Abstract
A novel class of acyclic nucleoside phosphonates has been discovered in which the base consists of a pyrimidine preferably containing an amino group at C-2 and C-4 and a 2-(phosphonomethoxy)ethoxy (PMEO) or a 2-(phosphonomethoxy)propoxy (PMPO) group at C-6. The 6-PMEO 2,4-diaminopyrimidine (compound 1) and 6-PMPO 2,4-diaminopyrimidine (compound 11) derivatives showed potent activity against human immunodeficiency virus (HIV) in the laboratory (i.e., CEM and MT-4 cells) and in primary (i.e., peripheral blood lymphocyte and monocyte/macrophage) cell cultures and pronounced activity against Moloney murine sarcoma virus in newborn NMRI mice. Their in vitro and in vivo antiretroviral activity was comparable to that of reference compounds 9-[(2-phosphonomethoxy)ethyl]adenine (adefovir) and (R)-9-[(2-phosphonomethoxy)-propyl]adenine (tenofovir), and the enantiospecificity of (R)- and (S)-PMPO pyrimidine derivatives as regards their antiretroviral activity was identical to that of the classical (R)- and (S)-9-(2-phosphonomethoxy)propyl purine derivatives. The prototype PMEO and PMPO pyrimidine analogues were relatively nontoxic in cell culture and did not markedly interfere with host cell macromolecular (i.e., DNA, RNA, or protein) synthesis. Compounds 1 and 11 should be considered attractive novel pyrimidine nucleotide phosphonate analogues to be further pursued for their potential as antiretroviral agents in the clinical setting.
Acyclic nucleoside phosphonates (ANPs) are nucleotide analogues in which a phosphonate group is linked to a purine or pyrimidine through an aliphatic chain via an ether linkage. A variety of ANPs are inhibitory to DNA virus and retrovirus infections (17-19, 27, 28, 37, 38). Several classes of ANPs can be distinguished, each with a different antiviral activity spectrum.
(S)-9-[3-Hydroxy-2-(phosphonomethoxy)propyl]adenine[(S)-HPMPA] (Fig. 1) is the prototype of the first class of ANPs endowed with activity against a broad spectrum of DNA viruses, including herpes simplex virus type 1 (HSV-1), HSV-2, cytomegalovirus (CMV), varicella-zoster virus, Epstein-Barr virus, human herpesvirus type 6, African swine fever virus, vaccinia virus (VV), and human adenoviruses (2, 3, 14, 15, 35, 41). It is not significantly active against RNA viruses, including human immunodeficiency virus (HIV). (S)-1-[3-hydroxy-2-(phosphonylmethoxy)propyl]cytosine [(S)-HPMPC] (cidofovir) (Fig. 1), the cytosine counterpart of (S)-HPMPA, proved to be a selective and potent anti-CMV agent (37, 38, 42) and has been approved (Vistide) for the treatment of CMV retinitis in AIDS patients.
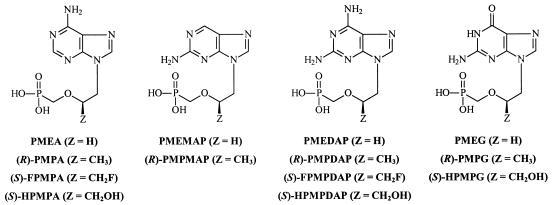
FIG. 1.
Structural formulas of a variety of PME, PMP, FPMP, and HPMP purine analogues that are endowed with antiviral activity.
The 9-[2-(phosphonomethoxy)ethyl] (PME) purines can be considered the second class of ANPs (Fig. 1). PMEA {9-[(2-phosphonomethoxy)ethyl]adenine; adefovir}, the prototype of this class, displays activity against DNA viruses, such as HSV-1, HSV-2, and VV (8, 14, 37, 38), and also against hepatitis B virus (HBV) (25, 26) and a broad variety of retroviruses, such as HIV type 1 (HIV-1), HIV-2, simian immunodeficiency virus, feline immunodeficiency virus (FIV), feline leukemia virus (FeLV), Visna virus, and Moloney murine sarcoma virus (MSV), both in cell culture and in animal models (1, 5, 8, 9, 15, 16, 20, 22-24, 32, 36, 39, 43-48, 50). A variety of base modifications, including the 2-amino and 2,6-diaminopurine derivatives and also the guanine derivative (Fig. 1) have been synthesized, and their antiviral activity has been reported. The oral prodrug PMEA for the treatment of HBV infections is currently in phase III clinical trials.
The third class of ANPs is characterized by little or no activity against DNA viruses but shows pronounced and selective activity against retroviruses and HBV. It is represented by two structural groups: (S)-9-[3-fluoro-2-(phosphonomethoxy)propyl] (FPMP) (7) and (R)-9-[(2-phosphonomethoxy)propyl] (PMP) purine (10, 11) derivatives (Fig. 1 and 2). Oral prodrug (R)-PMPA {tenofovir disoproxil; (R)-9-[(2-phosphonomethoxy)propyl]adenine; Viread} has recently been approved by the Food and Drug Administration for the treatment of HIV infections. Thus, minor structural modifications in the side chain of the ANP purine (or pyrimidine) derivatives {i.e., (S)-9-[3-hydroxy-2-(phosphonylmethoxypropyl)] (HPMP), PME, FPMP, and PMP} have a marked impact on the antiviral activity spectrum of the ANPs.
FIG. 2.
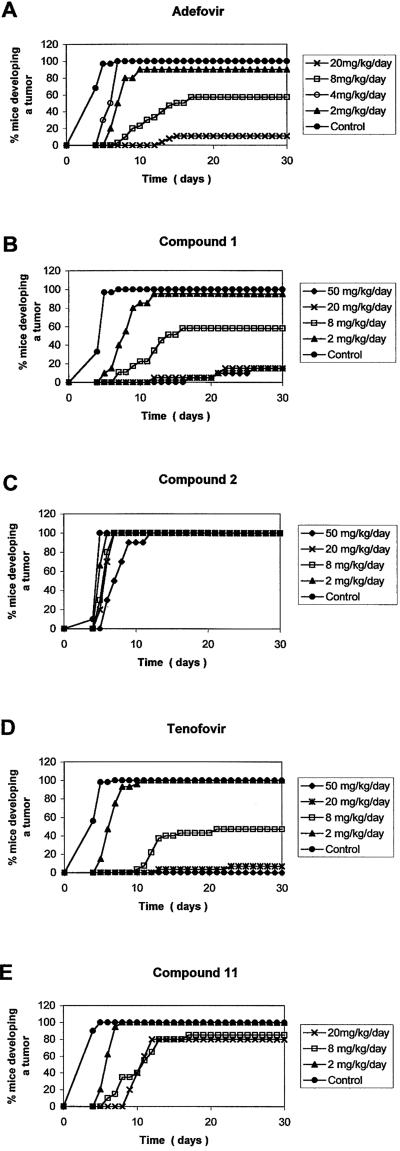
Delay of tumor formation in drug-treated MSV-infected newborn NMRI mice.
With the exception of that for cidofovir, all important biological activities found for the ANPs reside in compounds that contain a purine base such as adenine, 2,6-diaminopurine, and guanine (17-19, 27-30, 36-38) (see Fig. 2). We now report the discovery of a novel class of ANPs where the base part is a pyrimidine derivative and the aliphatic phosphonate side chain is linked to the C-6 position of the pyrimidine ring instead of the N-1 position of the pyrimidine base (as in cidofovir). These compounds possess interesting anti(retro)viral properties. The detailed synthesis of these compounds is described elsewhere (31). Here, we focus on the antiretrovirus activity of this novel class of pyrimidine nucleotide phosphonate analogues.
