Abstract
Nitazoxanide (NTZ) is a redox-active nitrothiazolyl-salicylamide prodrug that kills Helicobacter pylori and also many anaerobic bacterial, protozoan, and helminthic species. Here we describe development and use of a spectrophotometric assay, based on nitroreduction of NTZ at 412 nm, to identify H. pylori enzymes responsible for its activation and mode of action. Three enzymes that reduce NTZ were identified: two related NADPH nitroreductases, which also mediate susceptibility to metronidazole (MTZ) (RdxA and FrxA), and pyruvate oxidoreductase (POR). Recombinant His-tagged RdxA, FrxA, and POR, overexpressed in nitroreductase-deficient Escherichia coli, each rapidly reduced NTZ, whereas only FrxA and to a lesser extent POR reduced nitrofuran substrates (furazolidone, nitrofurantoin, and nitrofurazone). POR exhibited no MTZ reductase activity either in extracts of H. pylori or following overexpression in E. coli; RdxA exhibited no nitrofuran reductase activity, and FrxA exhibited no MTZ reductase activity. Analysis of mutation to rifampin resistance (Rifr) indicated that NTZ was not mutagenic and that nitrofurans were only weakly mutagenic. Alkaline gel DNA electrophoresis indicated that none of these prodrugs caused DNA breakage. In contrast, MTZ caused DNA damage and was strongly mutagenic. We conclude that POR, an essential enzyme, is responsible for most or all of the bactericidal effects of NTZ against H. pylori. While loss-of-function mutations in rdxA and frxA produce a Mtzr phenotype, they do not contribute much to the innate susceptibility of H. pylori to NTZ or nitrofurans.
Prodrugs are particularly appealing as therapeutic agents because of their selective toxicity for microorganisms possessing specific activating enzymes (7). Metronidazole (MTZ) is a nitroimidazole prodrug that is often used in combination therapies against Helicobacter pylori, the microaerobic bacterium that causes gastritis and peptic ulcer disease and that is an early risk factor for gastric cancer (6, 19, 30). However, resistance to MTZ is very common and reduces the efficacy of MTZ-containing regimes (10). MTZ is also highly mutagenic, and hence its use may accelerate development of resistance to other clinically important antibiotics, such as clarithromycin (24, 29). These considerations have stimulated interest in alternative redox-active prodrugs in either first-line or salvage (failed eradication) therapies, especially those against which resistance may be uncommon in H. pylori. Alternative prodrugs include the nitrofurans and nitrothiazoles, which exhibit antimicrobial activity against H. pylori and also against facultative anaerobes, anaerobic bacterial and protozoan species, and helminths (12, 19, 21, 30, 32, 33). For these drugs, the spectrum of bioactivity and the mechanisms of activation are largely a function of the redox potential (E′7) of the 5-nitro groups. For example, nitrofurans (nitrofurazone, nitrofurantoin, and furazolidone) display relatively high redox potentials (−250 to −270 mV) and are reductively activated by a wide range of enzymes including the NAD(P)H nitroreductases of enteric bacteria (22, 23); whereas MTZ (nitroimidazole) is only activated by anaerobic enzyme systems generating very low redox potentials (−480 mV), such as the pyruvate:ferredoxin oxidoreductases and hydrogenases of susceptible anaerobes and protozoan parasites (7, 32, 33).
Nitazoxanide (NTZ) is a nitrothiazolyl-salicylamide derivative with a predicted redox potential in the ∼−360-mV range that is active against H. pylori (21, 35), anaerobic bacteria (6), helminths, and protozoa (5, 25) but not against enteric bacteria or aerobes and that is well tolerated by humans (1). The drug was particularly efficacious in eradicating infection and curing disease in a hamster model of antibiotic-induced diarrhea caused by Clostridium difficile compared to the standard vancomycin and metronidazole treatments (20). The drug and its deacylated prodrug form (tizoxanide [TIZ]) are effective in treatment of persistent diarrhea caused by Cryptosporidium parvum, Giardia intestinalisis, and Entamoeba histolytica (25, 26, 27). These drugs display antimicrobial activity against Mtzr strains of H. pylori (19, 21), and high-level resistance to them has not been found clinically. The Mtzr phenotype results from mutational inactivation of rdxA and often frxA as well, genes that encode two nitroreductases of H. pylori (11, 17, 18). Some strains display low-level resistance to furazolidone and nitrofurantoin and, where studied, are also Mtzr. This suggests that these nitroreductases contribute to drug activation (19). However, frxA and rdxA knockout mutations constructed in laboratory strains are not resistant to furazolidone and nitrofurantoin, which indicates that mutations in other genes are also needed for resistance (19).
To better understand the role of redox active enzymes of H. pylori in the activation of MTZ, NTZ, and the nitrofurans, we purified RdxA, FrxA, and pyruvate oxidoreductase (POR) following overexpression in Escherichia coli and examined each enzyme's substrate specificity. We also tested whether these drugs are mutagenic or cause DNA fragmentation. Here we report that RdxA, FrxA, and POR reduce NTZ, while FrxA and to a lesser extent POR (but not RdxA) reduce the nitrofurans, and only RdxA exhibits MTZ reductase activity, as previously established (11). Reductive products of MTZ activation were mutagenic and DNA damaging (29), while reductive products of NTZ and nitrofuran activation caused no DNA damage and only nitrofuran substrates were weakly mutagenic. Our studies show that the selective toxicity of nitazoxanide for H. pylori is due to its efficient activation by POR, an essential key enzyme of central intermediary metabolism.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
H. pylori strains 26695 (Mtzs) (31), its Mtzr (rdxA deletion) mutant, HP950 (Mtzs), HP1061 (Mtzr), HP1061 containing the pDH26:rdxA plasmid (Mtzs), and HP439 (Mtzr) have been previously described (11). New clinical isolates were obtained from the Queen Elizabeth II Health Science Center (Halifax, Nova Scotia), and their MTZ resistance was determined by E-test as previously described (11, 35). The MICs for nitazoxanide and tizoxanide for each clinical isolate were determined by agar dilution on Brucella agar (BA) supplemented with 7.5% newborn calf serum (Sigma) (11) over a range of 0.064 to 64 μg/ml. E. coli strain CC104 [ara Δ(lac proB)/F′ lacI lacZ proAB+] contains a mutation in lacZ that renders it unable to ferment lactose (4). The rdxA gene from H. pylori strain 950 was amplified by PCR, and cloned into the pBluescript (pBSK) cloning vector, and introduced into E. coli CC104 as previously described (29). E. coli strains AB1157 (wild type) and JVQ2 (nfsA nfsB) were obtained from I. B. Lambert (34) and used as host strains for expression of POR from pBSK. MTZ susceptibility of E. coli strains expressing RdxA or FrxA was determined by dilution and plating on Luria-Bertani (LB) medium supplemented with various concentrations of MTZ (0 to 250 μg/ml).
