Abstract
The antituberculosis drug isoniazid (INH) is quickly oxidized by stoichiometric amounts of manganese(III) pyrophosphate. In the presence of nicotinamide coenzymes (NAD+, NADH, nicotinamide mononucleotide [NMN+]) and nicotinic acid adenine dinucleotide (DNAD+), INH oxidation produced the formation of INH-coenzyme adducts in addition to known biologically inactive products (isonicotinic acid, isonicotinamide, and isonicotinaldehyde). A pool of INH-NAD(H) adducts preformed in solution allowed the rapid and strong inhibition of in vitro activity of the enoyl-acyl carrier protein reductase InhA, an INH target in the biosynthetic pathway of mycolic acids: the inhibition was 90 or 60% when the adducts were formed in the presence of NAD+ or NADH, respectively. Under similar conditions, no inhibitory activity of INH-NMN(H) and INH-DNAD(H) adducts was detected. When an isolated pool of 100 nM INH-NAD(H) adducts was first incubated with InhA, the enzyme activity was inhibited by 80%; when present in excess, both NADH and decenoyl-coenzyme A are able to prevent this phenomenon. InhA inhibition by several types of INH-coenzyme adducts coexisting in solution is discussed in relation with the structure of the coenzyme, the stereochemistry of the adducts, and their existence as both open and cyclic forms. Thus, manganese(III) pyrophosphate appears to be an efficient and convenient alternative oxidant to mimic the activity of the Mycobacterium tuberculosis KatG catalase-peroxidase and will be useful for further mechanistic studies of INH activation and for structural investigations of reactive INH species in order to promote the design of new inhibitors of InhA as potential antituberculous drugs.
Tuberculosis, an infectious disease caused by Mycobacterium tuberculosis, remains one of the leading causes of death in the world. Isoniazid (INH), a frontline antibiotic used in the treatment of tuberculosis (3, 4), is considered to be a prodrug that requires activation by the M. tuberculosis catalase-peroxidase KatG (10, 28). However, none of the stable derivatives observed in KatG-dependent INH conversion, i.e., isonicotinic acid (product 1), isonicotinamide (product 2) and isonicotinaldehyde (product 3) (Fig. 1) have demonstrated a bactericidal effect (9). Studies (22, 23, 26) have suggested that the activated form of INH, probably an isonicotinoyl radical, is capable of reacting with the β-NAD (NAD+/NADH) which is the cofactor of the long-chain 2-trans-enoyl-acyl carrier protein reductase InhA (2, 20). InhA is a key enzyme involved in the biosynthesis of long-chain fatty acids and of mycolic acids, specific components of the mycobacterial cell wall (5, 15). The formation of the covalent adduct(s) INH-NAD(H) as a competitive inhibitor(s) might explain the loss of InhA activity and interruption of mycolic acid synthesis (20, 22). Despite recent efforts, the exact nature of the active form of INH and the precise activation mechanism of this drug are still a matter of debate. Several studies have been focused on the role of the KatG protein (8, 10, 14, 25, 28), a hemoprotein having a manganese-dependent peroxidase activity (13, 27). KatG catalyzes the conversion of Mn(II) to Mn(III) (13), the latter metal ion being probably able to oxidize INH (this mode of activation is reminiscent of the manganese peroxidase mechanism of the white-rot fungus Phanerochaete chrysosporium [24]). In addition, since Mn(II)/O2 is a poor activating system (7, 12), INH activation has been directly performed with Mn(III) salts (7) and was shown to give oxidation products 1 and 2 (13). The possibility of using Mn(III) to oxidize INH and form InhA inhibitors has been briefly mentioned, but without any experimental details (26).
FIG. 1.
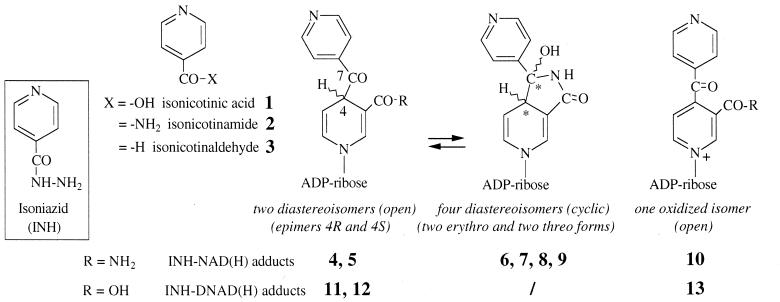
Structures of INH, stable oxidation products 1 to 3, and proposed structures for INH-NAD(H) or INH-DNAD(H) adducts observed in solution.
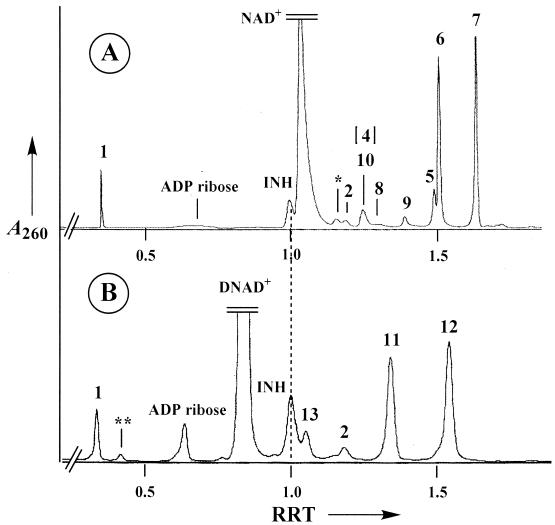
In the present work, we investigate the activation of INH by stoichiometric amounts of Mn(III) pyrophosphate, a stable form of Mn(III) ions in aqueous solutions previously used in our model studies of the manganese peroxidase of P. chrysosporium (6). Since Mn(III) is a strong oxidant which undergoes spontaneous dismutation in Mn(II) and Mn(IV) in water, we chose pyrophosphate as an oxidant-resistant chelating agent to stabilize Mn(III) in the pH range from 4 to 6. Other organic chelating agents such as malate, malonate, lactate, oxalate, or tartrate are not as stable over time and show storage problems. In a previous work (18), we demonstrated that a stoichiometric amount of Mn(III) pyrophosphate can replace either the use of Mn(II)/O2 or the catalysis by the KatG protein in the activation of INH. Formation of a series of adducts was detected and shown to be the result of acylation in position 4 of the nicotinoyl moiety of the coenzyme by the isonicotinoyl radical generated from INH (with creation of a new chiral center at position 4 and therefore formation of two epimeric adducts; see structures 4 and 5 or 11 and 12 in Fig. 1, for INH adducts with NAD+ and nicotinic acid adenine dinucleotide [DNAD+], respectively). An additional spontaneous cyclization process creates a second chiral center at position 7, which gives four new diastereoisomeric compounds having a hemiamidal structure (see structures 6 to 9 in Fig. 1). The coexistence in solution of these six dihydropyridine derivatives (two open and four cyclized) was clearly demonstrated for NAD+ (17). A typical high-performance liquid chromatography (HPLC) profile is shown in Fig. 2A. In the case of INH-DNAD adducts, the carboxylic group of the nicotinic moiety (instead of the amide group present in NAD+) does not allow the cyclization process, and only the two open structures, compounds 11 and 12, were observed (Fig. 2B). It should be noted that a small amount of oxidized adducts (the dihydropyridine ring being converted into a pyridinium ring) can also be detected (peak 10 in Fig. 2A, peak 13 in Fig. 2B, and compounds 10 and 13 in Fig. 1). In the case of reaction with NAD+, the two main adducts, compounds 6 and 7, analyzed by liquid chromatography-electrospray ionization-mass spectrometry (LC-ESI-MS), showed a molecular weight (770.1) identical and UV characteristics (λmax = 260 and 330 nm, ɛ260/ɛ330 = 3.2 to 3.5) similar to those reported for the adduct recently isolated after activation of INH in the presence of KatG and InhA (11). All these data are also consistent with the proposed formula of the bound inhibitor present in the crystal structure of the InhA-inhibitor complex (Fig. 3) (22), making it the most likely inhibitor of InhA.
