Abstract
The emergence of resistant hepatitis B virus (HBV) with the L528M mutation and/or the M552V and M552I mutations in the polymerase gene following long-term lamivudine treatment is becoming an important clinical problem. The aim of this study was to investigate the susceptibility of wild-type and lamivudine-resistant HBV to MCC-478 (LY582563), a novel nucleoside analogue derivative of phosphonomethoxyethyl purine. The susceptibility of wild-type HBV and lamivudine-resistant mutants (M552I, M552V, and L528M/M552V) to MCC-478 was examined by transient transfection of full-length HBV DNA into human hepatoma cells. HBV DNA replication was monitored by Southern blot hybridization, and the effective concentration required to reduce replication by 50% (EC50) was determined. The replicative intermediates of wild-type and lamivudine-resistant mutants were progressively diminished by treatment with increasing doses of MCC-478. The MCC-478 EC50s were 0.027 μM for wild-type HBV (about 20 times more efficient than lamivudine), 2.6 μM for M552I, 3.3 μM for M552V, and 2.0 μM for L528M/M552V. Wild-type HBV and lamivudine-resistant mutants are susceptible to MCC-478. MCC-478 appears to be a candidate for the treatment of HBV infection and exhibits potent activity against lamivudine-resistant HBV.
Hepatitis B is a serious disease, and according to a 1997 World Health Organization report (http://www.who.ch/whr/1997/presse.htm), it is among the 10 leading killer diseases. Interferon treatment is partially effective for chronic hepatitis B but has dose-limiting side effects (9, 19, 28). Recently, lamivudine became a standard oral therapy for hepatitis B treatment (6), and clinical data show that it rapidly reduces hepatitis B virus (HBV) DNA levels, is well tolerated, and improves liver histology (8, 12, 14, 20).
Short-term lamivudine therapy is not sufficient to clear the virus. However, long-term therapy is associated with the emergence of the lamivudine-resistant viruses M552I and M552V (the M552V mutation is often accompanied by a second L528M mutation). These viruses have amino acid substitutions in the tyrosine-methionine-aspartate-aspartate (YMDD) motif of viral DNA polymerase (1, 16, 26). The emergence rates of lamivudine-resistant HBV have ranged from 14 to 46% after 1 year to as high as 67 to 75% after 3 to 4 years of continuous therapy (6, 12; D. T. Y. Lau, M. F. Khokhar, M. G. Ghany, E. Doo, D. Herion, D. E. Kleiner, Y. Park, P. Schmid, T. J. Liang, and J. H. Hoofnagle, Abstr. 10th Int. Symp. Vir. Hepatitis Liver Dis., abstr. 076, 2000). Although the natural history of patients with lamivudine-resistant HBV is not yet well defined, this virus has been associated with an acute exacerbation of liver disease (15), advanced hepatic fibrosis and necroinflammatory process in patients receiving liver transplantation (2), and severe hepatitis in patients coinfected with human immunodeficiency virus (3). Thus, the need for new antiviral agents for hepatitis B treatment is becoming clear.
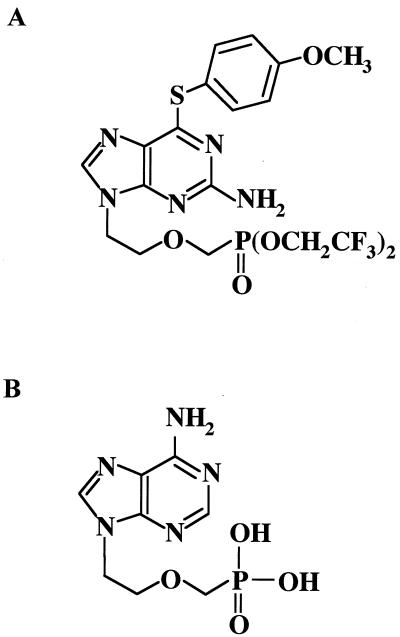
MCC-478 {LY582563, 2-amino-9-[2-(phosphonomethoxy)ethyl]-6-(4-methoxyphenylthio)purine bis(2,2,2-trifluoroethyl) ester}, a novel nucleoside analogue, was synthesized as a derivative of adefovir. In this study, we evaluated the susceptibility of wild-type HBV and lamivudine-resistant mutants to MCC-478 by a transient transfection of full-length HBV DNA into human hepatoma cells.
MATERIALS AND METHODS
Cells.
The human hepatoma cell line HuH-7 (18) was obtained from the Human Science Research Resource Bank (Osaka, Japan) and was cultured in Dulbecco's modified Eagle's medium (Invitrogen, Groningen, the Netherlands) supplemented with 10% heat-inactivated fetal bovine serum at 37°C in a 5% CO2 atmosphere.