DNA manipulations and cloning of rdxA, frxA, and porDGAB.
H. pylori genomic DNA was isolated from confluent cultures grown on BA plates using the cetyltrimethyl-ammonium bromide-phenol method (28). PCR was carried out in 20-μl volumes containing 10 ng of genomic DNA, 10 pmol of each primer, 1 U of Taq DNA polymerase (MBI Fermentas) or high-fidelity pwo Taq (Roche Diagnostics), and 0.25 mmol of each deoxynucleoside triphosphate in standard PCR buffer. Reaction mixtures were preincubated for 2 min at 94°C and subjected to 30 cycles of 94 oC (30 s), 50°C (30s), and 72 oC (30 s or 3 min for long sequences) and a final extension period of 7 min.
The rdxA and frxA genes were amplified from H. pylori strain 26695 or HP950 using primer pairs RDXBMHIF (5′ GCGGATCCGATGAAATTTTTGGATCAAG) and RdxXHO1R (5′ CCGCTCGAGCAACCAAGTAATCGC) for rdxA and FrxBMF (5′ GGATCGATGGACAGAGAACAAATT) and FrxXHO1R (5′ CCGCTCGAGTTCAATCACTTCATA) for frxA, and the amplicons were cloned into the expression vector pET29b (Novagen) to place six histidine residues at the C termini. The plasmids were transformed into E. coli strain BL21 by electroporation. The pyruvate oxidoreductase operon (porDGAB), contained in a 3.1-kbp DNA fragment, was amplified by PCR from strain HP439 using the primer pairs PORF NdeI (5′ AGGAGACATATTCATATGTTTCAAATTAG) and PORR EcoRI (5′ GTGAATTCACGCAAAAAGCGCCTTGA) and ligated into pBluescript (pBSK). The orientation was determined by PCR, and the nucleotide sequence of the POR operon cloned in pBSK was determined by automated sequencing at the NRC Institute for Marine Biosciences (accession number AF013980). The isopropyl-β-d-thiogalactopyranoside (IPTG) inducibility of pBSK:porDGAB was optimized and verified by sodium dodecyl sulfate-polyacrylamide gel electrophoresis as previously described (13).
Preparation of cell extracts and POR assays.
For enzyme assays, H. pylori strains were grown to late log phase in Brucella broth (BB) and harvested as previously described (14). For E. coli strains expressing POR, the bacteria were grown in LB broth at 37°C and following IPTG (final concentration, 1 mM) induction for 2 h (optical density at 660 nm, 0.5 to 0.8), the bacteria were harvested for the enzyme assay. Bacteria were pelleted by centrifugation (6,000 × g) for 5 min at 4°C, washed once in phosphate-buffered saline (PBS) (4°C), suspended in 100 mM potassium phosphate buffer (pH 7.0), and subjected to 10-s bursts from an ultrasonic probe (Heat Systems-Ultrasonics Inc. [Plainview, N.Y.] sonifier). Following five cycles of bursts interspersed by 1 min of cooling in an ice bath, the crude extract was centrifuged at 10,000 × g for 30 min at 4°C to remove unbroken cells and debris. The supernatant was supplemented with 10 mM dithiothreitol during preparation to protect oxygen-sensitive enzymes. POR enzyme assays were carried out at 25°C in 1-ml-volume cuvettes in a modified Cary-14 spectrophotometer equipped with an OLIS data acquisition system (On Line Instrument Co., Bogart, Ga.) (14). POR (EC 1.2.7.1) was assayed under anaerobic conditions with a solution containing 100 mM potassium phosphate (pH 7.0), 10 mM sodium pyruvate, 5 mM benzyl viologen (BV), 0.18 mM CoA, 1 mM MgCl2 and 5 μM thiamine pyrophosphate. A few grains of sodium dithionite was added to render the cuvette anaerobic, and pyruvate-dependent reduction of BV at 546 nm was monitored (Ε = 9.2 mM−1 cm−1). The reference cuvette contained no sodium pyruvate. POR was also assayed under aerobic conditions with NTZ as the electron acceptor. For this assay, benzyl viologen and dithionite were omitted, and nitroreduction of NTZ at 412 nm was monitored (Ε = 0.55 mM−1 cm−1). NTZ was prepared as a 20-mg/ml stock solution in dimethyl sulfoxide. Enzymatic activities are reported as nanomoles or micromoles per minute per milligram of protein. Protein determinations were done using the Bradford procedure (Bio-Rad) with bovine serum albumin as a standard. All assays were performed in triplicate, and a mean and standard deviation were computed. Variation in enzyme activity from batch to batch was also examined in triplicate in bacterial extracts prepared on different days.
Purification and enzyme assays for FrxA and RdxA.
FrxA and RdxA were purified by nickel interaction chromatography following overexpression from pET29 in BL21. Protein purification included denaturation of proteins in 6 M urea and slow renaturation and elution from the nickel interaction column (details to be published elsewhere). The substrate specificities of each enzyme were determined spectrophotometrically in a 700-μl reaction volume containing the following (final concentration in 1-ml volume): 50 mM Tris-HCl at pH 7.5, 0.1 mM NADPH, and 0.1 mM substrate. The reaction was initiated following addition of the enzyme, and the initial rate was measured with a Beckman DU 520 spectrophotometer at the appropriate wavelength for each substrate at 23°C. The oxidation of NADPH by RdxA or FrxA at 340 nm was monitored (Ε = 6.22 mM−1 cm−1). The reduction of various substrates was also monitored at the appropriate wavelengths, which include the following: MTZ, 320 nm (Ε = 9.0 mM−1 cm−1); nitrofurazone, 400 nm (Ε = 12.6 mM−1 cm−1); nitrofurantoin, 420 nm (Ε = 12 mM−1 cm−1); furazolidone, 400 nm (Ε = 18.8 mM−1 cm−1); and NTZ, 412 nm (E = 0.55 mM−1 cm−1). All extinction coefficient values were verified experimentally. Each assay was performed in triplicate, and the mean and standard deviation was determined. All enzyme activities reported are within a 5% error, and this includes variation from batch to batch purified by these methods.
Determination of mutation frequency and susceptibility to NTZ, MTZ, and the nitrofurans.