MATERIALS AND METHODS
Viruses.
The origins of MSV, HIV-1 (strains IIIB and BaL), HIV-2 (strain ROD), and FIV (strain Petaluma) have been described previously (9, 13, 20, 24, 40). HIV-1(IIIB) and HIV-2(ROD) stocks were obtained from supernatants of virus-infected MT-4 cell cultures. HIV-1(BaL) was expanded in human primary monocytes/macrophages (M/M), whose supernatants were collected, filtered, and stored at −80°C before use. Characteristics of viral stocks used for this study were 2.1 × 108 HIV RNA genomes/ml (corresponding to 35 ng of p24 antigen) and 5,000 50% tissue culture infectious doses/ml as assessed by virus titration in other primary M/M cultures. The isolation and characterization of clinical HIV-1 isolates L1S, L6S, and L6S/PMEA have been reported (48, 49). The HIV-1/L1S clinical isolate was derived from a patient not treated with nucleoside reverse transcriptase inhibitors (NRTIs) or ANPs and cultured without the selective pressure of any drugs. Therefore, it contained no obvious mutations that are characteristic of isolates from NRTI- or ANP-treated patients. HIV-1/L6S is a clinical isolate from a drug-treated individual cultured without the selective pressure of any drugs. As is characteristic for NRTI-treated patients, it contained S68G, K70T, V75I, F77L, F116Y, and Q151M mutations in its reverse transcriptase (RT). HIV-1/L6S/PMEA is clinical isolate HIV-1/L6S that has been isolated after culture for 11 passages in the presence of increasing concentrations of PMEA. It gained, in addition to the mutations mentioned for HIV-1/L6S, the K65R mutation characteristic of PMEA resistance in its RT.
Radiochemicals.
[methyl-3H]thymidine (dThd; specific radioactivity, 42 Ci/mmol), [5-3H]uridine (specific radioactivity, 26 Ci/mmol), and [4,5-3H]leucine (specific radioactivity, 52 Ci/mmol) were derived from Amersham Pharmacia Biotech (Buckinghamshire, United Kingdom).
Compounds.
The following compounds were used in this study: 1, 2,4-diamino-6-[2-(phosphonomethoxy)ethoxy]pyrimidine; 2, 2,4-diamino-6-123 [2-(phosphonomethoxy)ethyl]-sulfanyl 125 pyrimidine; 3, 4-amino-6-[2-(phosphonomethoxy)ethoxy]pyrimidine; 4, 2-amino-4-hydroxy-6-[2-(phosphonomethoxy)ethoxy]pyrimidine; 5, 2-amino-4-hydroxy-6-123 [2-(phosphonomethoxy)ethyl]sulfanyl 125 pyrimidine; 6, 2-amino-4-dimethylamino-6-[2-(phosphonomethoxy)-ethoxy]pyri-midine; 7, 2-amino-4-cyclopropylamino-6-[2-(phosphonomethoxy)ethoxy]pyri-midine; 8, 4-amino-2-methylsulfanyl-6-[2-(phosphonomethoxy)ethoxy]pyrimi-dine; 9, 2-amino-4-methyl-6-[2-(phosphonomethoxy)ethoxy]pyrimidine; 10, 2,4-diamino-6-(S)-[2-(phosphonomethoxy)propoxy]pyrimidine; 11, 2,4-diamino-6-(R)-[2-(phosphonomethoxy)propoxy]pyrimidine; PMEA; (R)-PMPA.
In vitro antiviral assays.
The activity against HIV-1- and HIV-2-induced cytopathicity in MT-4 cell cultures was examined at day 5 postinfection and was based on the determination of cell viability by trypan blue dye staining. Activity in CEM cell cultures was examined at day 4 to 5 postinfection and was based on the microscopical examination of virus-induced giant-cell formation. HIV-1 and HIV-2 were added at 100 50% cell culture infective doses (CCID50) to the cell cultures.
Peripheral blood mononuclear cells (PBMC) from healthy donors were isolated by density centrifugation (Lymphoprep; Nycomed Pharma, AS Diagnostics, Oslo, Norway) and stimulated with phytohemagglutinin (PHA) (Sigma Chemical Co., Bornem, Belgium) for 3 days. The activated cells (PHA-stimulated T-cell blasts) were washed with phosphate-buffered saline, and viral infections were done as described by the AIDS Clinical Trial Group protocols. Briefly, PBMC (2 × 105/200-μl well) were plated in the presence of serial dilutions of the test compound and were infected with HIV stocks at 1,000 CCID50 per ml. At day 4 postinfection, 125 μl of the supernatant of the infected cultures was removed and replaced with 150 μl of fresh medium containing the test compounds at the appropriate concentrations. At 7 days after plating the cells, p24 antigen was detected in the culture supernatant by an enzyme-linked immunosorbent assay (NEN, Paris, France).
Human primary M/M were prepared and purified as follows. PBMC obtained from healthy HIV-1-negative donors were separated over a Ficoll gradient and seeded in 48-well plates at 1.8 × 106 cells/well in 1 ml of RPMI 1640 containing 20% heat-inactivated, endotoxin- and mycoplasma-free fetal bovine serum (HyClone Laboratories, Inc., Logan, Utah), 4 mM l-glutamine (Life Technologies), and 50 U of penicillin/ml and 50 μg of streptomycin/ml (Life Technologies) (complete medium). Five days after plating and culturing the PBMC at 37°C in a humidified atmosphere enriched with 5% CO2, adherent cells were carefully removed by repeated washings with warmed RPMI 1640, leaving a monolayer of adherent cells, which were incubated in complete medium. Cells treated under these conditions have previously been shown to be >97% M/M, as determined by cytofluorometric analysis. M/M were treated for 30 min with the compounds and then challenged with 300 50% tissue culture infectious doses of HIV-1(BaL)/ml. Two hours after virus challenge, M/M were washed to remove the viral inoculum, complete medium containing the appropriate compound concentrations was replaced, and the M/M were then cultured for the duration of the experiments. Each compound concentration was run in triplicate, while positive controls were run in sextuplicate. Therefore, compounds were replaced each time the medium was changed. Supernatants were collected at day 14 after infection for assessment of virus production by analysis of HIV-1 p24 antigen.
For the anti-FIV assays, 105 Crandell feline kidney (CrFK) cells were seeded onto 24-well tissue culture plates. Cells were cultured with 2 ml of culture medium/well containing 2.5% fetal calf serum in the presence of various drug concentrations. The assays were carried out in triplicate. After a 1-h incubation period at 37°C, cells were infected with FIV. Virus was left in contact with the cultures for 1 day, after which the medium was removed and new medium containing the appropriate drug concentrations was added. After 6 days, the presence of FIV p24 antigen was examined by an antigen capture assay.
The inhibitory effect of the test compounds on MSV-induced transformation of murine embryo fibroblast C3H/3T3 cell cultures was examined microscopically at day 6 postinfection. MSV was added at 75 focus-forming units to the cell cultures. The detailed procedures for the antiretroviral evaluations have been described in detail before (9, 10, 13).
Anti-MSV activity in vivo.
The inhibitory effects of the compounds on the initiation of MSV-induced tumor formation and survival of MSV-inoculated mice were evaluated as previously described (5, 7,10). Briefly, 2- to 3-day-old NMRI mice were each inoculated subcutaneously in the left hind leg with MSV and treated intraperitoneally with a single dose of test compound at 4 h prior to virus infection (day 1), followed by a single dose of test compound at days 2, 3, 4, and 5. Drug doses were 50, 20, 8, 4, and/or 2 mg/kg of body weight/day for tenofovir [(R)-PMPA], adefovir (PMEA), and compounds 1, 2, and 11. No toxicity was observed for the highest dose of the test compound. The appearance and growth of MSV-induced tumors at the site of virus inoculation, as well as the survival of the mice for up to 30 days postinfection, were recorded daily.
