Abstract
In previous studies, we demonstrated the leishmanicide effect of coronaridine, a natural indole alkaloid isolated from stem bark of Peschiera australis (Delorenzi et al., Antimicrob. Agents Chemother. 45:1349-1354, 2001). In this study we show the leishmanicidal effect of the synthetic coronaridine and its racemic 18-methoxylated analog, 18-methoxycoronaridine. Both alkaloids revealed a potent leishmanicide effect against Leishmania amazonensis, a causative agent of cutaneous and diffuse cutaneous leishmaniasis in the New World. Despite their potent leishmanicide effect, both alkaloids were neither toxic to murine macrophages nor did they modulate their oxidative or cytokine production responses.
Leishmaniasis, a disease that affects 12 million people worldwide, presents broad clinical manifestations, ranging from a single localized ulcer to fatal hepatosplenomegaly, depending on the parasite species and the immunological status of the host (42). Recently, a dramatic increase in leishmaniasis cases was observed, specifically associated with human immunodeficiency virus infection (41, 42).
The drugs currently available for leishmaniasis treatment—pentavalent antimonials, amphotericin B, and pentamidine—present many severe side effects, are expensive, and are frequently ineffective. Furthermore, large-scale clinical antinomy resistance has been reported (5, 10, 21, 28). All these problems, together with the lack of a safe and effective vaccine, emphasize the importance of the development of new drugs against leishmaniasis.
Recently, we described the antiparasite effect of coronaridine (COR), isolated from the stem of the Peschiera australis shrub, against Leishmania amazonensis promastigotes and intracellular amastigotes (7, 12). COR is an iboga-type indole alkaloid found in many species of the plant kingdom. Like others iboga alkaloids, COR was investigated for a wide variety of pharmacological effects, such as antitumor, anti-inflammatory, and bactericidal activities (23, 37, 39), as well as a stimulatory action on the central nervous system. COR has been studied for its potential antiaddictive properties (11, 13, 14, 17, 29), showing an effective decrease in morphine, cocaine, ethanol, and nicotine self-administration in laboratory animals analogous to that observed with ibogaine (reviewed in references 13 and 14). Because of side effects such as tremor, cerebellar neurotoxicity, and bradycardia associated with ibogaine and COR, an 18-methoxylated COR analog was developed with the goal of reducing adverse effects. In preclinical studies, 18-methoxycoronaridine (18-MCOR) exerted few to none of the side effects associated with ibogaine or COR (reviewed in references 15, 16, and 32).
In the present study, we describe the leishmanicidal activities of the synthetic COR and its analog, 18-MCOR, which were similar to the antiparasite effect of the natural COR. The synthetic compounds, like the natural COR, did not induce nitric oxide production, nor did they stimulate the synthesis of interleukin 6 (IL-6), IL-12, and tumor necrosis factor alpha (TNF-α) by drug-treated macrophages.
(J. C. Delorenzi performed this work in partial fulfillment of his Ph.D. degree at the Instituto de Biofísica Carlos Chagas Filho, Universidade Federal do Rio de Janeiro, Rio de Janeiro, Brazil.)
MATERIALS AND METHODS
Parasite culture.
L. amazonensis (WHOM/BR/75/Josefa) promastigotes were cultured at 26°C in Schneider insect medium (Sigma Chemical Co. St. Louis, Mo.) plus 10% fetal calf serum (Gibco BRL, Gaithersburg, Md.) and gentamicin (15 μg/ml; Schering-Plough, Rio de Janeiro, Brazil).
Drugs.
Synthetic (±)-COR (hydrochloric salt) and (±)-18-MCOR (free base and water-soluble hydrochloric salt) were synthesized according to the methods of Bormann and Kuehne (6) and Bandarage et al. (3). The salts were dissolved in sterile water and the free base was dissolved in 1% dimethyl sulfoxide to prepare 10-mg/ml stock solutions.
Antiamastigote activity.