New mutations to rifampin resistance were quantified as a measure of drug-induced mutation in H. pylori strain 26695 (Mtzs and Mtzr) and in the E. coli tester strain CC104 as previously described (29). E. coli strains were grown for 12 h in LB broth containing furazolidone, nitrofurazone, nitrofurantoin, NTZ, and MTZ (0, 1, 3, 5, and 10 μg/ml, respectively), and aliquots were spread on LB agar containing 25 μg of rifampin/ml and on rifampin-free LB agar. For H. pylori strains, the bacteria were first grown for 3 days on BA plates supplemented with various concentrations of NTZ, furazolidone, or MTZ (0.5 to 15 μg/ml), and the growth was scraped off, suspended in PBS, and plated in triplicate on BA in the presence or absence of 5 μg of rifampin/ml. The data were normalized to 108 bacteria, and the mutation frequency was computed. Susceptibility of bacteria to the various drugs was determined by spotting 10-fold dilutions of exponentially growing cells, suspended in PBS, onto solid media (BA or LB) supplemented with various concentrations of NTZ, MTZ, or nitrofurans (0, 0.1, 0.25, 0.5, 1, 2, 3, 5, 10, and 25 μg/ml). A strain was considered susceptible to concentrations of a drug that caused at least a 10-fold decrease in the efficiency of colony formation by individual cells (efficiency of plating [EOP]). We have previously established that quantitative bacterial counts (EOP) were more reliable in scoring MTZ susceptibility and resistance of H. pylori than the traditional agar dilution method, which relies on growth versus nongrowth after spotting of suspensions of at least >105 cells on MTZ-containing medium (17, 18, 29). For consistency, the EOP protocol was employed for all the drugs used in this study.
Alkaline gel DNA analysis.
DNA fragmentation analysis was performed as described previously for MTZ (29). Briefly, H. pylori strain 26695 was grown overnight and used to inoculate 500-ml flasks containing 100 ml of BB to a standardized optical density at 600 nm of 0.25. A defined amount of nitrofurazone, NTZ, or MTZ was added to the culture and incubated with shaking at 37°C for 30 min. Aliquots (1 ml) of the cultures were then harvested and prepared for assessment of DNA fragmentation. As a control, 100 μl of cell suspension was treated with 20 mM hydrogen peroxide (H2O2) at 37°C for 15 min to fragment DNA.
Agarose plugs were prepared and treated as described previously (8) with modifications outlined previously (29). Alkaline agarose gel electrophoresis generally followed the protocols of Zirkle and Krieg (36). Briefly, the agarose plugs were placed into the preformed wells of a 0.8% agarose (Vector Biosystems or Roche Diagnostics) gel prepared under alkaline conditions (30 mM NaOH, 10 μM EDTA). The agarose plugs were sealed in the wells with molten agarose and then subjected to electrophoresis for 6 h at 25 mV. The gel was neutralized for 1 h in 30 mM NaCl-50 mM Tris-HCl (pH 6.0), stained for 1 h with 0.5 μg of ethidium bromide (Sigma)/ml, destained in distilled water (2 h), and visualized under UV light.
RESULTS
MICs of NTZ and TIZ for clinical isolates.
Thirty-six clinical isolates were examined initially by the E-test for susceptibility to MTZ, and 15 of them were determined to be Mtzr. This was confirmed by the ability to grow on BA supplemented with 18 μg of MTZ/ml. The MICs of NTZ and TIZ (the deacetylated form of NTZ) were then determined for both susceptible and resistant strains of H. pylori by agar dilution. For the 21 Mtzs strains, the MICs of NTZ and TIZ were 1.21 μg/ml (standard deviation, 0.75 μg/ml) and 1.06 μg/ml (standard deviation, 1.0 μg/ml), respectively; for the 15 Mtzr strains, the MICs of NTZ and TIZ were 4.93 μg/ml (standard deviation, 3.82 μg/ml) and 4.60 μg/ml (standard deviation, 2.67 μg/ml), respectively. P values for results with NTZ and TIZ were 0.002 and <0.001, respectively, by Student's t test (with two samples, assuming unequal variances). Mtzr isolates exhibited a nearly fourfold increase in resistance to NTZ and TIZ, to 4 to 5 μg/ml. However, this level is so low that these strains would still be considered susceptible to clinically relevant levels of these drugs. The Mtzr derivative HP1061, which contains a defined mutation in rdxA (11), displayed the higher level of Ntzr. Introduction of a wild-type rdxA nitroreductase gene on plasmid pDH26 decreased MTZ susceptibility from 32 to 1 μg/ml. This also reduced the intrinsic NTZ resistance from 8 to 1 μg/ml. This suggested that the RdxA nitroreductase is one of the enzymes that can activate this drug.
In related experiments, we examined the MIC for nitrofurantoin of H. pylori strains containing defined rdxA and frxA loss-of-function mutations. Strains carrying mutations in rdxA exhibited the same plating efficiency as the parental strain for nitrofurantoin, whereas some strains carrying mutations only in frxA exhibited a modest twofold increase in resistance (data not presented). This suggests a possible role for frxA in nitrofuran activation. Since H. pylori strains containing loss-of-function mutations in both rdxA and frxA were still quite susceptible to NTZ and the nitrofurans, other enzymes must be able to activate these drugs.
Cloning and expression of POR in E. coli.
The genes encoding subunits of POR are essential for survival of H. pylori (3, 15). To test the possible role of POR in activation of nitrofurans and NTZ, a 3.1-kbp DNA fragment containing the POR operon of H. pylori strain 439 was cloned into pBSK and introduced into E. coli. IPTG-induced POR activity was demonstrated in cell extracts of E. coli carrying pBSK-POR using an assay based on pyruvate-dependent reduction of BV as described previously (14). No BV reduction was detected in the absence of pyruvate (data not presented). DNA sequence analysis revealed near-identity (3 to 5% DNA difference) of POR operon sequences from H. pylori strain 439 (accession number AF013980) to those of strains J99 and 26695, as expected.
NTZ reduction by POR.
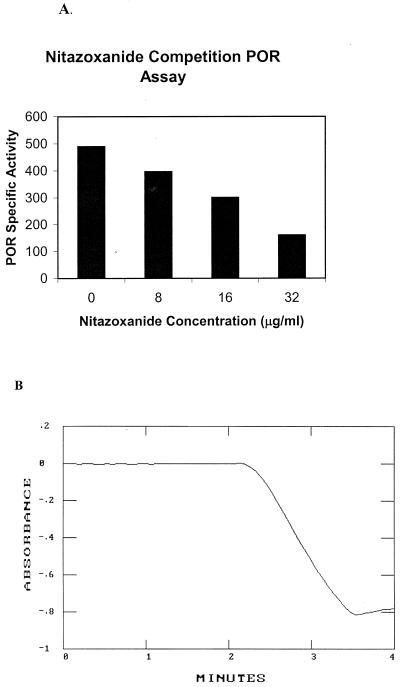
POR enzyme activity can be assayed spectrophotometrically with several redox-active viologen dyes (14, 15). To test whether NTZ could also serve as an electron acceptor in this assay, we first determined if NTZ could compete with BV for reducing equivalents generated during the oxidative decarboxylation of pyruvate by POR. As seen in Fig. 1A, POR activity in cell extracts prepared from H. pylori was ca. 500 nmol/min/mg of protein. Addition of NTZ led to a concentration-dependent decrease in the rate of BV reduction. There was no time lag in BV reduction (BV was in excess), indicating that NTZ did not directly reduce BV. These results indicated that NTZ effectively competed with BV for reducing equivalents (competitive inhibition), possibly by serving as an electron acceptor.