FIG. 2.
HPLC profiles of reaction mixtures containing INH (2 mM), NAD+ (2 mM) (A) or DNAD+ (2 mM) (B), and Mn(III) pyrophosphate (4 mM; introduced in 10 consecutive additions of 400 μM each, every 2 min) in phosphate buffer (100 mM), pH 7.5. See Fig. 1 for the compound formula corresponding to each peak. Compounds 4 and 10 coelute and compound 4 is the minor component. ∗ and ∗∗, nicotinamide and nicotinic acid coming from decomposition of NAD+ and DNAD+, respectively. RRT, relative retention time (with INH as reference).
FIG. 3.
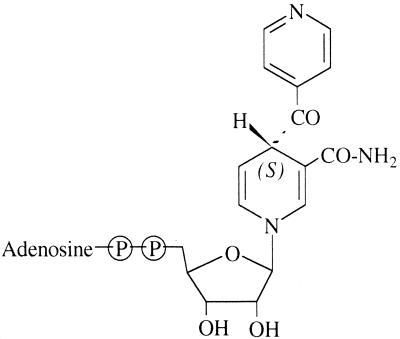
Structure of the INH-NAD(H) adduct within the active site of InhA as proposed on the basis of X-ray crystallography (22).
However, several questions remained; for instance, are adducts generated by oxidation in the presence of the chemical activator Mn(III) pyrophosphate able to inhibit InhA, and what is the incidence of the cyclization process observed for INH-NAD(H) adducts on their potential inhibitory activity? In the present work, we investigate (i) the ability of Mn(III) pyrophosphate to oxidize INH in the presence of various nicotinic coenzymes with formation of INH adducts and (ii) the potency of these adducts to inhibit InhA.
MATERIALS AND METHODS
Analytical methods. (i) HPLC analyses.
HPLC analyses were performed on a reverse-phase C18 column (particle size, 10 μm; 250 by 4.6 mm; Nucleosil) using a 5:95 (vol/vol) methanol-5 mM ammonium acetate (NH4OAc) (pH 4.5) solution as eluent (Table 1, runs 1 and 2) or a linear gradient from 0 to 100% acetonitrile in 70 mM NH4OAc aqueous solution for INH-coenzyme adduct {INH-NAD(H), INH-DNAD(H), INH-nicotinamide mononucleotide [NMN(H)] adducts} (Table 1, runs 3 to 6) analyses (flow rate: 1 ml min−1). The column was coupled to a diode array detector (Kontron) for the detection of products at 260 nm and the monitoring of UV-visible spectra. Yields were calculated for INH and compounds 1 to 3 by comparison with authentic sample calibration curves (in order to detect compound 3 as its O-carboxymethyl oxime derivative, some reaction samples were analyzed after 5 min of quenching with 20 mM NH2OCH2COOH) and, for INH adducts, by using the same ɛ260 values as for corresponding coenzymes. Collection of HPLC-separated peaks was performed on a reverse-phase C8 semipreparative column (particle size, 10 μm; 250 by 10 mm; Nucleosil) using detection at 260 and 330 nm (this last maximum absorbance was used as an indicator for dihydropyridine adduct formation). Elution conditions are precisely described under “Purification of INH-coenzyme adducts” below (flow rate, 2.5 ml min−1).
TABLE 1.
Determination of INH derivatives produced after nonenzymatic activation in the absence or presence of nicotinamide or nicotinic coenzymes
| Run no. | Coenzyme (2 mM) | Mn (III) pyrophosphate concn (mM) | INH concn | % INH converted after 15 min | % INH derivatives yielda
|
% INH adduct yielda (pool) | ||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | ||||||
| 1 | 1 | 500 μM | 93 | 45 | 12 | 4 | ||
| 2 | 500 μM | <3b | 0.1 | <1 | <1 | |||
| 3 | NAD+ | 4 | 2 mM | 60 | 10 | 5 | NDc | 41 |
| 4 | NADH | 4 | 2 mM | 53 | 16 | 3 | ND | 14 |
| 5 | NMN+ | 4 | 2 mM | 60 | 14 | 8 | ND | 5 |
| 6 | DNAD+ | 4 | 2 mM | 84 | 12 | 7 | ND | 6 |
Based on initial INH concentration after 20 min incubation.
No evolution even when incubated for 48 h.
ND, not determined.
LC-ESI-MS analyses
. LC separations were performed under the conditions indicated above but with a quaternary pump. Only 4% of the flow eluted from the column was introduced into the electrospray source after automated mixing with a 1% HCOOH solution using a Harvard Apparatus syringe pump. The ESI-MS spectrometer was a Perkin-Elmer SCIEX API 365, and analyses were performed in the positive mode.