Resident peritoneal cells from normal BALB/c mice were harvested and cultured as previously described (12). Adhered macrophages were infected with L. amazonensis promastigotes (stationary growth phase) at a 10:1 parasite/macrophage ratio and incubated at 37°C in 5% CO2. After 1 h of incubation, free promastigotes were removed by extensive washing with 0.01 M phosphate-buffered saline, and the cultures were incubated as described above for 24 h. Amastigote-infected macrophage cultures were treated with the different compounds for an additional 24 h. After that, monolayers were washed with phosphate-buffered saline at 37°C, fixed in methanol, and stained with Giemsa. The number of amastigotes was determined by counting at least 400 macrophages in duplicate cultures. The results were expressed as a percentage of survival in relation to controls. The survival indices were obtained by multiplying the percentage of infected macrophages by the number of amastigotes per infected macrophage. Only experiments with a survival index of ≥200 for the untreated macrophages were considered.
Cytotoxicity assay.
Macrophage cytotoxicity was tested by trypan blue exclusion and phagocytosis assays as described previously (12). In addition we tested drug cytotoxicity by the 2,3-bis-[2-methoxy-4-nitro-5-sulfophenyl]-2H-tetrazolium-5-carboxanilida (XTT; Sigma) as described by Bou-Habib et al. (8). The XTT formazan dye is metabolized by mitochondrial enzymes to a water-soluble dye form, indicating cell viability (8). For this test, 3% thioglycolate-elicited uninfected mouse peritoneal macrophages were used.
Nitric oxide production.
NO production was measured using J774.A1 murine macrophages activated or not with gamma interferon (IFN-γ) and lipopolysaccharide (LPS) (Escherichia coli O111:B4; Difco Labs Inc. Detroit, Mich.) by the Griess reaction as described previously (12).
Cytokine production.
Adhered J774.A1 murine macrophage cells (106/well in a 24-well plate) were activated or not as for the nitric oxide assay. After 24 h at 37°C in 5% CO2, the monolayers were treated with 10 μg of COR or 18-MCOR per ml. The production of IL-6, IL-12 (p40/p70), and TNF-α were assessed by sandwich enzyme-linked immunosorbent assay (ELISA) technique, using capture and detection antibodies purchased from Pharmingen (San Diego, Calif.), according to the manufacturer's instructions. Recombinant cytokines were used as standards, and assays were performed in duplicate or triplicate. The reactions revealed with streptavidin-alkaline phosphatase (Gibco BRL) and p-nitrophenylphosphate (Sigma) were read at 405 nm in a microplate reader (Bio-Rad Labs, Hercules, Calif.). Curve regression was performed with microplate manager software (Bio-Rad).
Statistical Analysis.
Results were statistically analyzed by the Student's t test. P values of ≤ 0.05 were considered significant.
RESULTS
Antiamastigote activity.
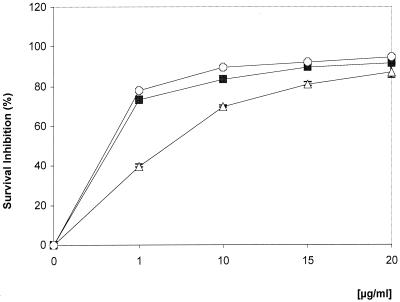
The leishmanicidal activities of synthetic COR and 18-MCOR (Table 1) were evaluated in L. amazonensis-infected macrophage cultures by adding the compounds only once 24 h after infection, without replacing the medium. The results in Fig. 1 show dose-dependent antiamastigote effects of COR and 18-MCOR. A 40% inhibition of amastigote survival was seen when COR was used at 1 μg/ml, and at 10 and 20 μg/ml, COR inhibited parasite survival by 70 and 87%, respectively. Treatment with 18-MCOR at 1, 10, or 20 μg/ml decreased the amastigote survival by 73, 84, and 92%, respectively. The 90% inhibitory concentrations (IC90s) of 22 and 16 μg/ml were calculated for COR and 18-MCOR, respectively. When infected monolayers were treated with N-methyl-d-glucamine antimoniate (1, 10, or 20 μg/ml; Glucantime; WRAIR BL09186), inhibitions of 78, 90, and 94% were observed, respectively, with an IC90 of 10 μg/ml. The free-base 18-MCOR revealed potential similar to that of the hydrochloric salt 18-MCOR, inhibiting the parasite survival in 80% of cases when used at 10 μg/ml but, due to its difficult solubilization, was not included in further tests (data not shown).