FIG. 1.
POR assay and competition with benzyl viologen. (A) Competitive inhibition of POR activity (benzyl viologen reduction) in H. pylori extracts as a function of NTZ concentration was monitored spectrophotometrically at 546 nm. The specific activity at each concentration of nitazoxanide was recorded. (B) POR was assayed in cell extracts of H. pylori by monitoring the pyruvate-dependent reduction of nitazoxanide at 412 nm (A) as described in the text.
To directly measure NTZ nitroreduction by POR and other enzymes, we developed a spectrophotometric assay based on spectral scan data identifying an absorption maximum for NTZ at 412 nm (attributed to the 5-nitro group), which decreased following chemical reduction by sodium hydrosulfite (dithionite). A molar extinction coefficient for the spectral change (oxidized minus reduced) was computed (0.55 mM−1 cm−1 at 412 nm). Pyruvate-dependent reduction of NTZ in crude extracts of H. pylori is depicted in Fig. 1B. The specific activities measured with NTZ generally paralleled those measured with BV (500 nmol/min/mg of protein) and did not require addition of dithionite to aid initiation of the reaction as is often required in the BV-based assay.
Substrate specificity of POR overexpressed in E. coli.
POR activity was determined in high-speed supernatants prepared from extracts of pBSK-POR plasmid-containing derivatives of E. coli strains AB1157 (wild type) and JVQ2, null alleles of nfsA and nfsB, the genes that encode nitroreductases that are also active with nitrofuran substrates (34). POR specific activity (pyruvate dependent) was first determined with BV for each batch of cell extract. Control extracts of E. coli strains not carrying cloned POR genes displayed no BV reductase activity, indicating that activity from strains expressing the POR operon was due to POR itself. As seen in Table 1, recombinant POR efficiently reduced NTZ (high specific activity, about 3,000 nmol/min/mg of protein). Pyruvate-dependent POR activity was also 100-fold lower with each of the nitrofurans, and no POR activity was detected when MTZ was used as the electron acceptor (Table 1). This result is consistent with previous reports that POR in extracts from H. pylori did not reduce MTZ (11, 14).
TABLE 1.
POR activity of PORGDAB expressed in E. colia
| Compound | Sp act (nmol/min/mg of protein)
|
|
|---|---|---|
| JVQ2:pBSKPOR | AB1157:pPBSKPOR | |
| NTZ | 3,325 ± 428 | 2,233 ± 117 |
| Nitrofurazone | 41.2 ± 5.4 | 37.9 ± 1.5 |
| Nitrofurantoin | 29.2 ± 4.1 | 21.4 ± 0.6 |
| Furazolidone | 25.6 ± 1.7 | 18.1 ± 0.4 |
| MTZ | <0.1 | <0.1 |
The E. coli strains used in this study are AB1157 (wild type) and JVQ2nfsAnfsB (deficient in nitroreductase activity). Plasmid pPORGDAB contains the POR operon in pBSK. Enzyme activities were determined under anaerobic conditions at the appropriate wavelength for each compound as detailed in the text.
Substrate specificity of purified RdxA and FrxA nitroreductases.
The substrate specificities of the RdxA and FrxA nitroreductases were also studied (Table 2). RdxA directly reduced MTZ and NTZ in an NADPH-dependent reaction (5.13 and 13.4 μmol/min/mg of protein, respectively) but displayed no enzyme activity with the nitrofurans using either NADPH or NADH as electron donors (< 0.2 nmol/min/mg of protein). The higher specific activity with NTZ over MTZ might be due to the lower redox potential required for MTZ reduction. The stoichiometry for MTZ reduction by NADPH was approximately 2:1, consistent with the predicted 4-electron reduction (2 mol of NADPH per mol of MTZ) (11). Similar stoichiometries were obtained with NTZ (data not presented).
TABLE 2.
Substrate specificity of RdxA and FrxA nitroreductases
| Substrate | Sp act (μmol/min/mg of protein)a
|
|
|---|---|---|
| RdxA nitroreductase | FrxA nitroreductase | |
| MTZ | 5.13 | <0.0002 |
| NADPH (MTZ)b | 9.01 | <0.0002 |
| NTZ | 13.4 | 22.2 |
| Nitrofurazone | <0.0002 | 0.50 |
| Furazolidone | <0.0002 | 1.50 |
| Nitrofurantoin | <0.0002 | 2.01 |
Specific activities are calculated as the means for five assays of each of two independent batches of enzyme for FrxA and RdxA. The error in these assays is <15%.
NADPH oxidation was followed at 340 nm with MTZ as the electron acceptor.
In contrast to RdxA, purified recombinant FrxA exhibited no activity with MTZ as the electron acceptor (Table 2), even though overexpressed frxA of E. coli rendered this normally resistant strain (MIC, >500 μg/ml) rather susceptible to MTZ (MIC, ∼60 μg/ml versus <10 μg/ml for recombinant expressed rdxA). However, FrxA reduced nitrofuran substrates (0.5 to 2 μmol/min/mg of protein) and was even more active with NTZ (22.2 μmol/min/mg of protein). Like RdxA, FrxA activity was NADPH dependent (data not presented). The nitrofuran substrate specificity of FrxA was similar to that of the classical nitroreductases of enteric bacteria (2).
NTZ- and furazolidone-induced mutagenicity tests.
Partially inhibitory (near MIC) concentrations of MTZ were highly mutagenic for Mtzs and also Mtzr strains of H. pylori (29) (Table 3). In contrast, NTZ, at or near its MIC, did not increase the frequency of mutation to Rifr (Table 3). NTZ at concentrations up to 5 μg/ml for HP26695 and 10 μg/ml for HP26695:ΔrdxA decreased viability by 2 or more logs without increasing the frequency of mutation to Rifr. In contrast, partially inhibitory concentrations of furazolidone were somewhat mutagenic for HP26695 (6-fold) and more mutagenic for the rdxA mutant (16-fold). In general, H. pylori was susceptible to lower concentrations of furazolidone (MIC, <1 μg/ml) than of NTZ (MICs, ∼2 to 5 μg/ml).
TABLE 3.