All InhA assays were performed on a Uvikon 923 spectrophotometer (Kontron) equipped with a circulating water bath for temperature control.
(ii) Materials.
Manganese(III) pyrophosphate, [MnIII(H2P2O7)3]Na3, was prepared according to the method of Archibald and Fridovich (1) and following the improved protocol previously described (17). M. tuberculosis InhA was overexpressed in Escherichia coli and purified as previously described (21). INH, nicotinamide coenzymes, and analogs were obtained from Sigma-Aldrich. The substrate 2-trans-decenoyl-coenzyme A (-CoA) was synthesized from 2-trans-decenoic acid using the mixed anhydride method and purified according to the procedure described elsewhere (19, 21). The concentration of the substrate was determined on the basis of ɛ260 being equal to 16,800 M−1 cm−1.
INH oxidation.
Run 1 (Table 1) involved oxidation with Mn(III) pyrophosphate; the reaction medium (final volume, 1 ml), which contained 100 mM phosphate buffer (pH 7.5), 500 μM INH, and 1 mM Mn(III) pyrophosphate, was stirred at room temperature (RT) for 10 min and then analyzed by HPLC after 5 min of quenching with 20 mM NH2OCH2COOH (200 μM 2-nitrobenzoic acid was used as the internal standard). The corresponding control reaction without oxidant (run 2) was incubated under the same conditions for 48 h.
INH-coenzyme adduct formation.
For runs 3 to 6 (Table 1), the reaction medium (final volume, 1 ml) contained 100 mM phosphate buffer (pH 7.5), 2 mM INH, 2 mM coenzyme (NAD+ in run 3, NADH in run 4, NMN+ in run 5, DNAD+ in run 6), and 4 mM Mn(III) pyrophosphate (introduced in 10 consecutive additions of 400 μM each, every 2 min). The reaction mixture was stirred at RT for 20 min after the last ingredient's addition. HPLC (detection at 260 nm) and LC-ESI-MS analyses were directly performed on the reaction mixture.
Adduct formation in the presence of InhA and simultaneous inhibition of InhA.
The standard incubation medium (total volume, 50 μl) consisted of 3 μM InhA, 100 μM INH, 100 μM NADH, and 200 μM Mn(III) pyrophosphate, in 100 mM sodium phosphate buffer (pH 7.5), at 25°C (Table 2, run 1). The addition of Mn(III) pyrophosphate initiated the reaction. Control reactions were carried out under the same conditions without Mn(III) pyrophosphate (run 2), without NADH (run 3), without INH, or without both Mn(III) pyrophosphate and INH (data not shown for the last two controls). Reaction aliquots (10 μl) were taken at defined times as specified on the x axis of Fig. 4 and analyzed for InhA activity using 2-trans-decenoyl-CoA (50 μM) as the substrate and NADH (100 μM) as the cofactor in 100 mM phosphate buffer, pH 7.5, at 25°C (total volume, 1 ml). In order to simplify the graphic, all control curves (except that of control without NADH) are included in the hatched zone plotted on Fig. 4.
TABLE 2.
Inhibition of InhA activity by adducts formed in the presence of InhA or by preformed adducts (INH, 100 μM; InhA, 3 μM)
| Run no. | Coenzyme (100 μM) | Mn (III) pyrophosphate concn of 200 μM | Preincubation time (min before InhA addition) | % InhA activity remaining after:
|
|
|---|---|---|---|---|---|
| 5 min | 12 min | ||||
| 1 | NADH | + | 0 | 43 ± 4 | 21 ± 2 |
| 2 | NADH | − | 0 | 90 ± 12 | 79 ± 18 |
| 3 | + | 0 | 86 ± 11 | 69 ± 7 | |
| 4 | NADH | + | 18 | 65 ± 10 | 40 ± 13 |
| 5 | + | 18 | 102 ± 11 | 86 ± 8 | |
| 6 | NADH | − | 18 | 116 ± 19 | 104 ± 6 |
| 7 | NAD+ | + | 18 | 8 ± 4 | 10 ± 3 |
| 8 | NAD+ | + | 6 | 20 | 13 |
| 9 | NAD+ | + | 2 | 14 | 14 |
| 10 | NAD+ | − | 18 | 92 ± 6 | 89 ± 16 |
| 11 | NMN+ | + | 18 | 99 | 106 |
| 12 | NMN+ | − | 18 | 105 | 100 |
| 13 | DNAD+ | + | 18 | 100 | 94 |
| 14 | DNAD+ | − | 18 | 100 | 97 |
FIG. 4.
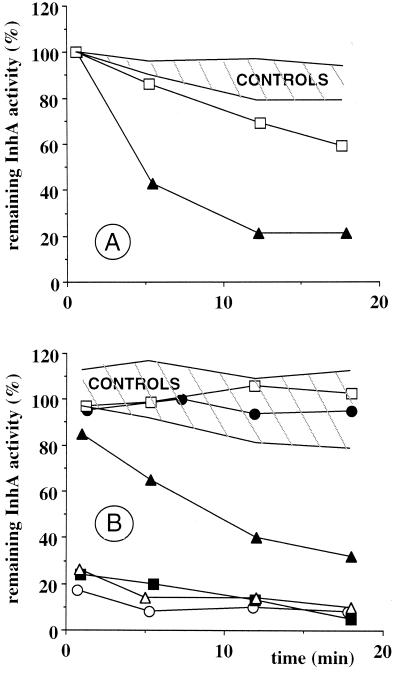
(A) Inhibition of InhA activity by adducts formed in the presence of InhA. The enzyme (3 μM) was incubated in 100 mM sodium phosphate buffer, pH 7.5, at 25°C, including 100 μM INH, 100 μM NADH, and 200 μM Mn(III) pyrophosphate (▴) or no NADH (□). (B) Inhibition of InhA activity by preformed adducts. Adducts were first formed by preincubation for 18 min in 100 mM sodium phosphate buffer, pH 7.5, at 25°C, containing 100 μM INH and 200 μM Mn(III) pyrophosphate and 100 μM NADH (▴), 100 μM NAD+ (▵, ▪, and ○) (▵ and ▪, only 2 and 6 min of preincubation time, respectively), 100 μM NMN+ (□), or 100 μM DNAD+ (•). Values of InhA activity obtained for each time point of a curve are expressed as a percentage of the initial enzymatic activity. Conditions are described in Materials and Methods. Hatched zones (control incubations), no INH and no Mn(III) pyrophosphate, no INH, no Mn(III) pyrophosphate.