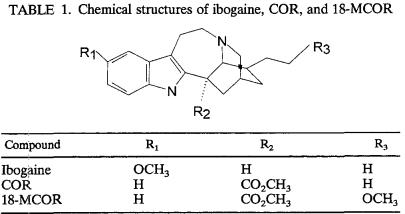
TABLE 1.
Chemical structures of ibogaine, COR, and 18-MCOR
FIG. 1.
Effect of COR (triangles), 18-MCOR (squares), and N-methylglucamine (GLU) (circles) on amastigote survival. L. amazonensis-infected mouse peritoneal macrophages were treated with different concentrations of drugs 24 h after infection, and amastigote survival was assessed 24 h later. Results from three experiments in duplicate are shown as mean percentages ± standard errors of the means (error bars) of survival inhibition in relation to untreated controls. All the results were significant (P < 0.05).
Nitric oxide production.
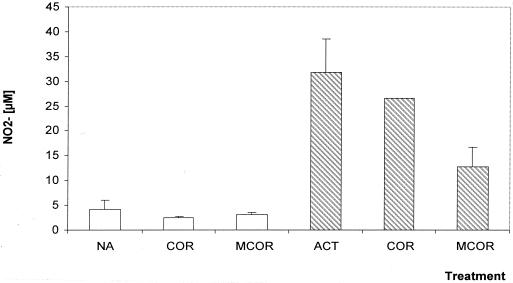
To confirm our previous results that natural COR did not up-regulate the NO production by macrophages (12), we measured NO production in macrophages treated with the racemic mixture hydrochloric salt of both COR and 18-MCOR. Nonactivated J774.A1 macrophages treated for 24 h with COR or 18-MCOR at 10 μg/ml were unable to produce more nitrite than untreated controls (Fig. 2). Activation of J774.A1 macrophages with LPS and IFN-γ induced a sixfold increase in their NO2− production in comparison with nonactivated macrophages (Fig. 2). Addition of COR at 10 μg/ml did not alter NO2− production, while treatment with 18-MCOR at 10 μg/ml reduces by 60% the NO2− production of these already-activated macrophages (Fig. 2).
FIG. 2.
Effect of synthetic indole alkaloids on the NO production by nonactivated (open bars) and IFN-γ-LPS-activated (hatched bars) J774.A1 macrophages. Supernatants were harvested 24 h after treatment, and nitrite concentration was estimated by Griess reaction. Data represent means with standard errors of the means (error bars) of two independent cultures done in triplicate. All compounds were tested at 10 μg/ml. Abbreviations: NA, untreated nonactivated cells; ACT, untreated activated cells.
Cytokine production.
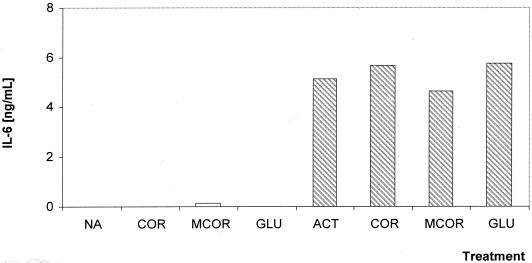
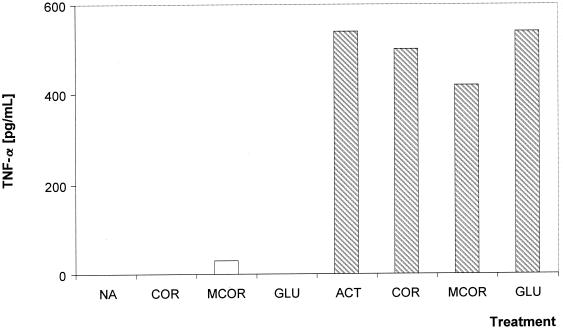
Both COR and 18-MCOR do not stimulate the macrophage production of any of the cytokines investigated (IL-6, IL-12, and TNF-α). Nonactivated J774.A1 macrophages treated for 24 h with COR or 18-MCOR at 10 μg/ml were unable to produce more IL-6 and TNF-α than untreated controls (Fig. 3 and 4). Activation of J774.A1 macrophages with IFN-γ-LPS induced a fivefold increase in their IL-6 and TNF-α production in comparison with nonactivated macrophages, but treatment of these macrophages with COR or 18-MCOR at 10 μg/ml did not alter their cytokine production (Fig. 3 and 4). Both drugs were unable to induce IL-12 in nonactivated macrophages or to change the production of this cytokine in activated macrophages (data not shown).