Cytostatic and antimetabolic effect of ANPs in vitro.
The assays to examine the inhibition of CEM cell growth by the test compounds have been described previously (9). The 50% cytostatic concentration (CC50) was defined as the concentration of compound that reduced the number of living cells by 50%. To measure the MT-4 cell toxicity of the test compounds, 5 × 104 MT-4 cells were incubated in the wells of 96-well microplates in the presence or absence of different concentrations of the test compounds for 5 days at 37°C. The CC50 was then calculated from the number of living cells counted under a microscope by the trypan blue exclusion method.
The incorporation of [methyl-3H]dThd, [3H]uridine, and [3H]leucine into the methanol-insoluble CEM cell fraction in microplates was also measured. To each well were added 105 CEM cells, 5.9 pmol (0.25 μCi) of [methyl-3H]dThd, 38 pmol (1.0 μCi) of [3H]uridine, and 19 pmol (1.0 μCi) of [4,5-3H]leucine and the test compound. The cells were allowed to proliferate for 20 h at 37°C in a humidified, CO2-controlled atmosphere. At the end of this incubation period, the contents of the wells (200 μl) were transferred to 25-mm-diameter glass fiber filters (type A/G; Gelman Instrument Co., Ann Arbor, Mich.) mounted on a Millipore 3025 sampling manifold apparatus. The filters were washed twice with cold phosphate-buffered saline, twice with cold 10% trichloroacetic acid, twice with cold 5% trichloroacetic acid, once with cold ethanol, and once with cold ether. The filters were then allowed to dry for 10 min at 60°C and assayed for radioactivity in a toluene-based scintillator.
RESULTS
Anti-HIV-1 activity of acyclic pyrimidine nucleoside phosphonate analogues in CEM cell cultures.
A variety of acyclic pyrimidine nucleoside phosphonate analogues have been synthesized as a novel class of ANPs and evaluated for their antiretroviral activity in cell culture. Characteristic of the new class of compounds is the linkage of the PME or PMP chain (preferably) through an ether (or thioether) bond to the C-6 position of the pyrimidine ring. We found that a prerequisite for potent antiretroviral activity is the presence of an amino group at C-2 of the pyrimidine ring together with an amino group at C-4 (compounds 1, 2, and 11) or a hydroxyl group at C-4 (compound 4) (Table 1). These 6-[2-(phosphonomethoxy)ethoxy] (6-PMEO)- and 6-[2-(phosphonomethoxy)propoxy] (6-PMPO)-substituted pyrimidine structures resulted in an anti-HIV activity with a 50% effective concentration (EC50) that was between 0.80 and 2.0 μg/ml. Lack of an amino group at C-2 (compound 3 or 8) or the presence of a dimethylamino or methyl or cyclopropylamino at C-4 (compounds 6, 9, and 7, respectively) resulted in complete annihilation of the antiretroviral activity (Table 1). Thioether derivatives were, as a rule, 5- to 10-fold less active than the corresponding ether derivatives (compare compound 2 with compound 1) or even completely inactive (compound 5). Interestingly, the antiretroviral activity of the novel pyrimidine ANPs showed marked enantiospecificity in their action against HIV-1. (R)-6-PMPO pyrimidine derivative 11 was markedly more inhibitory to these viruses than the corresponding (S)-6-PMPO pyrimidine derivative (10) (Table 1). The residual antiviral activity noted for the (S) enantiomer might be due to contamination with the (R) enantiomer originating from the chiral starting material (1 to 2%).
TABLE 1.
Anti-retroviral and cytostatic activity of acyclic pyrimidine nucleoside phosphonates in HIV-1-infected CEM cell cultures
Concentration required to inhibit HIV-1 (IIIB)-induced cytopathicity in CEM cell cultures by 50%.
Concentration required to inhibit CEM cell proliferation by 50%.
Cp, cyclopropyl.
For most of the compounds, poor, if any cytotoxicity was noted at a concentration of 100 μg/ml, with a striking exception for compound 4 (CC50: 2.5 μg/ml for CEM cells). The antivirally most active 6-PMEO-2,4-di-NH2 pyrimidine derivative (compound 1) showed a CC50 of 11 μg/ml for CEM cells, whereas its corresponding thioether analogue, compound 2, and the (R)-6-PMPO-2,4-di-NH2 pyrimidine derivative, compound 11, had CC50 values of around 60 μg/ml (Table 1). Compounds 1, 2, and 11 were not inhibitory to [methyl-3H]dThd, [3H]uridine, and [3H]Leu incorporation into trichloroacetic acid-insoluble CEM cell material within a 12-h incubation period at 200 μg/ml. Also, PMEA and (R)-PMPA were not inhibitory to macromolecular synthesis at this drug concentration (data not shown).
Antiretrovirus activity of acyclic pyrimidine nucleoside phosphonate analogues in several virus/cell systems.
6-PMEO derivatives, compounds 1 and 4, the 6-PME thioether (6-PMES), compound 2, and the (R)-6-PMPO derivative, compound 11, were evaluated for their inhibitory activity against several in vitro retrovirus models (Table 2). As a rule, the antiviral activity values of the test compounds found for HIV-1(IIIB) in CEM cell cultures (Table 1) matched very closely the antiviral activity values found for HIV-2(ROD) in CEM cells, for HIV-1 and HIV-2 in MT-4 cell cultures, and for FIV in CrFK cells. Thus, the EC50 values of compound 1 for HIV ranged between 0.29 and 0.80 μg/ml and those for compound 11 ranged between 1.3 and 3.0 μg/ml. These values were close to those observed for the reference compounds PMEA (adefovir) (EC50: 0.96 to 2.0 μg/ml) and (R)-PMPA (tenofovir) (EC50: 0.36 to 0.52 μg/ml). When the 6-PMEO and 6-(R)-PMPO derivatives were evaluated against HIV-1 in primary cells [HIV-1(IIIB) in peripheral blood lymphocytes (PBL) and HIV-1(BaL) in M/M], the antiretroviral potency was even more pronounced. Indeed, compounds 1 and 11 inhibited the virus in PBL at EC50s of 0.07 and 0.12 μg/ml, respectively, compared with 0.55 and 0.09 μg/ml for reference compounds PMEA and (R)-PMPA, respectively. The 6-PMEO and (R)-6-PMPO derivatives were even more inhibitory to HIV-1(BaL) in M/M, as also observed for PMEA and (R)-PMPA. As a rule, the compounds proved less cytotoxic in MT-4 cells than in CEM cells and not toxic at 100 μg/ml in M/M.
TABLE 2.