Inhibition of InhA by a reaction mixture of preformed adducts.
The preincubation reactions were performed in 50 μl (total volume) of 100 mM sodium phosphate buffer solution, pH 7.5, at 25°C containing 100 μM INH, 100 μM coenzyme (Table 2, NADH in run 4; NAD+ in run 7; NMN+ in run 11, or DNAD+ in run 13), and 200 μM Mn(III) pyrophosphate. After 2 and 6 min of preincubation (only for NAD+; runs 9 and 8, respectively) or after 18 min (all types of coenzyme), the addition of 3 μM InhA initiated the incubation reaction. Control incubation reactions were carried out under the same conditions as those described above without any coenzyme (run 5), without Mn(III) pyrophosphate (run 6 for NADH; run 10 for NAD+; run 12 for NMN+, and run 14 for DNAD+), without INH, or without both Mn(III) pyrophosphate and INH (data not shown). Reaction aliquots (10 μl) were taken at defined times as specified on the x axis of Fig. 4 and analyzed for InhA activity as described above.
Purification of INH-coenzyme adducts.
The formation of INH-coenzyme adducts was performed as described in Table 1, run 3 (pool of adducts 5 to 10 and isolated adducts 6 and 7) and run 6 (isolated adducts 11 and 12). The pool of adducts 5 to 10 was separated from other components of the reaction mixture (INH, NAD+, ADP-ribose, isonicotinic acid, isonicotinamide, nicotinamide, and buffer) by first washing the column for 80 min with a 70 mM NH4OAc aqueous solution and then by eluting the pool of adducts with water and collection. HPLC reinjection of the pool of INH-NAD(H) adducts 5 to 10 displays an HPLC profile similar to the adduct profile observed in Fig. 2A (peaks 5 to 10). The two major peaks, peaks 6 and 7, attributed to cyclic INH-NAD(H) adducts, were purified using a linear gradient from 0 to 100% acetonitrile in 70 mM NH4OAc aqueous solution. The peaks 11 and 12, corresponding to INH-DNAD(H) isomeric adducts, were purified using aqueous 17 mM NH4OAc as eluent. The chromatographic purity of each collected INH-coenzyme adduct was estimated to be >95%, based on subsequent HPLC reinjection. An alternative purification process of the pool of adducts consisted of passing the reaction mixture through a Sep-pak C18 cartridge and washing with 4 mM NH4OAc aqueous solution. Further careful washing with water followed by elution with acetonitrile and concentration under vacuum afforded the pool of adducts. After dilution in water, HPLC reinjection of this pool of INH-NAD(H) adducts 5 to 10 yielded an HPLC profile similar to the adduct profile observed for the crude reaction mixture.
The concentration of the pool of INH-coenzyme adducts or of isolated adducts collected either from HPLC or the Sep-pak cartridge was estimated by UV-visible spectrum analyses using a ɛ260 of 27,000 M−1 cm−1 and a ɛ326 of 6,900 M−1 cm−1 from data in the literature (11). The pool of INH-NAD(H) adducts 5 to 10 exhibited two absorption peaks at 260 nm and 326 to 328 nm and an A260/A326-328 ratio of 3.51. The isolated INH-NAD(H) adducts 6 and 7 showed two absorption peaks at 260 and 328 nm and an A260/A326-328 ratio of 3.30 (6) and 3.55 (7). Concerning the INH-DNAD(H) adducts, each fraction collected showed an absorption peak at 260 nm and a shoulder around 302 nm and an A260/A300-302 ratio of 3.55 (11) and 3.49 (12).
Inhibition of InhA by an isolated pool of adducts and by collected single adducts.
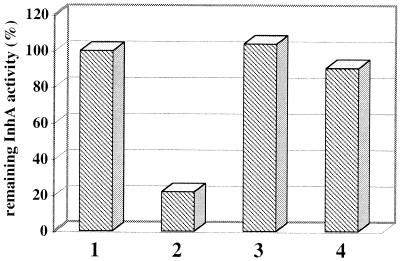
The HPLC-collected fractions containing the pool of peaks 5 to 10 or one of peaks 6, 7, 11, or 12, after determination of the concentration, were used in InhA assays. Reactions were initiated by adding 60 nM InhA into a solution (total volume, 1 ml) of 100 mM sodium phosphate buffer, pH 7.5, at 25°C containing 100 μM NADH, 50 μM 2-trans-decenoyl-CoA, and adequate concentrations of the pool or of single collected adducts as specified in Table 3 and, for the pool of adducts, on the x axis of Fig. 5. Control InhA assays were carried out under the same conditions as described above using the fractions collected at the same retention time as for the corresponding adducts, but in this case, the preincubations were performed without oxidant. Protection of InhA activity by the cofactor or the substrate (Fig. 6) was tested as follows. A 77 nM concentration of InhA was preincubated for 5 min at 25°C alone (column 1) or in the presence of (i) a 100 nM pool of adducts purified by Sep-pak (see above) (column 2), (ii) 100 μM NADH (column 3), or (iii) 100 μM 2-trans-decenoyl-CoA (column 4), in 100 mM sodium phosphate buffer (pH 7.5) in a total volume of 800 μl. Reactions were initiated by adding 100 μM decenoyl-CoA and 100 μM NADH (columns 1 and 2), a 100 nM concentration of the purified pool of adducts and 100 μM 2-trans-decenoyl-CoA (column 3), or a 100 nM concentration of the purified pool of adducts and 100 μM NADH (column 4). Controls without adducts assayed with or without the preincubation step showed a slightly lower activity of InhA in the absence of its cofactor (NADH), due to a partial denaturation.
TABLE 3.