HBV DNA.
HBV DNA was amplified and cloned as described previously (22). In short, HBV DNA was extracted from the serum of a patient with hepatitis B e antigen-positive cirrhosis. Then, HBV DNA was amplified by PCR according to the method described by Günther et al. (7). The PCR product was digested with ScaI and then cloned into pBluescript II SK+ (Stratagene, La Jolla, Calif.). Three types of lamivudine-resistant mutants were created by substituting nucleotides to change the codon for methionine in the YMDD motif to isoleucine (M552I) or valine (M552V) and codon 528 for leucine in the B-domain motif to methionine (L528M/M552V) by using the QuikChange Site-Directed Mutagenesis Kit (Stratagene) as described previously (23).
Transfection of full-length HBV DNA into HuH-7 cells.
HuH-7 cells (80 to 90% confluent in 60-mm-diameter dishes) were transfected with 0.9 μg of HBV DNA by using Effectene transfection reagent (Qiagen, Hilden, Germany) and incubated for 24 h. Then, cells were exposed to 0 to 10 μM of MCC-478 (a gift from Mitsubishi Pharma Corporation, Osaka, Japan) (Fig. 1) for 3 days. Cells were rinsed three times with ice-cold phosphate-buffered saline and then harvested. Transfection efficiency was monitored by cotransfecting 0.1 μg of β-galactosidase expression plasmid pCMVβ (Clontech Laboratories Inc., Palo Alto, Calif.). HuH-7 cell extracts were assayed for β-galactosidase as described previously (22). Experiments were performed at least in duplicate.
FIG. 1.
Chemical structures of MCC-478 (A) and adefovir (B).
Isolation of HBV DNA from transfected cells.
HBV DNA was purified from intracellular core particles by a method described by Günther et al. (7) with minor modifications. Briefly, cells were suspended in 500 μl of lysis buffer containing 50 mM Tris-HCl (pH 7.4), 1 mM EDTA, and 1% NP-40; transferred to an Eppendorf tube; vortexed; and allowed to stand on ice for 15 min. Nuclei were pelleted by centrifugation at 4°C and 15,000 × g for 1 min. The supernatant was transferred to a new tube, the MgCl2 concentration was adjusted to 10 mM, and the DNA was digested with 100 μg of DNase I/ml for 30 min at 37°C. To stop the reaction, EDTA was added to a final concentration of 25 mM. Then, 0.5 mg of proteinase K/ml and 1% sodium dodecyl sulfate were added and samples were incubated at 50°C for 4 h. Phenol-chloroform (1:1) extraction was performed, and the nucleic acids were ethanol precipitated along with a glycogen carrier.
Southern blot hybridization of HBV DNA.
HBV DNA was resolved on 1.5% agarose gel, transferred to a nylon membrane (Hybond N+; Amersham Pharmacia Biotech, Piscataway, N.J.) by Southern blotting, and hybridized with an alkaline-phosphatase-labeled full-length HBV DNA probe generated with the Gene Images AlkPhos Direct labeling system (Amersham Pharmacia Biotech). Chemiluminescent detection was performed with CDP-Star (Amersham Pharmacia Biotech) and analyzed by using a LAS1000 image analyzer (Fuji Photo Film, Tokyo, Japan).
RESULTS
Wild-type HBV is susceptible to MCC-478.
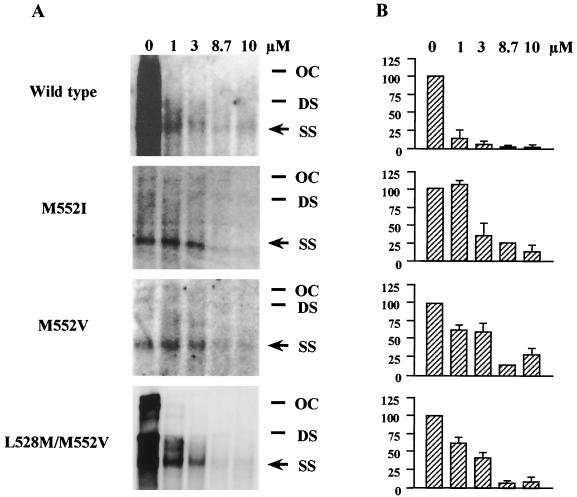
To assess the effect of MCC-478 on wild-type HBV replication in vitro, HuH-7 cells transfected with wild-type HBV DNA were incubated with different concentrations (0 to 10 μM) of MCC-478. Southern blot hybridization of DNA extracts showed a single-stranded band (representative of the HBV replicative intermediates) in the drug-free sample (Fig. 2). This band diminished with increasing concentrations of MCC-478, indicating that wild-type HBV was susceptible to MCC-478. We determined the inhibition of wild-type HBV DNA synthesis by using 0, 0.01, 0.1, 1, and 10 μM concentrations of MCC-478 and then calculated the effective concentration that inhibited HBV replication by 50% (EC50) (Table 1). Comparing the EC50s, MCC-478 was about 20 times more potent than lamivudine (Table 1).