FIG. 3.
Effect of synthetic indole alkaloids on IL-6 production by nonactivated (open bars) and IFN-γ-LPS-activated (hatched bars) J774.A1 macrophages. Supernatants were harvested 24 h after treatment, and IL-6 production was determined by ELISA. Results are from one representative experiment out of three, all done in triplicate. All compounds were tested at 10 μg/ml. Abbreviations: NA, untreated nonactivated cells; ACT, untreated activated cells; GLU, N-methylglucamine.
FIG. 4.
Effect of synthetic indole alkaloids on TNF-α production by nonactivated (open bars) and IFN-γ-LPS-activated (hatched bars) J774.A1 macrophages. Supernatants were harvested 24 h after treatment, and TNF-α production was determined by ELISA. Results are from one representative experiment out of three, all done in triplicate. All compounds were tested at 10 μg/ml. Abbreviations: NA, untreated nonactivated cells; ACT, untreated activated cells; GLU, N-methylglucamine.
Effects of the compounds on macrophages.
In order to test the safety of the compounds for mammalian cells, murine macrophages were treated with the drugs and their viabilities were checked by trypan blue dye exclusion. When treated with COR at 10 or 20 μg/ml for 24 h, 93 and 84% of cells remained viable; while treatment with 18-MCOR under the same conditions yielded 93 and 88% survival when concentrations of 10 and 20 μg/ml, respectively, were used. Treatment with N-methylglucamine at 20 μg/ml resulted in 89% viable cells (Table 2).
TABLE 2.
Drug effects on macrophage viabilitya
| Drug (concn [μg/ml]) | % Cell viabilityb as shown by assay
|
||
|---|---|---|---|
| Trypan blue | XTT | Phagocytosis | |
| GLUc (20) | 88.9 ± 1.0 | 72.0 ± 6.0 | 95.0 ± 9.3 |
| COR (10) | 93.0 ± 2.8 | 95.0 ± 1.9 | NDd |
| COR (20) | 83.5 ± 2.3 | 90.4 ± 1.9 | 90.0 ± 7.7 |
| 18-MCOR (10) | 92.5 ± 0.3 | 99.0 ± 3.0 | ND |
| 18-MCOR (20) | 88.1 ± 2.4 | 99.0 ± 2.0 | 98.0 ± 5.5 |
Resident or elicited mouse peritoneal macrophages were treated with the indicated concentrations of the drugs for 24 h as described in Materials and Methods.
Results are shown as means ± standard errors of the means of two experiments done in duplicate for trypan blue and phagocytosis assays and of five experiments done in duplicate for XTT tests.
GLU, N-methylglucamine.
ND, not done.
The capacity of the drugs to affect macrophage phagocytosis was also tested. Murine macrophages treated with 20 μg/ml of COR or 18-MCOR, showed, respectively, a 10% and 2% inhibition of the promastigotes phagocytosis in relation to untreated controls. N-Methylglucamine at 20 μg/ml inhibited 5% of the phagocytosis capacity in the same assay (Table 2). Like the natural COR, the synthetic alkaloids did not affect the viability or the phagocytosis capacity of the macrophages. Experiments carried out to determine the toxicity of the drug by the XTT method showed that 10 and 20 μg/ml of COR inhibited, respectively, 5 and 10% of the elicited macrophage mitochondrial activity (Table 2).
DISCUSSION
In this work we describe the antileishmanial activities of the synthetic indole alkaloids COR and its analog, 18-MCOR, against amastigotes of L. amazonensis. Our previous observations of the leishmanicidal activity of COR purified from P. australis stem extract (12) were confirmed with the congener synthetic drug, as well as with its 18-methoxylated analog.