Antiretrovirus activity of 6-PMEO and (R)-6-PMPO derivatives in different cell typesa
| Compound or drug | EC50b (μg/ml) for strain (cell type):
|
CC50c,e (μg/ml)
|
MICd,e (μg/ml) for C3H | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HIV-1(IIIB) (CEM) | HIV-2(ROD) (CEM) | HIV-1(IIIB) (MT-4) | HIV-2(ROD) (MT-4) | HIV-1(IIIB) (PBL) | HIV-1(BaL) (M/M) | FIV (CrFK) | MSV (C3H) | CEM | MT-4 | PBL | M/M | CrFK | ||
| 1 | 0.9 ± 0.4 | 0.66 ± 0.19 | 0.34 ± 0.03 | 0.29 | 0.07 ± 0.02 | 0.002 ± 0.001 | 0.25 ± 0.01 | 0.16 ± 0.04 | 11 ± 2.0 | 46 ± 7.4 | 2.2 ± 0.8 | >100 | 11 | >40 (200) |
| 2 | 4.6 ± 3.1 | 3.0 ± 1.4 | 2.5 ± 0.3 | 2.9 | 0.97 ± 0.40 | 1.7 ± 0.9 | 64 ± 9.8 | ≥100 | 42 | >40 (200) | ||||
| 11 | 1.9 ± 0.5 | 1.3 ± 0.4 | 3.0 ± 0.4 | 1.6 ± 0.6 | 0.12 ± 0.01 | 0.005 ± 0.0 | 0.66 ± 0.14 | 0.05 ± 0.01 | 62 ± 25 | ≥100 | 9.6 ± 2.5 | >100 | >100 | >40 (200) |
| 4 | 1.4 ± 0.7 | 1.4 ± 1.2 | 1.9 ± 0.4 | 2.1 | 0.08 ± 0.03 | 2.5 ± 0.2 | 10 ± 2.2 | ≥16 | ||||||
| PMEA | 0.96 ± 0.24 | 1.9 ± 1.1 | 1.3 ± 1.1 | 1.9 | 0.55 ± 0.41 | 0.006 | 0.46 ± 0.19 | 0.62 ± 0.27 | 16 ± 9.1 | 28 ± 12 | 1.7 ± 0.4 | >100 | 18 | >40 (200) |
| (R)-PMPA | 0.36 ± 0.24 | 0.43 ± 0.41 | 0.46 ± 0.006 | 0.52 | 0.09 ± 0.03 | 0.003 ± 0.001 | 0.13 | 1.4 ± 0.9 | 125 ± 26 | 72 ± 12 | >100 | >100 | >100 | >200 |
Data are means ± standard deviations (SD) of two to four separate experiments. Data without an SD are the result of a single experiment carried out in duplicate.
Concentration required to inhibit HIV-induced cytopathicity (giant-cell formation) in CEM and MT-4 cells or p24 antigen production in PBL and M/M or MSV-induced C3H cell transformation by 50%.
CC50, concentration required to inhibit cell proliferation (CEM cells) or reduce cell viability (MT-4 cells, PBL, and M/M) by 50%.
MIC, concentration required to cause a microscopically visible alteration of cell morphology.
Symbols: ≥, slight microscopically visible morphological change; >, no visible toxicity at the indicated concentration. Values in parentheses are concentrations at which morphological toxicity was observed.
Since it has been previously shown that PMEA and (R)-PMPA exhibit pronounced inhibitory activity against MSV in both cell culture and newborn mice, the novel 6-PMEO and (R)-6-PMPO derivatives were also evaluated for their in vitro activity against MSV. Compounds 1, 11, and 4 were highly inhibitory against MSV-induced C3H cell transformation. The EC50 values were as low as 0.05 to 0.15 μg/ml (Table 2).
Effect of natural nucleosides and nucleobases on the anti-HIV activity of the acyclic pyrimidine nucleoside phosphonate analogues in cell culture.
It is well known that the anti-HIV activity of pyrimidine nucleoside analogues such as 3′-azido-3′-deoxythymidine (zidovudine) and 2′,3′-dideoxycytidine (zalcitabine) can be affected (i.e., diminished) in the presence of natural nucleosides such as dThd and 2′-deoxycytidine (dCyd) (4, 10). Therefore, to estimate whether the presence of natural nucleosides and nucleobases could influence the anti-HIV activity of the novel 6-PMEO and (R)-6-PMPO pyrimidine derivatives, the effect of subtoxic concentrations of pyrimidine nucleosides dThd and dCyd, purine nucleosides adenosine and guanosine, and nucleobase adenine was examined (Table 3). None of the natural nucleosides and nucleobases had any measurable influence on the anti-HIV-1 activity of the test compounds in CEM cell cultures. In all cases, the antiretroviral activity of the compounds was fully preserved, as was also the case for the reference compounds PMEA, (R)-PMPA, and 9-[2-(phosphonylmethoxy)ethyl]guanine (PMEG) (Table 3).
TABLE 3.
Effect of natural nucleosides and nucleobases on the antiviral activity of 6-PMEO and (R)-PMPO derivatives
| Compound or drug | EC50a (μg/ml) upon addition of:
|
|||||
|---|---|---|---|---|---|---|
| Nothing | dThd (10 μM) | dCyd (1 mM) | Ado (400 μM) | Guo (10 μM) | Ade (100 μM) | |
| 1 | 0.77 ± 0.25 | 0.35 ± 0.07 | 0.55 ± 0.35 | 0.63 ± 0.29 | 0.65 ± 0.21 | 0.26 ± 0.16 |
| 2 | 1.9 ± 0.75 | 1.4 ± 0.21 | 2.1 ± 1.7 | 4.4 ± 2.8 | 1.9 ± 0.49 | 3.3 ± 3.2 |
| 11 | 1.2 ± 0.25 | 0.83 ± 0.35 | 1.1 ± 0.51 | 1.5 ± 0.70 | 0.77 ± 0.38 | 0.35 ± 0.17 |
| 4 | 1.4 ± 0.71 | 1.0 ± 0.3 | 2.4 ± 1.9 | 1.4 ± 0.93 | 1.9 ± 0.92 | 1.7 ± 1.2 |
| PMEA | 0.94 ± 0.24 | 1.4 ± 0.4 | 2.1 ± 0.19 | 1.6 ± 0.4 | 1.0 ± 0.8 | 1.9 ± 1.3 |
| (R)-PMPA | 0.57 ± 0.26 | 0.43 ± 0.0 | 0.53 ± 0.26 | 0.46 ± 0.23 | 0.43 ± 0.0 | 1.6 ± 1.6 |
| PMEG | >0.05 | >0.25 | >0.25 | >0.05 | >0.05 | >0.05 |
Concentration required to inhibit HIV-1(IIIB)-induced cytopathicity in CEM cell cultures. Data represent the means ± standard deviations of at least two or three separate experiments. Ado, adenosine; Guo, guanosine; Ade, adenine.
Efficacy of acyclic pyrimidine nucleoside phosphonate analogues against MSV-induced tumor formation in newborn NMRI mice.