Mutagenic action of sublethal concentrations of MTZ, NTZ, and furazolidone on Mtzs and Mtzr H. pylori
| Drug concn (μg/ml) | Mutation frequency (no. Rifr/108)
|
|||||
|---|---|---|---|---|---|---|
| Metronidazole
|
Nitazoxanide
|
Furazolidone
|
||||
| WT (EOP)a | rdxA (EOP) | WT (EOP) | rdxA (EOP) | WT (EOP) | rdxA (EOP) | |
| 0 | 12 (1) | 8 (1) | 2.27 (1) | 6.10 (1) | 1.0 (1) | 4.35 (1) |
| 0.25 | NTb | NT | 1.27 (1) | NT | 2.64 (0.1) | 16.43 (1) |
| 0.5 | NT | NT | 1.50 (1) | 2.94 (1) | 6.90 (0.1) | 64.91 (0.1) |
| 1.0 | 14 (1) | 5 (1) | 1.41 (0.5) | 4.46 (1) | 0 (10−3) | 0 |
| 2.0 | 69 (10−2) | 10 (1) | 0.97 (0.1) | 2.47 (1) | NT | NT |
| 3.0 | 144 (10−2) | 5 (1) | NT | NT | NT | NT |
| 5.0 | 0 (0) | 12 (1) | 0 (10−5) | 5.26 (0.5) | NT | NT |
| 10.0 | NT | NT | NT | 0 (10−8) | NT | NT |
| 25.0 | NT | 213 (10−2) | NT | NT | NT | NT |
Strains used were 26695 and the rdxA mutant. 0, insufficient survivors from which to calculate a mutation frequency. The data represent the means of three determinations each from two independent experiments. WT, wild type.
NT, not tested.
Drug-induced mutation in E. coli strains (± rdxA).
We also tested for mutagenic effects of NTZ and nitrofurans with E. coli (Rifr mutants) using the Rifs strain CC104. These results were compared with those obtained using E. coli strains producing RdxA nitroreductase in the case of NTZ (since RdxA activates NTZ). The nitrofurans are considered weakly mutagenic through the action of the hydroxylamino adduct with nucleic acids (34). Each of the nitrofurans was weakly mutagenic at or near its MIC, although none of the nitrofurans was as mutagenic as MTZ for CC104 expressing rdxA (<22-fold versus 500-fold; Table 4). Strain CC104 was particularly susceptible to being killed by the nitrofurans, especially furazolidone, and this made it difficult to accurately measure mutation frequencies. NTZ, in contrast, was not bactericidal at the concentrations tested (MIC, >64 μg/ml), even for strains expressing RdxA in a nitroreductase-proficient background (Table 4). E. coli strain JVQ2 expressing high specific activities of POR was similarly resistant to killing by NTZ (MIC, >64 μg/ml). The intrinsic resistance of E. coli to NTZ might stem from lack of uptake or mechanisms of inactivation that do not involve nitroreduction.
TABLE 4.
Frequency of rifampin resistance in E. coli tester strain CC104
| Strain, compound, and concn (μg/ml)a | Survivalb | Rifr frequency (per 108)c | Fold |
|---|---|---|---|
| CC104 | |||
| Nitazoxanide | |||
| 0 | 1 | 4.8 | |
| 5 | 1 | 6.7 | |
| 10 | 1 | 2.6 | |
| CC104(pGS950) | |||
| Nitazoxanide | |||
| 0 | 1 | 7.0 | |
| 5 | 1 | 5.5 | |
| 10 | 1 | 6.3 | |
| CC104 | |||
| Nitrofurazone | |||
| 0 | 1 | 3.36 | |
| 1 | 1 | 26.4 | 7.9 |
| 2 | 0.7 | 58.6 | 17.4 |
| 5 | 10−3 | 75.4 | 22.4 |
| Nitrofurantoin | |||
| 0 | 1 | 3.36 | |
| 1 | 0.9 | 6.14 | 1.8 |
| 2 | 0.9 | 8.59 | 2.6 |
| 5 | 2.5 × 10−2 | 32.58 | 9.7 |
| Furazolidone | |||
| 0 | 1 | 3.36 | |
| 0.1 | 10−2 | 64.0 | 19 |
| 1 | 10−3 | 0 | |
| Controld | |||
| Metronidazole | |||
| 0 | 1 | 0.75 | |
| 5 | 10−2 | 53 | 70 |
| 10 | 10−4 | 378 | 504 |
The bacteria (E. coli CC104 + pBSK or CC104 + pGS950rdxA+) were grown on LB agar with the appropriate concentration of drug and then plated on medium containing rifampin as indicated in the text.
EOP − efficiency of plating (EOP of 1 = no loss of viability).
The Rifr frequencies represent the means of three determinations and are reported as the number of Rifr colonies per 108 bacteria plated.
Similar to data of Sisson et al. (29); included for comparison. Control, CC104(pGS950).
DNA fragmentation as a measure of DNA damage.
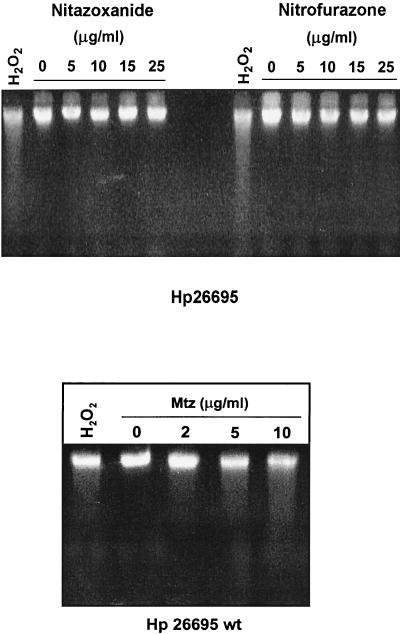
To test for NTZ- or nitrofurazone-induced DNA damage, H. pylori strain 26695 was incubated for 30 min in BB with different concentrations of NTZ, nitrofurazone or MTZ (0 to 25 μg/ml), and, for comparison, with 20 mM H202, an oxidant that causes DNA fragmentation (36). Figure 2 shows that neither NTZ nor nitrofurazone produced significant DNA fragmentation in H. pylori at concentrations up to 25 μg/ml (fivefold higher than MICs for these drugs). In contrast, MTZ at concentrations as low as 1 μg/ml produced substantial DNA fragmentation (see Fig. 2).
FIG. 2.
Lack of NTZ- or nitrofuran-induced DNA fragmentation in H. pylori. The MTZs strain H. pylori (Hp) 26695 was challenged with various concentrations of NTZ or nitrofurazone for 30 min as described in the text. The bacteria were suspended and lysed in agarose plugs, and agarose gels were run under alkaline conditions to display the extent of DNA fragmentation of denatured genomic DNA. Bacteria were treated with hydrogen peroxide (20 mM) for 15 min (positive controls). wt, wild type.