COR was first isolated by Gorman et al. in 1960 (20) and first synthesized by Kutney et al. in 1970 (27). The synthesis of this compound yielded sufficient amounts of COR to permit the study of its pharmacological effects. In the same manner, structure modifications produced congeners with improved pharmacological activities and reduced side effects. COR and its related compound ibogaine seem to interact with opioid receptors, especially κ-opioids, and block N-methyl-d-aspartic acid and nicotinic ion channels (14, 30, 35). Ibogaine, notably lacking the carbomethoxy substituent of COR, provokes adverse effects, such as hallucinations and tremors, that limit its use in clinics (15, 32). However, COR presents dramatically reduced, but not abolished, side effects in comparison with ibogaine (13, 18, 19). Therefore, modifications in COR chemical structure were made in order to improve its efficacy and to reduce even more its side effects, which was achieved with a methoxylation in carbon-18. The resulting analog, 18-MCOR, retained the pharmacological activity of COR without inducing toxic effects (15, 16, 18, 40).
In our experiments, 18-MCOR was more effective than COR in killing amastigotes in infected murine macrophages, showing an IC90 of 16 μg/ml, in comparison with an IC90 of COR of 22 μg/ml. Like the natural COR, synthetic COR and 18-MCOR neither damaged macrophages nor inhibited fundamental physiological functions of these cells, as determined by trypan blue dye exclusion and phagocytosis tests (Table 2). The effect of alkaloid exposure was also assessed by XTT assay, in which mitochondrial dehydrogenases metabolize the XTT reagent to a water-soluble formazan dye, indicating preserved mitochondrial activity and cell viability (8).
Leishmania organisms are obligate intracellular parasites that develop inside macrophages, and nitric oxide production by these cells is considered the most important mechanism in immunologically mediated amastigote killing (31). In order to verify the ability of COR and 18-MCOR to activate the NO synthase pathway, we measured nitric oxide production in COR- or 18-MCOR-treated macrophages, activated or not with IFN-γ-LPS. Like natural COR, synthetic COR did not up regulate NO production, either in activated or in nonactivated macrophages (Fig. 2). Although NO production was not altered by 18-MCOR in nonactivated macrophages, in activated macrophages it reduces 60% of this activity. Interestingly, it was reported that ibogaine, a COR- and 18-MCOR-related alkaloid, reduced nitric oxide synthase activity in the brains of parenterally treated mice (34). Taken together these results suggest that the observed parasite killing may not be mediated by increased activity of the NO synthase pathway.
The control of leishmaniasis requires the induction of an immune response capable of activating macrophages to a microbicidal state. The most-potent cytokine for the induction of leishmanicidal activity in macrophages is IFN-γ, which is associated with a Th1-cell response (33, 36). The role that IL-12 plays in the development of Th1 cells, potentiating cell-mediated immune responses against leishmanial infection, is well characterized (4, 9, 38). TNF-α is also required early on to control intracellular growth of Leishmania (25). On the contrary, macrophage treatment with IL-6, a cytokine recognized by its proinflammatory properties, induces a suppression of IFN-γ and TNF-α activation for the killing of Leishmania (22). Likewise, IL-10 decreases IL-12 and TNF-α production by macrophages, preventing parasite killing (26). In our analysis we found that none of the tested cytokines (IL-6, IL-12, and TNF-α) were either stimulated or inhibited by both compounds in macrophages preactivated or not with IFN-γ-LPS, suggesting that the amastigote killing by the alkaloids cannot be explained by alterations in the production of these cytokines. It has also been reported that IL-6 production by thioglycolate-elicited peritoneal macrophages was not affected by ibogaine exposure (24).
The results shown in this work, besides supporting the antileishmanial activity of COR, present a congener, 18-MCOR, which effectively kills intracellular amastigotes at when used at concentrations nontoxic to human and murine macrophages. Moreover, the use of ibogaine, a related COR and 18-MCOR compound, as antiaddictive therapy in humans (1, 2) is indicative that COR and 18-MCOR may be safely used for treating leishmaniasis. Other advantages of these compounds are their simple chemical structure and their already-described synthesis, which may facilitate not only their production but also the synthesis of derivatives with increased efficacy for treatment of leishmaniasis.
A newly described form of leishmaniasis transmission, directly from person to person through the sharing of needles, is becoming frequent among injecting drug users with human immunodeficiency virus coinfections (42). Thus, COR and 18-MCOR could have a double benefit in these patients based on their antiaddictive and antileishmanial properties.
Acknowledgments
This work was supported in part by UNDP/World Bank/WHO-TDR, CNPq, PRONEX, and FAPERJ.