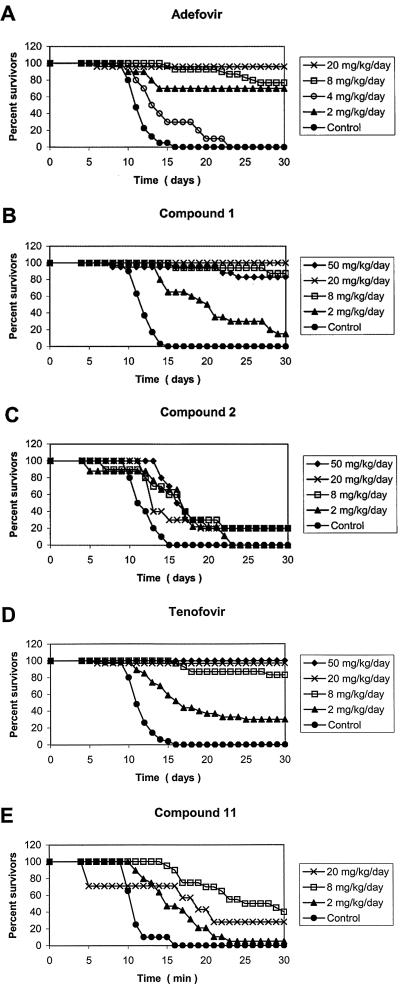
6-PMEO derivative compound 1 and its thioether analogue, compound 2, and the (R)-6-PMPO derivative, compound 11, were evaluated for their inhibitory effect on MSV-induced tumor formation and associated death in newborn NMRI mice (Table 4; Fig. 2 and 3). PMEA (adefovir) and (R)-PMPA (tenofovir) were included as reference compounds. Of the novel 6-PMEO and (R)-6-PMPO derivatives, compound 1 proved to be the most efficient in preventing MSV-induced tumor formation and associated death of newborn NMRI mice (Table 4). At least 80% of mice were protected from tumor formation at 50 and 20 mg/kg, and the remaining mice that developed tumors survived for more than 30 days postinfection. At a dose as low as 2 mg/kg, compound 1 could still prevent tumor formation in 5% of the mice and produced 15% long-term survivors. Compound 1 had an ability to prevent tumor formation and associated animal death comparable to those of PMEA and (R)-PMPA (Table 4). In contrast, the corresponding thioether derivative, compound 2, was unable to prevent tumor formation at all doses tested, and it produced 20% long-term survivors at a dose of 8 to 50 mg/kg. The capacity of the (R)-6-PMPO derivative, compound 11, to prevent tumor formation and associated death was intermediate between those observed for compounds 1 and 2. The delay of tumor formation in drug-treated mice is shown in more detail in Fig. 2. All drugs delayed MSV-induced tumor formation in a dose-dependent manner. Compound 1 had an inhibitory effect that was comparable to that of (R)-PMPA and PMEA. Compound 11 was inferior to compound 1, and compound 2 produced a pronounced delay in tumor formation only at a dose of 50 mg/kg. In Fig. 3, the effect of the drugs on survival of the mice is shown in detail. As noted for tumor formation, delay of associated animal death was also dose dependent, and compound 1 was at least as efficient in delaying death as the reference drugs, PMEA and (R)-PMPA.
TABLE 4.
Anti-MSV activity of acyclic pyrimidine nucleoside phosphonates in newborn NMRI mice
| Compound or drug | Dose (mg/kg)a | No. of mice used in expt | % of mice not develop- ing a tumor | % of long- term survivors (>30 days) |
|---|---|---|---|---|
| 1 (PMEO-2,4-di-NH2-Pym) | 50 | 19 | 84 | 100b |
| 20 | 20 | 80 | 100 | |
| 8 | 18 | 44 | 89 | |
| 2 | 20 | 5 | 15 | |
| 2 (PMES-2,4-di-NH2-Pym) | 50 | 10 | 0 | 20 |
| 20 | 10 | 0 | 20 | |
| 8 | 10 | 0 | 20 | |
| 2 | 8 | 0 | 0 | |
| 11 (PMPO-2,4-di-NH2-Pym) | 20 | 10 | 20 | 40c |
| 8 | 20 | 15 | 40 | |
| 2 | 18 | 0 | 5 | |
| PMEA | 100 | 10 | 100d | 0d |
| 20 | 29 | 89 | 94 | |
| 8 | 30 | 44 | 77 | |
| 4 | 20 | 5 | 35 | |
| (R)-PMPA | 50 | 10 | 100 | 100 |
| 20 | 28 | 92 | 100 | |
| 8 | 27 | 65 | 83 | |
| 2 | 28 | 0 | 31 |
Dose given 2 h prior to MSV infection followed by four additional daily doses during the 4 subsequent days postinfection. The mean day of tumor development in control MSV-infected mice was 4.5 days, whereas the mean day of animal death in the MSV-infected mice was 12.3 days.
At the indicated drug dose (50 mg/kg), 16% of mice died prematurely (<24 days), likely due to toxicity of the drug and without signs of tumor formation. Premature death was not taken into account to calculate the percentage of long-term survivors.
At the indicated drug dose (20 mg/kg), 50% of mice died prematurely (<6 days), likely due to toxicity of the drug and without signs of tumor formation. Premature death was not taken into account to calculate the percentage of long-term survivors.
All mice died before day 6, likely due to toxicity of the drug. None of the mice showed tumor formation before death.
FIG. 3.
Delay of death in drug-treated MSV-infected newborn NMRI mice.
Sensitivity of mutated HIV-1 strains to acyclic pyrimidine nucleoside phosphonate analogues in CEM cell cultures.
Compounds 1, 2, and 11 were evaluated for their inhibitory activity against HIV-1(IIIB) strains with nonnucleoside RT inhibitor-specific L100I, K103N, Y181C, and Y188H mutations in the RT. All three compounds retained full activity against these mutant virus strains (data not shown). The same compounds were also evaluated on their inhibitory effect on clinical isolates HIV-1/L1S, HIV-1/L6S, and HIV-1/L6S/PMEA (Table 5). PMEA and (R)-PMPA were included as reference compounds. Compound 1 retained pronounced antiviral activity against the three virus strains. Compounds 2 and particularly 11 showed clearly decreased activity against the HIV-1/L6S/PMEA isolate, and so did PMEA and (R)-PMPA. Thus, the multi-NRTI resistance mutations (S68G, K70T, V75I, F77L, F116Y, and Q151 M) and the K65R mutation, characteristic of PMEA resistance, present in HIV-1/L6S/PMEA did not markedly affect the antiviral potency of compound 1 and PMEA (∼3.6- to 4.5-fold-increased EC50) but decreased sensitivity to the other ANPs to a greater extent (Table 5).
TABLE 5.
Inhibitory activity of acyclic pyrimidine nucleoside phosphonate analogues against clinical HIV-1 isolates
| Compound or drug | Relative resistancea of:
|
||
|---|---|---|---|
| HIV-1/L1Sb | HIV-1/L6Sc | HIV-1/L6S/PMEAd | |
| 1 | 0.8 | 1.5 | 3.6 |
| 2 | 2.1 | 1.4 | 14 |
| 11 | 1.9 | 3.5 | 30 |
| PMEA | 2.0 | 2.7 | 7.3 |
| (R)-PMPA | 2.3 | 11 | 54 |
Resistance relative to that of laboratory HIV-1(IIIB) strain in CEM cell cultures.
Clinical isolate derived from a patient not treated with NRTIs or ANPs and not containing NRTI- or ANP-specific mutations in the RT.
Clinical isolate derived from a drug-treated patient. The RT contains the S68G, K70T, V75I, F77L, F116Y, and Q151M mutations.
HIV-1/L6S isolate cultured in the presence of PMEA and containing the K65R mutation in addition to the NRTI-specific mutations listed in footnote c.