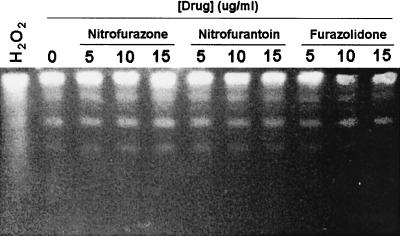
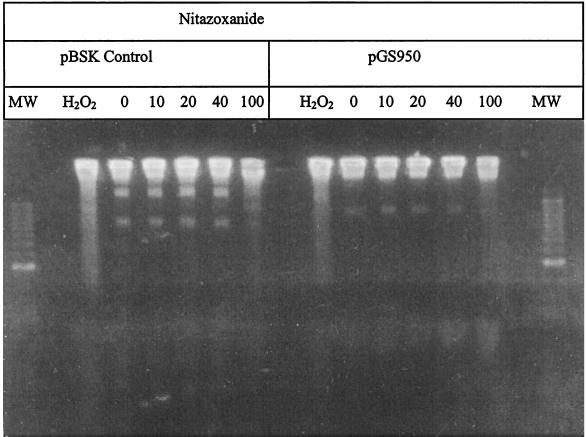
Since the nitrofurans are also toxic for E. coli, we also tested whether they might cause DNA damage in this species. However, none of the nitrofuran drugs produced detectable DNA fragmentation in E. coli CC104, even at concentrations that were in fivefold excess of their MIC (see Fig. 3). Similarly, except at the highest concentration of NTZ tested (100 μg/ml), there was little NTZ-induced DNA fragmentation in E. coli, even when a functional rdxA gene was present (see Fig. 4), which does potentiate MTZ-induced DNA fragmentation (29). Thus, both the nitrofurans and NTZ differ fundamentally from MTZ in that they cause bacterial killing without causing major DNA strand breakage and in the case of NTZ without causing mutation.
FIG. 3.
Lack of drug-induced DNA fragmentation of E. coli strain CC104 carrying pBSK. The bacteria were grown in the presence of the nitrofuran drugs as described in the text. The preparation of agarose plugs is as described in Fig. 2. Hydrogen peroxide was added at a 20 mM concentration as a positive control. The distinct bands noted in each of the lanes are pBSK plasmid DNA.
FIG. 4.
Lack of nitazoxanide-induced DNA fragmentation of E. coli strains carrying rdxA of H. pylori. E. coli strain CC104 containing either pBSK (control) or pGS950 (rdxA+) was grown in the presence of NTZ, and bacteria were suspended and lysed in agarose plugs and electrophoresed as described for Fig. 2 and detailed in the text. Hydrogen peroxide was added at a 20 mM concentration as a positive control. The distinct bands noted in the various lanes are plasmid DNA.
DISCUSSION
Here we report that NTZ, a promising prodrug with in vitro activity against H. pylori, is activated via nitroreduction by three enzymes: pyruvate oxidoreductase (PorGDAB), RdxA, an oxygen-insensitive NADPH nitroreductase, and FrxA, another oxygen-insensitive flavin NADPH nitroreductase. Since Mtzr H. pylori bacteria are generally sensitive to NTZ and nitrofuran (19, 21), we sought to establish the biochemical basis for their antimicrobial activities. In tests of substrate preferences, we found that POR, an essential enzyme of H. pylori, was most active with NTZ as the electron acceptor, was nearly 100-fold less reactive with nitrofuran substrates, and exhibited no MTZ reductase activity (as seen before [11,14]). The substrate preferences of RdxA and FrxA were dependent on the redox potential of the 5-nitro group of the prodrug, with RdxA reducing MTZ (low redox potential, −485 mV) and FrxA reducing nitrofurans (higher redox potential, −270 mV). The two enzymes reduced NTZ equally (mid-range redox potential, ∼−360 mV), indicating functional overlap in this redox range. The substrate preferences of FrxA resemble those of the classical nitroreductases of enteric bacteria, to which FrxA is most closely related (11, 34). While some classical nitroreductases exhibit weak MTZ reductase activity as indicated by mutagenicity of MTZ in the Ames test and the increased susceptibility of E. coli to MTZ when expressing a cloned frxA gene (17), the substrate preference and high specific activity displayed by the RdxA nitroreductase for MTZ is unprecedented and apparently unique to this H. pylori enzyme. The expression of several nitroreductases of differing substrate specificities is rather common among bacteria, and they likely contribute to maintenance of cytoplasmic redox potential or to the regeneration of NAD(P) through the oxidation of a wide range of redox active substrates (2, 11, 34).
In H. pylori POR, ferredoxin is a subunit of the holoenzyme and does not serve as a carrier of reducing equivalents (14, 15). The POR operon is essential for viability, which explains why null mutations that might produce an Ntzr phenotype have not been isolated. In contrast, null mutations in the nonessential rdxA and then in frxA genes are responsible for most or all of the high-level Mtz resistance observed with clinical isolates (17, 18). These Mtzr variants remain susceptible to NTZ and the nitrofurans because they must retain POR activity.
Mutations in FrxA and RdxA appear to influence the relative MICs of nitrofurans and nitazoxanide for a strain, but these increases are small and not sufficient to confer clinically significant resistance. In general, clinical isolates with confirmed MTZ resistance displayed a fourfold-lower susceptibility to NTZ (MIC of ∼5 μg/ml) and are assumed (based on previous findings [17,18]) to be rdxA or both rdxA and frxA deficient. Tests with isogenic mutant lab strains showed that frxA inactivation caused at best a twofold increase in nitrofuran resistance, while rdxA inactivation did not affect nitrofuran resistance. FrxA levels vary among strains (17), and we note here that variability was similarly seen in resistance to nitrofurans. These strain susceptibility data agree with enzymological data showing that only FrxA is active with nitrofurans. MIC levels for furazolidone- and nitrofurantoin-resistant strains of H. pylori are in the 4-μg/ml range (16, 19), suggesting that genes other than frxA are involved in this low-level resistance to nitrofurans.
We isolated H. pylori mutants resistant to NTZ (MIC, 8 to 16 μg/ml). One of these strains contained an rdxA null allele (frame shift) and displayed high-level Mtz resistance (32 μg/ml). Susceptibility to both NTZ and MTZ was restored by introduction of a functional rdxA gene on a shuttle plasmid, indicating that cross-resistance to NTZ is conferred by mutations that cause MTZ resistance, a result that disagrees with an earlier assessment of NTZ (21). No cross-resistance between nitrofurans and MTZ was found however, since laboratory-induced mutations in rdxA and frxA did not increase resistance to nitrofurans (19). It is curious, however, that all nitrofuran-resistant clinical isolates studied to date are also resistant to MTZ (19). Perhaps rdxA inactivation confers some selective advantage during exposure to nitrofurans in vivo, or perhaps these strains have acquired additional mutations affecting drug transport or efflux or having subtle effects on POR substrate ranges that do not abolish its essential enzyme activity.
Mechanism of action of NTZ and nitrofurans.