REFERENCES
- 1.Alper, K. R., H. S. Lotsof, G. M. Frenken, D. J. Luciano, and J. Bastiaans. 2000. Ibogaine in acute opioid withdrawal. An open label case series. Ann. N. Y. Acad. Sci. 909:257-259. [DOI] [PubMed] [Google Scholar]
- 2.Alper, K. R., H. S. Lotsof, G. M. Frenken, D. J. Luciano, and J. Bastiaans. 1999. Treatment of acute opioid withdrawal with ibogaine. Am. J. Addict. 8:234-242. [DOI] [PubMed] [Google Scholar]
- 3.Banderage, U. K., M. E. Kuehne, and S. D. Glick. 1999. Total synthesis of racemic albifloranine and its anti-addictive congeners, including 18-methoxycoronaridine. Tetrahedron 55:9405-9424. [Google Scholar]
- 4.Belkaid, Y., B. Bucher, and D. L. Sacks. 1998. Analysis of cytokine production by inflammatory mouse macrophages at single-cell level: selective impairment of IL-12 induction in Leishmania-infected cells. Eur. J. Immunol. 28:1389-1400. [DOI] [PubMed] [Google Scholar]
- 5.Berman, J. D. 1997. Human leishmaniasis: clinical diagnostic and chemotherapeutic developments in the last 10 years. Clin. Infect. Dis. 24:684-703. [DOI] [PubMed] [Google Scholar]
- 6.Bormann, W. G., and M. E. Kuehne. 1992. A common intermediate providing synthesis of ψ-tabersonine, coronaridine, iboxyphylline, vinamidine and vinblastine. J. Org. Chem. 57:1752. [Google Scholar]
- 7.Bou-Habib, D. C., E. M. B. Saraiva, J. C. Delorenzi, G. A. Ferraro, A. C. Pinto, C. M. Rezende, and M. T. Andrade. October 1998. Brazil patent PI 9804032-4.
- 8.Bou-Habib, D. C., G. Roderiquez, T. Oravecz, P. W. Berman, P. Lusso, and M. A. Norcross. 1994. Cryptic nature of envelope V3 region epitopes protects primary monocytotropic human immunodeficiency virus type 1 from antibody neutralization. J. Virol. 68:6006-6013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carrera, L., R. T. Gazzinelli, R. Badolato, S. Hieny, W. Müller, R. Kühn, and D. L. Sacks. 1996. Leishmania promastigotes selectively inhibit IL-12 induction in bone marrow-derived macrophages from susceptible and resistant mice. J. Exp. Med. 183:515-526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen, M., S. B. Christensen, J. Blom, E. Lemmich, L. Nadelmann, K. Fich, T. G. Theander, and A. Kharazmi. 1993. Licochalcone A, a novel antiparasitic agent with potent activity against human pathogenic protozoan species Leishmania., Antimicrob. Agents Chemother. 37:2550-2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deecher, D. C., M. Teitler, D. M. Soderlund, W. G. Bormann, M. E. Kuehne, and S. D. Glick. 1992. Mechanisms of action of ibogaine and harmaline congeners based on radioligand biding studies. Brain Res. 571:242-247. [DOI] [PubMed] [Google Scholar]
- 12.Delorenzi, J. C., M. Attias, C. R. Gattass, M. Andrade, C. Rezende, C. A. Pinto, A. T. Henriques, D. C. Bou-Habib, and E. M. B. Saraiva. 2001. Antileishmanial activity of an indole alkaloids from Peschiera australis. Antimicrob. Agents Chemother. 45:1349-1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Glick, S. D. M. E. Kuehne, J. Raucci, T. E. Wilson, D. Larson, R. W. Keller, Jr., and J. N. Carlson. 1994. Effects of iboga alkaloids on morphine and cocaine self-administration in rats: relationship to tremorigenic effects and to dopamine release in nucleus accumbens and striatum. Brain Res. 657:14-22. [DOI] [PubMed] [Google Scholar]