DISCUSSION
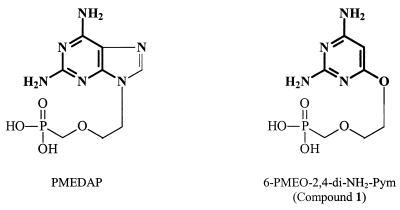
The novel class of ANPs reported in this study have as salient features a pyrimidine ring that obligatorily contains a C-2 amino group and (preferably) also a C-4 amino group and a PME or PMP moiety linked to the C-6 position of the pyrimidine ring via an ether function (preferably oxyether). The most active compounds among these series of structures, i.e., 6-PMEO derivative compound 1 and the (R)-6-PMPO derivative, compound 11, had pronounced antiretroviral activity both in cell culture and in an in vivo murine model. The antiviral activity of the novel compounds was comparable to that found for the established reference drugs adefovir and tenofovir. The structure-activity relationship studies for the 6-PMEO and (R)-6-PMPO pyrimidine derivatives revealed that the concomitant presence of amino groups at the C-2 and C-4 positions of the pyrimidine base is a prerequisite for optimal antiviral activity. In addition to PMEA and (R)-PMPA, PMEDAP, (R)-PMPDAP, and PMEG ranked among the most potent and selective antiretroviral drugs (10). It should be noted that the novel 6-PMEO and (R)-6-PMPO pyrimidine derivatives reported in this study can be considered mimics of the corresponding 9-(2-phosphonylmethoxyethyl)-2,6-diaminopurine (PMEDAP) and (R)-9-[2-(phosphonylmethoxy)propyl]-2,6-diaminopurine [(R)-PMPDAP] derivatives in that the 2,4-diamino substituted pyrimidine ring may be considered an analogue of the pyrimidine ring in the 2,6-diaminopurine ANP derivatives (Fig. 4). Thus, the pyrimidine moiety of the novel 6-PMEO and (R)-6-PMPO derivatives may act as an incomplete purine ring that retains the most important part of the purine base for recognition by the phosphorylating (activating) enzymes, with RT being the most likely target enzyme for their antiretroviral activity. Due to the need of metabolic activation (phosphorylation) (6, 33, 34), the phosphonate oxygens should be freely accessible for phosphorylation. Although it seems obvious to test compounds that contain an amine linker instead of the ether or thioether linker between the base part and the acyclic side chain, such compounds, which have been synthesized in the past, have been found to lack anti-HIV activity (21, 51). PMEA and (R)-PMPA are administered to patients as their oral bioavailable adefovir dipivoxyl and tenofovir disoproxil prodrugs. Therefore, it would be interesting to synthesize the corresponding PMEO and (R)-PMPO pyrimidine derivatives to investigate the pharmacokinetic properties of such prodrugs.
FIG. 4.
Structural similarities (boldface) between PMEDAP and compound 1.
It is interesting that neither the anti-HIV activity of the newly described 6-PMEO and (R)-6-PMPO derivatives nor that of PMEA, PMPA, and PMEG could be reversed by natural nucleosides and nucleobases. This behavior is different from that of nucleoside analogues (i.e., zidovudine, zalcitabine, stavudine, and lamivudine), whose antiviral activity can be reversed by their corresponding natural nucleosides (i.e., dThd and dCyd), presumably by competition for phosphorylation by the required nucleoside kinases (i.e., dThd kinase, dCyd kinase) (12). Therefore, it is not unexpected that the antiviral potency of the novel ANPs [as also shown for their corresponding parent PMEA and (R)-PMPA derivatives] has not been very much influenced by endogenous nucleoside levels.
The structural similarity between the novel 6-PMEO and (R)-6-PMPO pyrimidine derivatives with the known PME- and PMP-purine derivatives (Fig. 2 and 4) is clearly in agreement with the observed biological activities of the novel drugs. Indeed, as for PMEA and PMEDAP, which inhibit both DNA viruses (i.e., HSV and VV) and retroviruses (i.e., HIV, FIV, and MSV), the 6-PMEO pyrimidine derivatives show similar spectra of antiviral activity (31). Also, the (virtually exclusive) preference of PMP-purine derivatives for antiretroviral activity was also observed for 6-PMPO pyrimidine derivative compound 11, although some anti-DNA virus activity was observed for this compound (31). Even more, the same enantiospecificity [preference of the (R) enantiomer over the (S) enantiomer] could be found for both series of PMP and PMPO analogues, again pointing to a likely and striking similarity in activation pattern and mechanism of anti(retro)viral action between the novel series of pyrimidine ANPs described herein and established purine ANPs PMEA and (R)-PMPA. However, the molecular basis of the antiretroviral activity of the novel compounds has not been revealed yet.
Our drug sensitivity findings for the drug-resistant isolates revealed that compound 1 (and also PMEA) retained pronounced antiviral activity against multi-NRTI/PMEA-resistant HIV-1 while the other ANPs showed higher levels of resistance against the clinical virus isolates. However, it should be mentioned that these data were generated for only one clinical isolate with mutations characteristic of resistance to multi-NRTI and PMEA. More in depth studies need to be performed with a variety of other NRTI-resistant clinical HIV-1 isolates before firm conclusions on the sensitivity spectrum of H-3404 against NRTI-resistant virus strains can be drawn.
In conclusion, we have demonstrated in this study that the 6-PMEO and (R)-6-PMPO (and thioether) pyrimidine derivatives possess a pronounced antiretroviral activity in a variety of virus/cell models, including HIV-1-infected PBL and M/M, a marked antiretroviral (MSV) activity in vivo, and a potency equivalent to that of clinically used adefovir and tenofovir. This novel class of pyrimidine ANPs should be further pursued for their potential as antiretroviral agents in the clinical setting.
Acknowledgments
We thank Ann Absillis, Cindy Heens, Lizette van Berckelaer, and Ria Van Berwaer for their excellent technical assistance and Christiane Callebaut for dedicated editorial help.
This work was supported by grants from the Fonds voor Geneeskundig Wetenschappelijk Onderzoek—Vlaanderen (G.0104.98), the Geconcerteerde Onderzoeksacties (GOA) Krediet no. 00/12, the Descartes Prize-2001 of the European Commission, and the Czech State Grant Agency (203/94/K001).
Footnotes
This study constitutes a part of research project 4055905 of the Institute of Organic Chemistry and Biochemistry, Prague, Czech Republic.
REFERENCES
- 1.Aduma, P. P., M. C. Connelly, R. V. Srinivas, and A. Fridland. 1995. Metabolic diversity and antiviral activities of acyclic nucleoside phosphonates. Mol. Pharmacol. 47:816-822. [PubMed] [Google Scholar]
- 2.Andrei, G., R. Snoeck, D. Schols, P. Goubau, J. Desmyter, and E. De Clercq. 1991. Comparative activity of selected antiviral compounds against clinical isolates of human cytomegalovirus. Eur. J. Clin. Microbiol. Infect. Dis. 10:1026-1033. [DOI] [PubMed] [Google Scholar]
- 3.Andrei, G., R. Snoeck, P. Goubau, J. Desmyter, and E. De Clercq. 1992. Comparative activity of various compounds against clinical strains of herpes simplex virus. Eur. J. Clin. Microbiol. Infect. Dis. 11:143-151. [DOI] [PubMed] [Google Scholar]
- 4.Balzarini, J., and E. De Clercq. 1994. Biochemical pharmacology of nucleoside analogs active against HIV, p. 751-772. In S. Broder, T. C. Merigan, and D. Bolognesi (ed.), Textbook of AIDS medicine. Williams & Wilkins, Baltimore, Md.