The antimicrobial action of nitrofurans, nitrothiazoles, and nitroimidazoles is generally believed to result from a 4-electron reduction of the 5-nitro group to short-lived redox active intermediates, including hydroxylamine adducts that are biologically active (7, 12, 22). We had found that MTZ reduction leads to transversion and transition base mutations and sufficient DNA fragmentation to cause lethality (29). The lack of DNA fragmentation by nitrofurans and NTZ suggests that the activated forms of these drugs act differently from MTZ. Furthermore, NTZ was not mutagenic for either H. pylori or E. coli strains expressing enzymes that activate NTZ. We confirmed here previous studies showing that the nitrofurans were weakly mutagenic (Ames test), but less so than MTZ (7, 32). Based on the relatively high specific activity of POR, RdxA, and FrxA for NTZ, we suggest that NTZ killing involves competition for reducing equivalents and disruption of bioenergetic processes. For example, NTZ reduction by the nitroreductases might drain cellular NADPH pools; in the case of POR, reducing equivalents generated from the oxidative decarboxylation of pyruvate would not be available for NADP reduction, the recipient of its generation (15). Also tenable, however, are models in which NTZ or nitrofuran activation generates novel biologically active intermediates that target a different enzyme function or process.
NTZ exhibits broad-spectrum activity against anaerobic bacteria in general (6) and against parasites such as Giardia, Entamoeba, Trichomonas, Cryptosporidium (27), and helminths. This suggests a common mechanism of action: our preliminary studies indicate that pyruvate:ferredoxin oxidoreductase is the target of action of NTZ in Trichomonas vaginalis, E. histolytica, and Clostridium perfringens (9).
In summary, we have identified three enzymes in H. pylori that activate NTZ, a promising therapeutic agent with a broad spectrum of activity against microaerobic bacteria, anaerobic protozoa, anaerobic bacteria, and helminths. Our development of a spectrometric assay for NTZ reduction should aid the identification of redox active enzymes in other susceptible pathogens that also mediate reduction of NTZ. Our work also suggests that the mode of action of NTZ differs from that of MTZ but may overlap with that of the nitrofurans. The inability of H. pylori to mutate to high or clinically significant levels of resistance to NTZ and the lack of mutagenicity of this agent especially emphasize its potential utility in combination therapies to treat H. pylori infections worldwide.
Acknowledgments
We thank Marc Ayers and Ipemida Adagu for helpful discussions and Ken Chisholm for technical assistance.
This research was supported grants from the Canadian Institutes for Health Research (MT11318, RP14292, and ROP37514), Astrazeneca Canada, and Romark Laboratories to P.S.H. and grants from the U.S. Public Health Service (AI38166, AI49161, DK53727, and P30 DK52574) to D.E.B.
REFERENCES
- 1.Broekhuysen, J., A. Stockis, R. L. Lins, J. De Graeve, and J. F. Rossignol. 2000. Nitazoxanide: pharmacokinetics and metabolism in man. Int. J. Clin. Pharmacol. Ther. 38:387-394. [DOI] [PubMed] [Google Scholar]
- 2.Bryant, C., and M. DeLuca. 1991. Purification and characterization of an oxygen-insensitive NAD(P)H nitroreductase from Enterobacter cloacae. J. Biol. Chem. 266:4119-4125. [PubMed] [Google Scholar]
- 3.Chalker, A. F., H. W. Minehart, N. J. Hughes, K. K. Koretke, M. A. Lonetto, K. K. Brinkman, P. V. Warren, A. Lupas, M. J. Stanhope, J. R. Brown, and P. S. Hoffman. 2001. Systematic identification of selective essential genes in Helicobacter pylori by genome prioritization and allelic replacement mutagenesis. J. Bacteriol. 183:1259-1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cupples, C. G., and J. H. Miller. 1989. A set of lacZ mutations in Escherichia coli that allow rapid detection of each of the six base substitutions. Proc. Natl. Acad. Sci. USA 86:5345-5349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Doumbo, O., J. F. Rossignol, E. Pichard, H. A. Traore, T. M. Dembele, M. Diakite, F. Traore, and D. A. Diallo. 1997. Nitazoxanide in the treatment of cryptosporidial diarrhea and other intestinal parasitic infections associated with acquired immunodeficiency syndrome in tropical Africa. Am. J. Trop. Med. Hyg. 56:637-639. [DOI] [PubMed] [Google Scholar]
- 6.Dubreuil, L., I. Houcke, Y. Mouton, and J.-F. Rossignol. 1996. In vitro evaluation of activities of nitazoxanide and tizoxanide against anaerobes and aerobic organisms. Antimicrob. Agents Chemother. 40:2266-2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Edwards, D. I.. 1993. Nitroimidazole drugs--action and resistance mechanisms. I. Mechanisms of action. J. Antimicrob. Chemother. 31:9-20. [DOI] [PubMed] [Google Scholar]
- 8.Gauton, R. K. 1997. Rapid pulsed-field gel electrophoresis protocol for typing of Escherichia coli O157:H7 and other gram-negative organisms in 1 day. J. Clin. Microbiol. 35:2977-2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gilles, H. M., and P. S. Hoffman. 2002. Treatment of intestinal parasitic infections: a review of nitazoxanide. Trends Parasitol. 18:95-97. [DOI] [PubMed] [Google Scholar]
- 10.Glupczynski, Y. 1998. Antimicrobial resistance in Helicobacter pylori: a global overview. Acta Gastroenterol. Belgium 61:357-366. [PubMed] [Google Scholar]
- 11.Goodwin, A., D. Kersulyte, G. Sisson, S. J. O. Veldhuyzen van Zanten, D. E. Berg, and P. S. Hoffman. 1998. Metronidazole resistance in Helicobacter pylori is due to null mutations in a gene (rdxA) that encodes an oxygen-insensitive NADPH nitroreductase. Mol. Microbiol. 28:383-393. [DOI] [PubMed] [Google Scholar]