Inhibition of InhA activity by an isolated pool of INH-NAD(H) adducts and by collected single INH-NAD(H) and INH-DNAD(H) adducts
| Run no. | Adduct | HPLC-collected peak | % Activity of InhA remaining
|
|
|---|---|---|---|---|
| Adduct (1 μM) | Adduct (5 μM) | |||
| 1 | INH-NAD(H) | Pool | 65 ± 9 | 18 ± 1 |
| 2 | INH-NAD(H) | 6 | 67 ± 1 | 29 ± 4 |
| 3 | INH-NAD(H) | 7 | 102 ± 7 | 69 ± 3 |
| 4 | INH-DNAD(H) | 11 | 106 ± 2 | 96 ± 3 |
| 5 | INH-DNAD(H) | 12 | 91 ± 3 | 74 ± 3 |
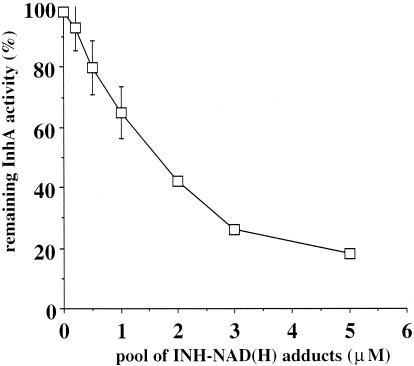
FIG. 5.
Inhibition of InhA activity by an isolated pool of adducts collected from HPLC. Experiments were performed in triplicate except for concentrations 2 and 3 μM (one experiment). Error bars, standard deviations.
FIG. 6.
Protection of InhA activity by NADH or decenoyl-CoA against INH-NAD(H) adducts. Column 1, control experiment in the absence of adducts with 5-min preincubation of InhA (column 1; 100%); columns 2 to 4, remaining InhA activities when InhA was preincubated with either 100 nM of the isolated pool of adducts (column 2; 22%), 100 μM NADH (column 3; 104%), or 100 μM decenoyl-CoA (column 4; 90.5%). Experiments were performed in duplicate (errors of < 7%).
InhA assay.
Enoyl reductase activity of InhA was assayed by monitoring the oxidation of NADH at 340 nm (25°C), and initial velocities were determined. Results are expressed as a percentage of remaining InhA activity determined on the basis of control experiments.
RESULTS AND DISCUSSION
Mn(III) pyrophosphate-dependent oxidation of INH and formation of INH-NAD(H) adducts and related compounds.
As shown in Table 1, the oxidation of INH by two equivalents of Mn(III) pyrophosphate (run 1) gave a high conversion rate of INH (93%) within a few minutes [in the absence of Mn(III) pyrophosphate, conversion of INH was below 3%, even after 48 h of incubation (run 2)]. The stoichiometry of this two-electron oxidation is consistent with that previously observed with Mn(III) malonate (13). This efficient chemical oxidation method gave the same stable products, compounds 1 to 3 (Fig. 1), as those obtained with KatG (10). However, since none of these products inhibits InhA (9), one key point remained the ability of the Mn(III) pyrophosphate system to produce the short-life reactive INH oxidation intermediates (e.g., the isonicotinoyl radical or ion) involved in the formation of the adducts with the NAD+/NADH cofactor of InhA and to lead to the inhibition of this enzyme.
Thus, in a second step, we used Mn(III) pyrophosphate in the presence of both INH and either NAD+, NADH, NMN+, or DNAD+ for testing its ability to produce various INH-coenzyme adducts. Monitoring by HPLC, we observed in all cases (Table 1, runs 3 to 6), in addition to the stable INH oxidation compounds 1 to 3, the formation of a family of products with UV spectra characteristic for dihydropyridine derivatives (absorbance peak at 320 to 333 nm), like those we recently observed with NAD+ and DNAD+ (17) and also like those observed by Johnsson et al. with the use of the KatG/MnCl2 as activating system (26). LC-ESI-MS analyses showed that six products having the same molecular mass could be detected in reactions performed with either NADH (molecular weight, 770) or with NMN+ (molecular weight, 441), as it was also the case for the previously described adducts with NAD+ (molecular weight, 770). For DNAD+ adducts, only two products with a mass of 771 were detected, as previously reported (17). The overall yield of the pool of adducts ranged from 35 to 45% in the case of NAD+, depending on the reaction conditions, but was only 5 to 14% when NADH, NMN+, or DNAD+ was used. Based on their HPLC behavior, UV data, and mass data, the six INH adducts formed either with NAD+ or NADH were shown to be identical. The most likely mechanism of INH-NAD(H) adduct formation consists of the addition of an isonicotinoyl radical, produced by Mn(III) pyrophosphate INH oxidation, to the electron-deficient pyridinium ring of NAD+, following a Minisci reaction (16). The resulting radical cation then looses an electron to form the final adduct product. The lower yield observed for the formation of adducts in the presence of NADH may be explained by an initial conversion of NADH into NAD+. Indeed, we observed that Mn(III) pyrophosphate (100 μM) alone was not able to oxidize NADH (100 μM), but after a 10-min incubation in the presence of INH (500 μM), NAD+ was formed in significant amounts (27% yield). Such NADH oxidation was also observed by using KatG/MnCl2 as the oxidant system (26). The even-lower yields observed for INH adducts with NMN+ or DNAD+ (5 to 6% [Table 1]), while the conversion of INH was high (60 to 84%), evidenced the critical role of structural parameters such as the presence of the adenylic moiety or the amide function at position 3 of the pyridinium ring.
The multiple peaks observed in HPLC profiles might be ascribed to the coexistence in solution of cyclized diastereoisomers (cases of INH adducts with NADH and NMN+ are similar to the case of adducts with NAD+) in addition to two epimeric open structures (which were the only ones present for DNAD+ adducts) (17). Oxidized adducts with a pyridinium ring instead of the dihydropyridine structure were also present in small amounts (see peaks 10 and 13 in Fig. 2 and the corresponding structures in Fig. 1; these products did not show an absorption peak around 320 to 330 nm). Since compound 10, isolated in the case of NAD+, was shown to be devoid of any inhibitory activity (26), UV analyses at 320 to 333 nm (absorption wavelength of the dihydropyridine ring) provide a rapid and convenient test for following the INH activation and detecting the INH-coenzyme adducts likely to behave as inhibitors of InhA. In conclusion, the oxidation with a stoichiometric amount of Mn(III) pyrophosphate appears clearly to be an efficient and fast way of activating INH in order to produce INH adducts in the presence of the nicotinic or nicotinamide coenzymes. These adducts represent potential inhibitors of the InhA target.
Formation of adducts in the presence of InhA and simultaneous inhibition of the enzyme activity.