FIG. 2.
Susceptibility of wild-type HBV and lamivudine-resistant mutants to MCC-478. (A) Southern blot hybridization analysis of HBV replication. Lanes correspond to DNA extracted from viral core particles derived from HuH-7 cells that were transfected with full-length HBV DNA with indicated concentrations of MCC-478. Arrow indicates the single-stranded band. OC, open circular HBV DNA; DS, double-stranded HBV DNA; SS, single-stranded HBV DNA. (B) Diagram comparing the susceptibility (means ± standard deviations) of wild-type HBV and M552I, M552V, and L528M/M552V mutants to MCC-478 at the indicated concentrations. Experiments were performed two to four times. Single-stranded bands were quantified using a LAS1000 fluorescent image analyzer (Fuji Photo Film) and normalized for transfection efficiency based on β-galactosidase activity. Intensities are given on the left of the graphs. The intensity of the single-stranded band of control (0 μM) was defined as 100.
TABLE 1.
EC50s of compounds and replication levelsa for wild-type and lamivudine-resistant HBV mutants following a 10 μM treatment
| Compound | Wild type
|
M552I
|
M552V
|
L528M/M552V
|
||||
|---|---|---|---|---|---|---|---|---|
| EC50 | Replication level | EC50 | Replication level | EC50 | Replication level | EC50 | Replication level | |
| Lamivudine | 0.56b | 1.3 ± 0.2b | >80c | 176.8 ± 17.9b | 33c | 76.9 ± 15.8b | >80c | 126.7 ± 9.6b |
| Adefovir | 0.58b | 10.5 ± 6.1b | 4.5c | 30.8 ± 20.0b | 4.9c | 32.5 ± 2.9b | 2.2c | 23.8 ± 13.3b |
| MCC-478 | 0.027 | 9.0 ± 14.4 | 2.6 | 13.1 ± 9.5 | 3.3 | 32.1 ± 10.4 | 2.0 | 9.1 ± 6.9 |
Numbers indicate the mean ± standard deviation from two to four experiments in replication percent for wild-type and mutant HBV transfected into HuH-7 cells and treated with 10 μM concentrations of the indicated compounds. Single-stranded bands were quantified using a LAS1000 image analyzer (Fuji Photo Film) and normalized for transfection efficiency based on β-galactosidase activity. Single-stranded control bands (without treatment) were set as 100%.
See reference 21.
See reference 23.
Lamivudine-resistant HBV is susceptible to MCC-478.
To analyze the in vitro antiviral effect of MCC-478 on lamivudine-resistant HBV, HuH-7 cells transfected with M552I, M552V, and L528M/M552V HBV mutants were incubated with different concentrations (0 to 10 μM) of MCC-478. Southern blot hybridization of DNA extracts showed a single-stranded band (representative of the HBV replicative intermediates) in the drug-free samples (Fig. 2). This band diminished with increasing concentrations of MCC-478 in the M552I, M552V, and L528M/M552V samples, indicating that all three lamivudine-resistant HBV mutants were susceptible to the drug. We determined the inhibition of lamivudine-resistant HBV DNA synthesis by using 0, 1, 3, 8.7, and 10 μM concentrations of MCC-478 and calculated the EC50s (Table 1). Although MCC-478 inhibited the replication of all three lamivudine-resistant mutants, a much higher dose of the drug (about 100 times more) was necessary to inhibit the replication of mutants than was required for wild-type HBV inhibition (Table 1).
DISCUSSION
The discovery of lamivudine was a breakthrough for the treatment of hepatitis B. Clinical trials show that a 1-year course of lamivudine antiviral therapy results in the suppression of viral replication and substantial histological improvement in patients with chronic hepatitis B (12). Moreover, a recent study revealed that a 2-year course of lamivudine therapy is well tolerated and more efficacious than a 1-year course (14). In fact, a 2-year course resulted in incremental HBeAg seroconversion from 17% at week 52 to 27% at week 104 (14). However, 38% of patients receiving a 2-year course developed drug-resistant mutant HBV compared to 14% of those receiving a 1-year course (12, 14). The longer treatment with lamivudine is continued, the more frequently drug-resistant HBV appears.