- 12.Hof, H., J. Stroder, J.-P. Buisson, and R. Royer. 1986. Effect of different nitroheterocyclic compounds on aerobic, microaerophilic, and anaerobic bacteria. Antimicrob. Agents Chemother. 30:679-683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoffman, P. S., C. A. Butler, and F. D. Quinn. 1989. Cloning and temperature-dependent expression in Escherichia coli of a Legionella pneumophila gene coding for a genus-common 60-kilodalton antigen. Infect. Immun. 57:1731-1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoffman, P. S., A. Goodwin, J. Johnsen, K. Magee, and S. J. O. Veldhuyzen van Zanten. 1996. Metabolic activities of metronidazole-sensitive and -resistant strains of Helicobacter pylori: repression of pyruvate oxidoreductase and expression of isocitrate lyase activity correlate with resistance. J. Bacteriol. 178:4822-4829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hughes, N. J., C. L. Clayton, P. A. Chalk, and D. J. Kelly. 1998. Helicobacter pylori porCDAB and oorDABC genes encode distinct pyruvate:flavodoxin and 2-oxoglutarate:acceptor oxidoreductases which mediate electron transport to NADP. J. Bacteriol. 180:1119-1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jenks, P. J., R. L. Ferrero, J. Tankovic, J.-M. Thiberge, and A. Labigne. 2000. Evaluation of nitrofurantoin combination therapy of metronidazole-sensitive and -resistant Helicobacter pylori infections in mice. Antimicrob. Agents Chemother. 44:2623-2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jeong, J. Y., A. K. Mukhopadhyay, J. K. Akada, D. Dailidiene, P. S. Hoffman, and D. E. Berg. 2001. Roles of FrxA and RdxA nitroreductases of Helicobacter pylori in susceptibility and resistance to metronidazole. J. Bacteriol. 183:5155-5162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jeong, J.-Y., A. K. Mukhopadhyay, D. Dailidiene, Y. Wang, B. Velapatiño, R. H. Gilman, A. J. Parkinson, G. B. Nair, B. C. Y. Wong, S. K. Lam, R. Mistry, I. Segal, Y. Yuan, H. Gao, T. Alarcon, M. L. Brea, Y. Ito, D. Kersulyte, H.-K. Lee, Y. Gong, A. Goodwin, P. S. Hoffman, and D. E. Berg. 2000. Sequential inactivation of rdxA (HP0954) and frxA (HP0642) nitroreductase genes causes moderate and high level metronidazole resistance in Helicobacter pylori. J. Bacteriol. 182:5082-5090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kwon, D. H., M. Lee, J. J. Kim, J. G. Kim, F. A. K., F. A. El-Zaatari, M. S. Osato, and D. Y. Graham. 2001. Furazolidone- and nitrofurantoin-resistant Helicobacter pylori: prevalence and role of genes involved in metronidazole resistance. Antimicrobial. Agents Chemother. 45:306-308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McVay, C. S., and R. D. Rolfe. 2000. In vitro and in vivo activities of nitazoxanide against Clostridium difficile. Antimicrob. Agents Chemother. 44:2254-2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Megraud, F., A. Occhialini, and J.-F. Rossignol. 1998. Nitazoxanide, a potential drug for eradication of Helicobacter pylori with no cross-resistance to metronidazole. Antimicrob. Agents Chemother. 42:2836-2840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nivinskas, H., R. L. Koder, Z. Anusevicius, J. Sarlauskas, A. F. Miller, and N. Cenas. 2001. Quantitative structure-activity relationships in two-electron reduction of nitroaromatic compounds by Enterobacter cloacae NAD(P)H:nitroreductase. Arch. Biochem. Biophys. 385:170-178. [DOI] [PubMed] [Google Scholar]
- 23.Orna, M. V., and R. P. Mason. 1989. Correlation of kinetic parameters of nitroreductase enzymes with redox properties of nitroaromatic compounds. J. Biol. Chem. 264:12379-12384. [PubMed] [Google Scholar]
- 24.Pilotto, A., M. Franceschi, M. Rassu, G. Leandro, L. Bozzola, F. Furlan, and F. Di Mario. 2000. Incidence of secondary Helicobacter pylori resistance to antibiotics in treatment failures after 1-week proton pump inhibitor-based triple therapies: a prospective study. Dig. Liver Dis. 32:667-672. [DOI] [PubMed] [Google Scholar]
- 25.Romero Cabello, R., L. R. Guerrero, M. R. Munoz Garcia, and A. Geyne Cruz. 1997. Nitazoxanide for the treatment of intestinal protozoan and helminthic infections in Mexico. Trans. R. Soc. Trop. Med. Hyg. 91:701-703. [DOI] [PubMed] [Google Scholar]
- 26.Rossignol, J. F. A., A. Ayoub, and M. S. Ayers. 2001. Treatment of diarrhea caused by Cryptosporidium parvum: a prospective randomized, double blind, placebo-controlled study of nitazoxanide. J. Infect. Dis. 184:103-106. [DOI] [PubMed] [Google Scholar]
- 27.Rossignol, J. F. A., A. Ayoub, and M. S. Ayers. 2001. Treatment of diarrhea caused by Giardia intestinalis and Entamoeba histolytica or E. dispar: a randomized, double-blind, placebo-controlled study of nitazoxanide. J. Infect. Dis. 184:381-384. [DOI] [PubMed] [Google Scholar]
- 28.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 29.Sisson, G., J.-Y. Jeong, A. Goodwin, L. Bryden, N. Rossler, S. Lim-Morrison, A. Raudonikiene, D. E. Berg, and P. S. Hoffman. 2000. Metronidazole activation is mutagenic and causes DNA fragmentation in Helicobacter pylori and in Escherichia coli containing a cloned H. pylori rdxA+ (nitroreductase) gene. J. Bacteriol. 182:5091-5096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith, M. A., and D. I. Edwards. 1995. Redox potential and oxygen concentration as factors in the susceptibility of Helicobacter pylori to nitroheterocyclic drugs. J. Antimicrob. Chemother. 35:751-764. [DOI] [PubMed] [Google Scholar]
- 31.Tomb, J. F., O, White, A. R. Kerlavage, R. A. Clayton, G. G. Sutton, R. D. Fleischmann, K. Ketchum, H. Klenk, S. Gill, B. Dougherty, K. Nelson, J. Quackenbush, L. Zhou, E. Kirkness, S. Peterson, B. Loftus, D. Richardson, R. Dodson, H. Khalak, A. Glodek, K. McKenney, L. Fitzegerald, N. Lee, M. Adams, E. Hickey, D. Berg, J. Gocayne, T. Utterback, J. Peterson, J. Kelley, M. Cotton, J. Weidman, C. Fujii, C. Bowman, L. Watthey, E. Wallin, W. Hayes, M. Borodovsky, P. Karp, H. Smith, C. Fraser, and J. Venter. 1997. The complete genome sequence of the gastric pathogen Helicobacter pylori. Nature 388:539-547. [DOI] [PubMed] [Google Scholar]
- 32.Townson, S. M., P. F. L. Boreham, P. Upcroft, and J. A. Upcroft. 1994. Resistance to the nitroheterocyclic drugs. Acta Tropica 56:173-194. [DOI] [PubMed] [Google Scholar]
- 33.Upcroft, P., and J. A. Upcroft. 2001. Drug targets and mechanisms of resistance in anaerobic protozoa. Clin. Microbiol. Rev. 14:150-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Whiteway, J., P. Koziarz, J. Veall, N. Sandhu, P. Kumar, B. Hoecher, and I. B. Lambert. 1998. Oxygen-insensitive nitroreductases: analysis of the roles of nsfA and nfsB in development of resistance to 5-nitrofuran derivatives in Escherichia coli. J. Bacteriol. 180:5529-5539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yamamoto, Y., A. Hakki, H. Friedman, S. Okubo, T. Shimamura, P. S. Hoffman, and J. F. Rossignol. 1999. Nitazoxanide, a nitrothiazolide antiparasitic drug, is an anti-Helicobacter pylori agent with anti-vacuolating toxin activity. Chemotherapy 45:303-312. [DOI] [PubMed] [Google Scholar]
- 36.Zirkle, R. E., and N. R. Krieg. 1996. Development of a method based on alkaline gel electrophoresis for estimation of oxidative damage to DNA in Escherichia coli. J. Appl. Bacteriol. 81:133-138. [DOI] [PubMed] [Google Scholar]