, J. M. Leonard, J. J. Plattenr, and D. Norbeck. 1998. ABT-378, a highly potent inhibitor of the human immunodeficiency virus protease. Antimicrob. Agents Chemother. 42:3218-3224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Trembly, C., D. P. Merrill, T. C. Chou, and M. S. Hirsch. 1999. Interactions among combinations of two and three protease inhibitors against drug-susceptible and drug-resistant HIV-1 isolates. J. Acquir. Immune Defic. Syndr. 22:430-436. [DOI] [PubMed] [Google Scholar]
- 17.Zolopa, A. R., R. W. Shafer, A. Warford, J. G. Montoya, P. Hsu, D. Katzenstein, T. C. Merigan, and B. Efron. 1999. HIV-1 genotypic resistance patterns predict response to saquinavir-ritonavir therapy in patients in whom previous protease inhibitor therapy had failed. Ann. Intern. Med. 131:813-821. [DOI] [PMC free article] [PubMed] [Google Scholar]