We investigated the INH-dependent inhibition of InhA in the presence of Mn(III) pyrophosphate and NADH, the natural cofactor of InhA (Fig. 4A; Table 2, runs 1 to 3) to verify that the adducts generated by this chemical activation of INH were efficient inhibitors. As shown in Fig. 4A and Table 2 (run 1), Mn(III) pyrophosphate efficiently mediated the inhibition of InhA activity by INH in the presence of NADH in a time-dependent process (79% loss of InhA activity after 12 min of incubation). Such a kinetic profile may correspond to either a slow kinetic of NAD+ formation from NADH prior to adduct formation (see discussion above) or to the time necessary to reach the equilibrium, since in these experiments, the adduct has to compete with an excess of NADH for binding to the InhA active site. Significant inhibition was not observed when either Mn(III) pyrophosphate (run 2) or INH or both of these two reagents were omitted (Fig. 4A). In the absence of NADH (Fig. 4A; Table 2, run 3), partial time-dependent inhibition of InhA was observed (31% loss of InhA activity after 12 min of incubation), possibly due to the instability of InhA in the absence of its cofactor. However, we could not rule out the possibility of an InhA inactivation due to a nonspecific interaction of activated INH species with the enzyme (e.g., through alkylation of amino acid residues). In order to distinguish between these two possibilities, INH was first preincubated with Mn(III) pyrophosphate in the absence of InhA under the same conditions described above. After preincubation at RT for 18 min, the enzyme was added. No significant loss of activity was observed in this way after a further 12-min incubation (data not shown), arguing against any instability of the enzyme in the absence of its cofactor but rather supporting a nonspecific interaction of transient reactive INH oxidation intermediates with the enzyme.
These results altogether show that it is possible to activate INH with a simple nonenzymatic process and, through the formation of INH adducts with the NADH cofactor of InhA, to nearly completely inhibit this enzyme within a few minutes.
Inhibition of InhA by a pool of preformed adducts.
We studied the inhibition of InhA by a pool of preformed adducts in two consecutive steps: first, we oxidized 100 μM INH in the presence of 100 μM NADH, NAD+, NMN+, or DNAD+ at RT under the same conditions described above for 18 min, a reaction time sufficient for complete INH adduct formation; second, the reaction medium containing the preformed pool of INH adducts was mixed with 3 μM InhA to study the kinetic data. The aim was to examine whether INH adducts produced in the absence of InhA are able to show the same inhibition activity as those formed in the presence of InhA and to investigate the possible role of the enzyme target in formation of the inhibitors. In the curves corresponding to evaluation of INH adducts with NADH prepared in the presence (Fig. 4A; Table 2, run 1) and in the absence of InhA (Fig. 4B; Table 2, run 4), we note a similar profile, with a time-dependent inhibition. In the second experiment, the adducts were preformed, so no kinetic of adduct formation can explain the time-dependent inhibition, which can be proposed in the first case. In addition, we have verified that after 2 h of preincubation of INH, NADH, and Mn(III) pyrophosphate, the concentration of INH adducts was the same and that the subsequent inhibition kinetic profile of InhA was unchanged. Thus, the time-dependent profile in Fig. 4B might correspond to the time necessary to reach the equilibrium between binding of NADH and binding of the competitive inhibitor to the active site. The InhA inhibition appeared slightly lower with preformed adducts than with adducts formed in the presence of InhA (Fig. 4; Table 2, runs 4 and 1, respectively).
In the case of adducts obtained in the presence of the oxidized coenzyme NAD+, formed in higher yields (Table 1, run 3, 41%), the inhibition was very fast and almost complete (Fig. 4B; Table 2, runs 7 to 9), even for short preincubation times (preincubation for 2 min was sufficient to form adducts giving up to 86% inhibition of InhA activity). Here, the initial higher concentrations of the inhibitory adducts and the known lower affinity of NAD+ for InhA (compared to NADH) (21) probably allowed to quickly reach the equilibrium.
INH adducts with other nicotinamide or nicotinic coenzymes were similarly evaluated as possible InhA inhibitors. NMN+ contrasts with NAD+ by the absence of the adenine nucleotide part and DNAD+ by the replacement of the carboxamide function of NAD+ by a carboxylic group. In both cases (Fig. 4B; Table 2, runs 11 and 13, respectively) and using strictly the same experimental conditions as with NAD+, no InhA inhibition was observed. This absence of reactivity, keeping however in mind the low level of adducts yielded (Table 1), might indicate that both the nicotinamide function and the adenylic moiety play a crucial role in the inhibitor binding and contribute to its affinity for the InhA target.
These results mainly show that INH-NAD(H) adducts can be formed using the Mn(III) pyrophosphate activation system in the absence of InhA. These adducts have the same potency to inhibit InhA activity as the adducts formed in the presence of the enzyme, signifying that preformed adducts are able to bind to the InhA active site. The rather small differences of inhibition rate observed between experiments in which adducts were either preformed or formed in the presence of InhA do not allow us to estimate whether, in vivo, the inhibitory adduct forms itself between activated INH and NAD(H) within the InhA active site or forms outside the active site and then binds to it.
Inhibition of InhA by an isolated pool of adducts or by collected single adducts.
The isomeric adducts described above are rather stable as long as they are kept as a mixture in the buffered reaction medium at RT. One of the main difficulties in evaluating their biological activity is related to separation and isolation problems. As an example, when the main and well-separated compounds 6 and 7 (Fig. 2A) were collected from HPLC and then lyophilized and redissolved in water, the subsequent reinjection of the collected samples in the HPLC column displayed a mixture of the different initial isomers as well as some unidentified degradation products. Such a phenomenon has already been observed (26). Salt and/or pH effects during concentration of samples might explain this behavior, with a possible epimerization occurring at position 4 through a pH-dependent keto-enolic equilibrium. Hence, we established a protocol allowing us to test the inhibition of InhA activity directly from the fractions collected from HPLC. Three kinds of experiments were performed: (i) one with a pool of INH-NAD(H) adducts isolated by HPLC, (ii) one with the isolated compounds 6 and 7, and (iii) one with the isolated compounds 11 and 12. Adducts formed in amounts that were too small were not collected. Adduct concentrations of the collected HPLC fractions were determined using a ɛ260 of 27 mM−1 cm−1, a value estimated by Lei et al. for an INH-NAD(H) adduct (11). The same ɛ value was used for INH-DNAD(H) adducts 11 and 12, considering that the absorption of these compounds should be close to that of INH-NAD(H) adducts (the starting cofactors NAD+ and DNAD+ have the same ɛ260, 18 mM−1 cm−1).