- 14.Glick, S. D., I. M. Maisonneuve, and S. M. Pearl. 1997. Evidence for roles of kappa opioid and NMDA receptors in the mechanism of action of ibogaine. Brain Res. 749:340-343. [DOI] [PubMed] [Google Scholar]
- 15.Glick, S. D., I. M. Maisonneuve, and K. K. Szumlinski. 2000. 18-Methoxycoronaridine (18-MC) and ibogaine: comparison of anti-addictive efficacy, toxicity and mechanisms of action. Ann. N. Y. Acad. Sci. 914:369-386. [DOI] [PubMed] [Google Scholar]
- 16.Glick, S. D., I. M. Maisonneuve, L. B. Hough, M. E. Kuehne, and U. K. Bandarage. 1999. (±)-18-Methoxycoronaridine: a novel iboga alkaloid conger having potential anti-addictive efficacy. CNS Drug Rev. 5:27-42. [Google Scholar]
- 17.Glick, S. D., K. Rossman, N. C. Rao, I. M. Maisonneuve, and J. N. Carlson. 1992. Effects of ibogaine on acute signs of morphine withdrawal in rats: independence from tremor. Neuropharmacology 31:497-500. [DOI] [PubMed] [Google Scholar]
- 18.Glick, S. D., M. E. Kuehne, I. M. Maisonneuve, U. K. Bandarage, and H. H. Molinari. 1996. 18-methoxycoronaridine, a non-toxic iboga alkaloid conger: effects on morphine and cocaine self-administration and on mesolimbic dopamine release in rats. Brain. Res. 719:29-35. [DOI] [PubMed] [Google Scholar]
- 19.Glick, S. D., S. M. Pearl, Z. Cai, and I. M. Maisonneuve. 1996. Ibogaine-like effects of nor-ibogaine in rats. Brain Res. 713:294-297. [DOI] [PubMed] [Google Scholar]
- 20.Gormam, M., N. Neuss, N. J. Cone, and J. A. Deyrup. 1960. Alkaloids from apocynaceae III. Alkaloids of Tabernaemontana and Ervatamia. The structure of coronaridine, a new alkaloid related to ibogamine. J. Am. Chem. Soc. 82:1142-1145. [Google Scholar]
- 21.Grogl, M., T. N. Thomason, and E. D. Franke. 1992. Drug resistance in leishmaniasis: its implication in systemic chemotherapy of cutaneous and mucocutaneous disease. Am. J. Trop. Med. Hyg. 47:117-126. [DOI] [PubMed] [Google Scholar]
- 22.Hatzigeorgiou, D. E., S. He, J. Sobel, K. H. Grabstein, A. Hafner, and J. L. Ho. 1993. IL-6 down-modulates the cytokine-enhanced antileishmanial activity in human macrophages. J. Immunol. 151:3682-3692. [PubMed] [Google Scholar]
- 23.Henriques, A. T., A. A. Melo, P. R. Moreno, L. L. Ene, J. A. Henriques, and E. E. Schapoval. 1996. Ervatamia coronaria: chemical constituents and some pharmacological activities. J. Ethnopharmacol. 50:19-25. [DOI] [PubMed] [Google Scholar]
- 24.House, R. V., P. T. Thomas, and H. N. Bhargava. 1995. Comparison of the hallucinogenic indole alkaloids ibogaine and harmaline for potential immunomodulatory activity. Pharmacology 51:56-65. [DOI] [PubMed] [Google Scholar]
- 25.Jüttner, S., J. Bernhagen, C. N. Metz, M. Röllinghoff, R. Bucala, and A. Gessner. 1998. Migration inhibitory factor induces killing of Leishmania major by macrophages: dependence on reactive nitrogen intermediates and endogenous TNF-α. J. Immunol. 161:2383-2390. [PubMed] [Google Scholar]
- 26.Kane, M. M., and D. M. Mosser. 2001. The role of IL-10 in promoting disease progression in leishmaniasis. J. Immunol. 166:1141-1147. [DOI] [PubMed] [Google Scholar]
- 27.Kutney, J. P., W. J. Cretney, P. Le Quesne, B. McKague, and E. Piers. 1970. Total synthesis of indole and dihydroindole alkaloids. IV. The total synthesis of dl-dihydrocleavamine, dl-carbomethoxydihydrocleavamine, dl-coronaridine, dl-dihydrocatharanthine, and dl-ibogamine. A general entry into the iboga and vinca alkaloids. J. Am. Chem. Soc. 92:1712-1726. [DOI] [PubMed] [Google Scholar]