- 5.Balzarini, J., L. Naesens, P. Herdewijn, I. Rosenberg, A. Holý, R. Pauwels, M. Baba, D. G. Johns, and E. De Clercq. 1989. Marked in vivo antiretrovirus activity of 9-(2-phosphonylmethoxyethyl)adenine, a selective anti-human immunodeficiency virus agent. Proc. Natl. Acad. Sci. USA 86:332-336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Balzarini, J., Z. Hao, P. Herdewijn, D. G. Johns, and E. De Clercq. 1991. Intracellular metabolism and mechanism of anti-retrovirus action of 9-(2-phosphonylmethoxyethyl)-adenine, a potent anti-human immunodeficiency virus compound. Proc. Natl. Acad. Sci. USA 88:1499-1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Balzarini, J., A. Holý, J. Jindrich, H. Dvoráková, Z. Hao, R., Snoeck, P. Herdewijn, D. G. Johns, and E. De Clercq. 1991. 9-[(2RS)-3-Fluoro-2-phosphonylmethoxypropyl] derivatives of purines: a class of highly selective antiretroviral agents in vitro and in vivo. Proc. Natl. Acad. Sci. USA 88:4961-4965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Balzarini, J., C.-F. Perno, D. Schols, and E. De Clercq. 1991. Activity of acyclic nucleoside phosphonate analogues against human immunodeficiency virus in monocyte/macrophages and peripheral blood lymphocytes. Biochem. Biophys. Res. Commun. 178:329-335. [DOI] [PubMed] [Google Scholar]
- 9.Balzarini, J., L. Naesens, J. Slachmuylders, H. Niphuis, I. Rosenberg, A. Holý, H. Schellekens, and E. De Clercq. 1991. 9-(2-Phosphonylmethoxyethyl)adenine (PMEA) effectively inhibits retrovirus replication in vitro and simian immunodeficiency virus infection in rhesus monkeys. AIDS 5:21-28. [DOI] [PubMed] [Google Scholar]
- 10.Balzarini, J., A. Holý, J. Jindrich, L. Naesens, R. Snoeck, D. Schols, and E. De Clercq. 1993. Differential antiherpesvirus and antiretrovirus effects of the (S) and (R) enantiomers of acyclic nucleoside phosphonates: potent and selective in vitro and in vivo antiretrovirus activities of (R)-9-(2-phosphonomethoxypropyl)-2,6-diaminopurine. Antimicrob. Agents Chemother. 37:332-338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Balzarini, J., S. Aquaro, C.-F. Perno, M. Witvrouw, A. Holý, and E. De Clercq. 1996. Activity of the (R)-enantiomers of 9-(2-phosphonyl-methoxypropyl)adenine and 9-(2-phosphonyl-methoxypropyl)-2,6-diaminopurine against human immunodeficiency virus in different human cell systems. Biochem. Biophys. Res. Commun. 219:337-341. [DOI] [PubMed] [Google Scholar]
- 12.Cooney, D. A., M. Dalal, H. Mitsuya, J. B. McMahon, M. Nadkarni, J. Balzarini, S. Broder, and D. G. Johns. 1986. Initial studies on the cellular pharmacology of 2′,3′-dideoxycytidine, an inhibitor of HTLV-III infectivity. Biochem. Pharmacol. 35:2065-2068. [DOI] [PubMed] [Google Scholar]
- 13.De Clercq, E., and T. C. Merigan. 1971. Moloney sarcoma virus-induced tumors in mice: inhibition or stimulation by (poly rI):(poly rC). Proc. Soc. Exp. Biol. Med. 137:590-594. [Google Scholar]
- 14.De Clercq, E., A. Holý, I. Rosenberg, T. Sakuma, J. Balzarini, and P. C. Maudgal. 1986. A novel selective broad-spectrum anti-DNA virus agent. Nature (London) 323:464-467. [DOI] [PubMed] [Google Scholar]
- 15.De Clercq, E., T. Sakuma, M. Baba, R. Pauwels, J. Balzarini, I. Rosenberg, and A. Holý. 1987. Antiviral activity of phosphonylmethoxyalkyl derivatives of purine and pyrimidines. Antivir. Res. 8:261-272. [DOI] [PubMed] [Google Scholar]
- 16.De Clercq, E., A. Holý, and I. Rosenberg. 1989. Efficacy of phosphonylmethoxyalkyl derivatives of adenine in experimental herpes simplex virus and vaccinia virus infections in vivo. Antimicrob. Agents Chemother. 33:185-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Clercq, E. 1991. Broad-spectrum anti-DNA virus and antiretrovirus activity of phosphonylmethoxyalkylpurine and -pyrimidines. Biochem. Pharmacol. 42:963-972. [DOI] [PubMed] [Google Scholar]
- 18.De Clercq, E. 1997. Acyclic nucleoside phosphonates in the chemotherapy of DNA virus and retrovirus infections. Intervirology 40:295-303. [DOI] [PubMed] [Google Scholar]
- 19.De Clercq, E. 1997. In search of a selective antiviral chemotherapy. Clin. Microbiol. Rev. 10:674-693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Egberink, H., M. Borst, H. Niphuis, J. Balzarini, H. Neu, H. Schellekens, E. De Clercq, M. Horzinek, and M. Koolen. 1990. Suppression of feline immunodeficiency virus infection in vivo by 9-(2-phosphonylmethoxyethyl)adenine. Proc. Natl. Acad. Sci. USA 87:3087-3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eger, K., E. M. Klunder, and M. Schmidt. 1994. Synthesis of new acyclic pyrimidine nucleoside analogues as potential antiviral drugs. J. Med. Chem. 37:3057-3061. [DOI] [PubMed] [Google Scholar]
- 22.Gangemi, J. D., R. M. Cozens, E. De Clercq, J. Balzarini, and H.-K. Hochkeppel. 1989. 9-(2-Phosphonylmethoxyethyl)adenine in the treatment of murine acquired immunodeficiency disease and opportunistic herpes simplex virus infections. Antimicrob. Agents Chemother. 33:1864-1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hartmann, K., A. Donath, B. Beer, H. F. Egberink, M. C. Horzinek, H. Lutz, G. Hoffmann-Fezer, I. Thum, and S. Thefeld. 1992. Use of two virustatica (AZT, PMEA) in the treatment of FIV and of FeLV seropositive cats with clinical symptoms. Vet. Immunol. Immunopathol. 35:167-175. [DOI] [PubMed] [Google Scholar]
- 24.Hartmann, K., J. Balzarini, J. Higgins, E. De Clercq, N. C. Pedersen. 1994. In vitro activity of acyclic nucleoside phosphonate derivatives against feline immunodeficiency virus in Crandell feline kidney cells and feline peripheral blood lymphocytes. Antivir. Chem. Chemother. 5:13-19. [Google Scholar]
- 25.Heijtink, R. A., G. A. De Wilde, J. Kruining, L. Berk, J. Balzarini, E. De Clercq, A. Holý, and S. W. Schalm. 1993. Inhibitory effect of 9-(2-phosphonylmethoxyethyl)adenine (PMEA) on human and duck hepatitis B virus infection. Antivir. Res. 21:141-153. [DOI] [PubMed] [Google Scholar]
- 26.Heijtink, R. A., J. Kruining, G. A. De Wilde, J. Balzarini, E. De Clercq, and S. W. Schalm. 1994. Inhibitory effects of acyclic nucleoside phosphonates on human hepatitis B virus and duck hepatitis B virus infections in tissue culture. Antimicrob. Agents Chemother. 38:2180-2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Holý, A., and I. Rosenberg. 1987. Synthesis of isomeric and enantiomeric O-phosphonylmethyl derivatives of 9-(2,3-dihydroxypropyl)adenine. Collect. Czech. Chem. Commun. 52:2775-2791. [Google Scholar]
- 28.Holý, A., and I. Rosenberg. 1987. Synthesis of 9-(2-phosphonylmethoxyethyl)adenine and related compounds. Collect. Czech. Chem. Commun. 52:2801-2809. [Google Scholar]