The inhibition of InhA by increasing concentrations of the pool of adducts is displayed in Fig. 5 and shows a dose-dependent inhibition. From the crystallographic data (22) on the structure of the complex InhA/INH-NAD(H) adduct, the NAD part of the adduct logically binds to the NADH binding site and the isonicotinoyl moiety binds to the substrate binding site. Thus, the complete adduct must compete with NADH and possibly with the enoyl substrates for binding to the InhA active site. This point is supported by results shown in Fig. 6. An isolated 100 nM pool of INH-NAD(H) adducts first incubated for 5 min with 77 nM InhA was shown to give a 78% inhibition of the enzyme activity (column 2). This inhibition was completely (column 3) or almost completely (column 4) reversed when the inhibitor was only added after preincubation of InhA in the presence of 100 μM NADH or 100 μM decenoyl-CoA, respectively. This phenomenon of protection by the NADH cofactor or the enoyl-CoA substrate explains also why, under the conditions of Fig. 5, where the inhibitor has to compete with both compounds, the concentration of INH-NAD(H) adducts necessary to observe an inhibition of about 80% was clearly higher (about 5 μM).
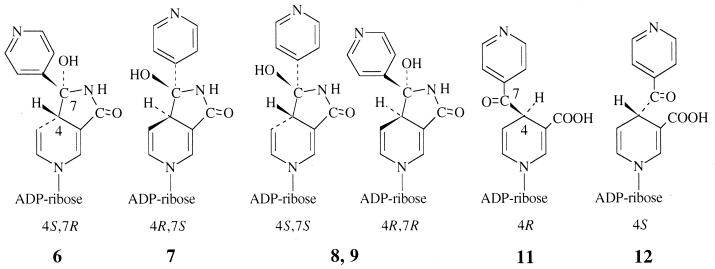
Concerning the inhibition observed with the isolated adduct forms, results shown in Table 3 indicate that compound 6 was clearly more efficient than compound 7 and was as efficient as the total pool. Among DNAD+ adducts, adduct 12 was a poor inhibitor, while adduct 11 was devoid of any inhibitory activity. Considering the adduct formula shown in Fig. 7, compounds 11 and 12 have the two possible configurations R and S at position 4 and were formed in a ratio of nearly 1:1. So, the alkylation process of the coenzyme by the activated form of INH must be considered to occur equally on each face of the pyridinium ring. Compounds 6 and 7, and similarly the pair of compounds 8 and 9, were also formed in similar amounts, and we can suppose that each pair contains one R isomer and one S isomer at the C-4 position. Since the pair of compounds 6 and 7 was formed in much higher yield than the pair of compounds 8 and 9, one hypothesis is that the cyclization process occurs preferentially with the two bulky substituents at C-4 and C-7 in trans position (Fig. 7). So, the compound pair 6-7 might be the couple of isomers 4S,7R and 4R,7S, and the compound pair 8-9 might be the couple 4S,7S and 4R,7R. A more precise attribution for compounds 6 and 7, as also for compounds 11 and 12, might be deduced from the biological results (Table 3). The most-likely structure for the most-active compounds 6 and 12 should be that with configuration S at C-4, which is the configuration proposed for the bound inhibitor present in the crystal structure of the InhA-inhibitor complex (22). Taken together, these arguments led us to propose for each of the compounds 6, 7, 11, and 12 the absolute configurations at C-4 and C-7 as ascribed in Fig. 7.
FIG. 7.
Structures of compounds 6 to 9 and compounds 11 and 12 and proposed stereochemistry of chiral centers for compounds 6, 7, 11, and 12. Adapted from reference 17.
It is noteworthy that one of the cyclic forms of the INH-NAD(H) adduct (form 6) has a relatively strong inhibitory potency on InhA activity, while the crystal structure of the adduct bound to InhA in the binary complex InhA-adduct was interpreted as an open form (Fig. 3); (22). Thus, it is legitimate to raise the question about the effective active form of the INH-NAD(H) adduct(s). Is there a single active form, or are several forms able to inhibit the InhA activity, with different levels of inhibitory potency?
In conclusion, we provide a method for a very fast and efficient inhibition of InhA in the presence of NAD+/NADH and INH, using Mn(III) pyrophosphate as a simple nonenzymatic oxidative INH activator. This chemical oxidant provides a simple way to synthesize various INH derivatives, mimicking the bioactivation pathway of this drug in M. tuberculosis. The structure-activity relationships of the different types of INH-coenzyme adducts evidenced in this work will be useful to facilitate the design of new inhibitors of InhA, with potential use as antituberculous drugs.
Acknowledgments
We thank Catherine Claparols and Suzy Richelme for their collaboration on all ESI-MS analyses, which were performed at the University Paul Sabatier in the Service de Spectrométrie de Masse FR14-LCC. We thank Gilbert Laneelle and Mamadou Daffé for helpful discussions.
REFERENCES
- 1.Archibald, F. S., and I. Fridovich. 1982. The scavenging of superoxide radical by manganous complexes: in vitro. Arch. Biochem. Biophys. 214:452-463. [DOI] [PubMed] [Google Scholar]
- 2.Banerjee, A., E. Dubnau, A. Quémard, V. Balasubramanian, K. S. Um, T. Wilson, D. Collins, G. de Lisle, and W. R. Jacobs, Jr. 1994. InhA, a gene encoding a target for isoniazid and ethionamide in Mycobacterium tuberculosis. Science 263:227-230. [DOI] [PubMed] [Google Scholar]
- 3.Bartmann, K., H. Iwainsky, H. H. Kleeberg, P. Mison, H. A. Offe, H. Otten, D. Tettenborn, and L. Trnka. 1988. Antituberculosis drugs. Springer-Verlag, Berlin, Germany.