- 28.Lira, R., S. Sundar, A. Makharia, R. Kenney, A. Gam, E. Saraiva, and D. Sacks. 1999. Evidence that the high incidence of treatment failures in Indian kala-azar is due to the emergence of antimony-resistant strains of Leishmania donovani. J. Infect. Dis. 180:564-567. [DOI] [PubMed] [Google Scholar]
- 29.Maisonneuve, I. M., G. L. Mann, C. R. Deibel, and S. D. Glick. 1997. Ibogaine and the dopaminergic response to nicotine. Psycopharmacology 129:206-214. [DOI] [PubMed] [Google Scholar]
- 30.Mash, D. C., J. K. Staley, J. P. Pablo, A. M. Holohean, J. C. Hackman, and R. A. Davidoff. 1995. Properties of ibogaine and its principal metabolite (12-hydroxyibogamine) at the MK-801 bidding site of the NMDA receptor complex. Neurosci. Lett. 192:53-56. [DOI] [PubMed] [Google Scholar]
- 31.Mauel, J., and A. Ransijn. 1997. Leishmania spp. Mechanisms of toxicity of nitrogen oxidation products. Exp. Parasitol. 87:98-111. [DOI] [PubMed] [Google Scholar]
- 32.Molinari, H. H., I. M. Maisonneuve, and S. D. Glick. 1996. Ibogaine neurotoxicity: a re-evaluation. Brain. Res. 737:255-262. [DOI] [PubMed] [Google Scholar]
- 33.Nabors, G. S., L. C. C. Afonso, J. P. Farbell, and P. Scott. 1995. Switch from a type 2 to a type 1 T helper cell response and cure of established Leishmania major infection in mice is induced by combined therapy with interleukin 12 and pentostam. Proc. Natl. Acad. Sci. USA 92:3142-3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Popik, P., and P. Skolnick. 1999. Pharmacology of ibogaine and ibogaine-related alkaloids. Alkaloids 52:197-231. [Google Scholar]
- 35.Popik, P., R. T. Layer, L. H. Fossom, M. Benveniste, B. Geter-Douglass, J. M. Witkin, and P. Skolnick. 1995. NMDA antagonist properties of the putative antiaddictive drug, ibogaine. J. Pharmacol. Exp. Ther. 275:753-760. [PubMed] [Google Scholar]
- 36.Proudfoot, L., A. Nicolaev, G. Feng, X. Wei, M. A. J. Ferguson, J. S. Brimacombe, and F. Y. Liew. 1996. Regulation of the expression of nitric oxide synthase and leishmanicidal activity by glycoconjugates of Leishmania lipophosphoglycan in murine macrophages. Proc. Natl. Acad. Sci. USA 93:10984-10989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rates, S. M. K., E. E. S. Schapoval, I. A. Souza, and A. T. Henriques. 1993. Chemical constituents and pharmacological activities of Peschiera australis. Int. J. Pharmacognosy 31:288-294. [Google Scholar]
- 38.Sartori, A., M. A. P. Oliveira, P. Schott, and G. Trinchieri. 1997. Metacyclogenesis modulates the ability of Leishmania promastigotes to induce IL-12 production in human mononuclear cells. J. Immunol. 159:2849-2857. [PubMed] [Google Scholar]
- 39.Sharma, P., and G. A. Cordell. 1988. Heyneanine hydroxyindolenine, a new indole alkaloid from Ervatamia coronaria var. plena. J. Natl. Prod. 51:528-531. [DOI] [PubMed] [Google Scholar]
- 40.Szumlinski, K. K., C. A. McCafferty, I. M. Maisonneuve, and S. D. Glick. 2000. Interactions between 18-methoxycoronaridine (18-MC) and cocaine dissociation of behavioral and neurochemical sensitization. Brain Res. 871:245-258. [DOI] [PubMed] [Google Scholar]
- 41.Wolday, D., N. Berhe, H. Akuffo, and S. Britton. 1999. Leishmania-HIV interaction: immunopathogenic mechanism. Parasitol. Today 15:182-187. [DOI] [PubMed] [Google Scholar]
- 42.World Health Organization. 1998. Leishmania and HIV in gridlock. UNAIDS, Geneva, Switzerland.