- 29.Holý, A., I. Votruba, A. Merta, J. Cerný, J. Veselý, J. Vlach, K. Sedivá, I. Rosenberg, M. Otmar, H. Hrebabecký, M. Trávnícek, V. Vonka, R. Snoeck, and E. De Clercq. 1990. Acyclic nucleotide analogues: synthesis, antiviral activity and inhibitory effects on some cellular and virus-encoded enzymes in vitro. Antivir. Res. 13:295-312. [DOI] [PubMed] [Google Scholar]
- 30.Holý, A., J. Günter, H. Dvoráková, M. Masojídková, G. Andrei, R. Snoeck, J. Balzarini, and E. De Clercq. 1999. Structure-antiviral activity relationship in the series of pyrimidine and purine N-[2-(2-phosphonomethoxy)ethyl] nucleotide analogues. 1. Derivatives substituted at the carbon atoms of the base. J. Med. Chem. 42:2064-2086. [DOI] [PubMed] [Google Scholar]
- 31.Holý, A., I. Votruba, M. Masojídková, G. Andrei, R. Snoeck, E. De Clercq, and J. Balzarini. 2002. 6-[2-(Phosphonomethoxy)alkoxy]pyrimidines with antiviral activity. J. Med. Chem. 45:1918-1929. [DOI] [PubMed] [Google Scholar]
- 32.Hoover, E. A., J. P. Ebner, N. S. Zeidner, and J. I. Mullins. 1991. Early therapy of feline leukemia virus infection (FeLV-AIDS) with 9-(2-phosphonylmethoxyethyl)adenine (PMEA). Antivir. Res. 16:77-92. [DOI] [PubMed] [Google Scholar]
- 33.Krejcová, R., K. Horská, I. Votruba, and A. Holý. 2000. Interaction of guanine phosphonomethoxyalkyl derivatives with GMP kinase isoenzymes. Biochem. Pharmacol. 60:1907-1913. [DOI] [PubMed] [Google Scholar]
- 34.Krejcová, R., K. Horská, I. Votruba, and A. Holý. 2000. Phosphorylation of purine(phosphonomethoxy)alkyl derivatives by mitochondrial AMP kinase (AK2 type) from L1210 cells. Collect. Czech. Chem. Commun. 65:1653-1668. [Google Scholar]
- 35.Lin, J. C., E. De Clercq, and J. S. Pagano. 1987. Novel acyclic adenosine analogs inhibit Epstein-Barr virus replication. Antimicrob. Agents Chemother. 31:1431-1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Naesens, L., J. Balzarini, I. Rosenberg, A. Holý, and E. De Clercq. 1989. 9-(2-Phosphonylmethoxyethyl)-2,6-diaminopurine (PMEDAP): a novel agent with anti-human immunodeficiency virus activity in vitro and potent anti-Moloney murine sarcoma virus activity in vivo. Eur. J. Clin. Microbiol. Infect. Dis. 8:1043-1047. [DOI] [PubMed] [Google Scholar]
- 37.Naesens, L., and E. De Clercq. 1997. Therapeutic potential of HPMPC (cidofovir), PMEA (adefovir) and related acyclic nucleoside phosphonate analogues as broad-spectrum antiviral agents. Nucleosides Nucleotides 16:983-992. [Google Scholar]
- 38.Naesens, L., R. Snoeck, G. Andrei, J. Balzarini, J. Neyts, and E. De Clercq. 1997. HPMPC (cidofovir), PMEA (adefovir) and related acyclic nucleoside phosphonate analogues: a review of their pharmacology and clinical potential in the treatment of viral infections. Antivir. Chem. Chemother. 8:1-23. [Google Scholar]
- 39.Pauwels, R., J. Balzarini, D. Schols, M. Baba, J. Desmyter, I. Rosenberg, A. Holý, and E. De Clercq. 1988. Phosphonylmethoxyethyl purine derivatives, a new class of anti-human immunodeficiency virus agents. Antimicrob. Agents Chemother. 32:1025-1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Popovic, M., M. G. Sarngadharan, E. Read, and R. C. Gallo. 1984. Detection, isolation, and continuous production of cytopathic retroviruses (HTLV-III) from patients with AIDS and pre-AIDS. Science 224:497-500. [DOI] [PubMed] [Google Scholar]
- 41.Reymen, D., L. Naesens, J. Balzarini, A. Holý, H. Dvoráková, and E. De Clercq. 1995. Antiviral activity of selected acyclic nucleoside analogues against human herpes virus 6. Antivir. Res. 28:343-357. [DOI] [PubMed] [Google Scholar]
- 42.Snoeck, R., T. Sakuma, E. De Clercq, I. Rosenberg, and A. Holý. 1988. (S)-1-(3-Hydroxy-2-phosphonylmethoxypropyl)cytosine, a potent and selective inhibitor of human cytomegalovirus replication. Antimicrob. Agents Chemother. 32:1839-1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Soike, K. F., J.-L. Huang, J.-Y. Zhang, R. Bohm, M. J. M. Hitchcock, and J. C. Martin. 1991. Evaluation of infrequent dosing regimens with (S)-1-[3-hydroxy-2-(phosphonylmethoxy)propyl]cytosine [(S)-HPMPC] on simian varicella infections in monkeys. Antivir. Res. 16:17-28. [DOI] [PubMed] [Google Scholar]
- 44.Srinivas, R. V., B. L. Robbins, M. C. Connelly, Y.-F. Gong, N. Bischofberger, and A. Fridland. 1993. Metabolism and in vitro antiretroviral activities of bis(pivaloyloxymethyl) prodrugs of acyclic nucleoside phosphonates. Antimicrob. Agents Chemother. 37:2247-2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Starrett, J. E., D. R. Tortolani, M. J. M. Hitchcock, J. C. Martin, and M. M. Mansuri. 1992. Synthesis and in vitro evaluation of a phosphonate prodrug: bis(pivaloyloxymethyl) 9-(2-phosphonylmethoxyethyl)adenine. Antivir. Res. 19:267-273. [DOI] [PubMed] [Google Scholar]
- 46.Starrett, J. E., D. R. Tortolani, J. Russell, M. J. M. Hitchcock, V. Whiterock, J. C. Martin, and M. M. Mansuri. 1994. Synthesis, oral bioavailability determination and in vitro evaluation of prodrugs of the antiviral agent 9-[2-(phosphonomethoxy)ethyl]adenine (PMEA). J. Med. Chem. 37:1857-1864. [DOI] [PubMed] [Google Scholar]
- 47.Thormar, H., J. Balzarini, A. Holý, J. Jindrich, I. Rosenberg, Z. Debyser, J. Desmyter, and E. De Clercq. 1993. Inhibition of visna virus replication by 2′,3′-dideoxynucleosides and acyclic nucleoside phosphonate analogs. Antimicrob. Agents Chemother. 37:2540-2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thormar, H., G. Georgsson, P. A. Palsson, J. Balzarini, L. Naesens, S. Torsteinsdottir, and E. De Clercq. 1995. Inhibitory effect of 9-(2-phosphonylmethoxyethyl)adenine on visna virus infection in lambs: model for in vivo testing of candidate anti-human immunodeficiency virus drugs. Proc. Natl. Acad. Sci. USA 92:3283-3287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Van Laethem, K., M. Witvrouw, C. Pannecouque, B. Van Remoortel, J.-C. Schmit, R. Esnouf, J.-P. Kleim, J. Balzarini, J. Desmyter, E. De Clercq, and A.-M. Vandamme. 2001. Mutations in the non-nucleoside binding-pocket interfere with the multi-nucleoside resistance phenotype. AIDS 15:553-561. [DOI] [PubMed] [Google Scholar]
- 50.Van Rompay, K. K. A., J. M. Cherrington, M. L. Marthas, C. J. Berardi, A. S. Mulato, A. Spinner, R. P. Tarara, D. R. Canfield, S. Telm, N. Bischofberger, and N. C. Pedersen. 1996. 9-[2-(Phosphonylmethoxy)adenine] therapy of established simian immunodeficiency virus infection in infant rhesus macaques. Antimicrob. Agents Chemother. 40:2586-2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wormstädt, F., U. Brinckemann, M. Gutschow, and K. Eger. 2000. Synthesis of acyclic nucleoside phosphonates as antiviral compounds. J. Heterocycl. Chem. 37:1187-1191. [Google Scholar]