- 4.Blanchard, J. S. 1996. Molecular mechanisms of drug resistance in Mycobacterium tuberculosis. Annu. Rev. Biochem. 65:215-239. [DOI] [PubMed] [Google Scholar]
- 5.Daffé, M., and P. Draper. 1998. The envelope layers of mycobacteria with reference to their pathogenicity. Adv. Microb. Physiol. 39:131-203. [DOI] [PubMed] [Google Scholar]
- 6.Defrance, S., J.-L. Séris, and B. Meunier. 1992. Metalloporphyrin-catalysed oxidation of manganese pyrophosphate. Methoxylated benzene derivatives as mediators in a chemical model of manganese peroxidase. New J. Chem. 16:1015-1016. [Google Scholar]
- 7.Ito, K., K. Yamamoto, and S. Kawanishi. 1992. Manganese-mediated oxidative damage of cellular and isolated DNA by isoniazid and related hydrazines: non-Fenton-type hydroxyl radical formation. Biochemistry 31:11606-11613. [DOI] [PubMed] [Google Scholar]
- 8.Johnsson, K., W. A. Froland, and P. G. Schultz. 1997. Overexpression, purification, and characterization of the catalase-peroxidase KatG from Mycobacterium tuberculosis. J. Biol. Chem. 272:2834-2840. [DOI] [PubMed] [Google Scholar]
- 9.Johnsson, K., D. S. King, and P. G. Schultz. 1995. Studies on the mechanism of action of isoniazid and ethionamide in the chemotherapy of tuberculosis. J. Am. Chem. Soc. 117:5009-5010. [Google Scholar]
- 10.Johnsson, K., and P. G. Schultz. 1994. Mechanistic studies of the oxidation of isoniazid by the catalase-peroxidase from Mycobacterium tuberculosis. J. Am. Chem. Soc. 116:7425-7426. [Google Scholar]
- 11.Lei, B., C. J. Wei, and S. C. Tu. 2000. Action mechanism of antitubercular isoniazid. Activation by Mycobacterium tuberculosis KatG, isolation, and characterization of InhA inhibitor. J. Biol. Chem. 275:2520-2526. [DOI] [PubMed] [Google Scholar]
- 12.Magliozzo, R. S., and J. A. Marcinkeviciene. 1996. Evidence for isoniazid oxidation by oxyferrous mycobacterial catalase-peroxidase. J. Am. Chem. Soc. 118:11303-11304. [Google Scholar]
- 13.Magliozzo, R. S., and J. A. Marcinkeviciene. 1997. The role of Mn(II)-peroxidase activity of mycobacterial catalase-peroxidase in activation of the antibiotic isoniazid. J. Biol. Chem. 272:8867-8870. [DOI] [PubMed] [Google Scholar]
- 14.Marcinkeviciene, J. A., R. S. Magliozzo, and J. S. Blanchard. 1995. Purification and characterization of the Mycobacterium smegmatis catalase-peroxidase involved in isoniazid activation. J. Biol. Chem. 270:22290-22295. [DOI] [PubMed] [Google Scholar]
- 15.Marrakchi, H., G. Laneelle, and A. Quémard. 2000. InhA, a target of the antituberculous drug isoniazid, is involved in a mycobacterial fatty acid elongation system, FAS-II. Microbiology 146:289-296. [DOI] [PubMed] [Google Scholar]
- 16.Minisci, F., E. Vismara, and F. Fontana. 1989. Recent developments of free-radical substitutions of heteroaromatic bases. Heterocycles 28:489-519. [Google Scholar]
- 17.Nguyen, M., C. Claparols, J. Bernadou, and B. Meunier. 2001. A fast and efficient metal-mediated oxidation of isoniazid and identification of isoniazid-NAD(H) adducts. ChemBiochem. 2:877-883. [DOI] [PubMed] [Google Scholar]
- 18.Nguyen, M., A. Quémard, H. Marrakchi, J. Bernadou, and B. Meunier. 2001. The nonenzymatic activation of isoniazid by MnIII-pyrophosphate in the presence of NADH produces the inhibition of the enoyl-ACP reductase InhA from Mycobacterium tuberculosis. C. R. Acad. Sci. Paris Ser. IIc 4:35-40. [Google Scholar]
- 19.Parikh, S., D. P. Moynihan, G. Xiao, and P. J. Tonge. 1999. Roles of tyrosine 158 and lysine 165 in the catalytic mechanism of InhA, the enoyl-ACP reductase from Mycobacterium tuberculosis. Biochemistry 38:13623-13634. [DOI] [PubMed] [Google Scholar]
- 20.Quémard, A., A. Dessen, M. Sugantino, W. R. Jacobs, J. C. Sacchettini, and J. S. Blanchard. 1996. Binding of the catalase-peroxidase-activated isoniazid to wild-type and mutant Mycobacterium tuberculosis enoyl-ACP reductases. J. Am. Chem. Soc. 118:1561-1562. [Google Scholar]
- 21.Quémard, A., J. C. Sacchettini, A. Dessen, C. Vilcheze, R. Bittman, W. R. Jacobs, Jr., and J. S. Blanchard. 1995. Enzymatic characterization of the target for isoniazid in Mycobacterium tuberculosis. Biochemistry 34:8235-8241. [DOI] [PubMed] [Google Scholar]
- 22.Rozwarski, D. A., G. A. Grant, D. H. R. Barton, W. R. Jacobs, Jr., and J. C. Sacchettini. 1998. Modification of the NADH of the isoniazid target (InhA) from Mycobacterium tuberculosis. Science 279:98-102. [DOI] [PubMed] [Google Scholar]
- 23.Sinha, B. K. 1983. Enzymatic activation of hydrazine derivatives. A spin-trapping study. J. Biol. Chem. 258:796-801. [PubMed] [Google Scholar]
- 24.Sollewijn Gelpke, M. D., P. Moenne-Loccoz, and M. H. Gold. 1999. Arginine 177 is involved in Mn(II) binding by manganese peroxidase. Biochemistry 38:11482-11489. [DOI] [PubMed] [Google Scholar]
- 25.Wang, J. Y., R. M. Burger, and K. Drlica. 1998. Role of superoxide in catalase-peroxidase-mediated isoniazid action against mycobacteria. Antimicrob. Agents Chemother. 42:709-711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wilming, M., and K. Johnsson. 1999. Spontaneous formation of the bioactive form of the tuberculosis drug isoniazid. Angew. Chem. Int. Ed. Engl. 38:2588-2590. [DOI] [PubMed] [Google Scholar]
- 27.Zabinski, R. F., and J. S. Blanchard. 1997. The requirement for manganese and oxygen in the isoniazid-dependent inactivation of Mycobacterium tuberculosis enoyl reductase. J. Am. Chem. Soc. 119:2331-2332. [Google Scholar]
- 28.Zhang, Y., B. Heym, B. Allen, D. Young, and S. Cole. 1992. The catalase-peroxidase gene and isoniazid resistance of Mycobacterium tuberculosis. Nature 358:591-593. [DOI] [PubMed] [Google Scholar]