Lamivudine-resistant viruses harbor substitutions from methionine (M) to isoleucine (I) or valine (V) in the YMDD motif in the C domain of the polymerase, and the M552V mutant contains a substitution from leucine (L) to M in the B domain (L528M) (1, 16, 26). L528M has been associated with famciclovir resistance (17, 25). It was previously shown that the L528M mutation cooperates with the M552V mutation, increasing HBV replication and drug resistance to entecavir, l-D4FC, and l-FMAU (21).
The in vitro full-length HBV DNA transfection system used in this study is suitable for evaluating antiviral agents, especially those that inhibit the DNA polymerase of this virus (21-23). This system is advantageous, since it facilitates the testing of various antiviral agents against different HBV mutants. Using this system, we demonstrated that adefovir could be a good treatment option for patients who fail lamivudine therapy due to the emergence of resistant virus (23). In fact, adefovir dipivoxil (the oral prodrug of adefovir) was shown to be effective against lamivudine-resistant mutants in vivo (24). Therefore, in this study, we tried to evaluate a novel reverse transcriptase inhibitor, MCC-478, against wild-type and lamivudine-resistant HBV mutants by using this system. MCC-478 is effective against not only wild-type HBV but also lamivudine-resistant mutants. Moreover, MCC-478 was equally effective against M552I, M552V, and L528M/M552V. However, it must be noted that the dose required to inhibit the replication of the lamivudine-resistant mutants was 100 times higher than that for the wild-type virus.
By using this system, the effects of 15 reverse transcriptase inhibitors (including MCC-478) against wild-type and lamivudine-resistant HBV mutants were previously evaluated (21, 23). Of 15 antiviral agents, 8 were effective against wild-type HBV. Comparing the EC50s of the eight antiviral agents, MCC-478 (0.027 μM) was the second most potent next to entecavir (0.00036 μM) (21). Only four (adefovir, lobucavir, entecavir, and MCC-478) were effective against all three types of lamivudine-resistant HBV, suggesting that lamivudine-resistant mutants are cross resistant to other nucleoside analogues. Because clinical studies of lobucavir were suspended due to oncogenicity in rodents (Bristol-Myers Squibb, New York, N.Y. [http://www.bms.com/news/press/data/fg_press_release_824.html]), MCC-478 has been one of three valuable candidates used to treat lamivudine-resistant HBV infection so far. It is also worth noting that the EC50s of MCC-478 (2.6 μM for M552I, 3.3 μM for M552V, and 2.0 μM for L528M/M552V) against lamivudine-resistant HBV were lower than those of adefovir (4.5 μM for M552I, 4.9 μM for M552V, and 2.2 μM for L528M/M552V) (23).
Although results from clinical trials of MCC-478 are not yet available, human study data are accumulating on adefovir. A recent phase III clinical study showed that no HBV mutations associated with adefovir resistance could be identified at week 48 (C. E. Westland, H. Yang, W. E. Delany IV, C. S. Gibbs, M. D. Miller, R. Fallis, J. Fry, C. L. Brosgart, H. Namini, and S. Xiong, Abstr. 52nd Ann. Meet. Am. Assoc. Study Liver Dis., abstr. 1099, 2001). It is possible that MCC-478 resistance is also difficult to develop because MCC-478 and adefovir had similar drug resistance profiles in our study. The most common toxicity associated with adefovir is nephrotoxicity manifested by an elevation in serum creatinine levels, which occurs in 35% of patients treated with 120 mg of adefovir/day (10). Moreover, dose-related decreases in serum carnitine levels have been noted in treated patients due to renal excretion of carnitine, although clinical significance of this biochemical change is uncertain (5). Therefore, 10- and 30-mg/day doses for hepatitis B treatment are being evaluated in clinical trials with adefovir. MCC-478 toxicity in humans should be carefully examined, although MCC-478 had no cytotoxicity up to 1,000 μM in HB611 cells (27), a human hepatoma-derived cell line in which the wild-type HBV genome is integrated and continuously produces HBV (N. Kamiya, A. Kubota, Y. Iwase, K. Sekiya, M. Ubasawa, and S. Yuasa, submitted for publication).
Optimal antiviral therapy should provide sufficient suppression of HBV replication. Treatment strategies that are safe and suitable for long-term use and suppress the emergence of drug-resistant strains are urgently needed. As shown in human immunodeficiency virus treatment, the goal may be reached by using combinations of anti-HBV drugs. It was previously shown that a combination of lamivudine and penciclovir has synergistic effects against HBV in vitro (4, 11). In clinical trials, hepatitis B patients treated with lamivudine in combination with famciclovir (the oral form of penciclovir) showed better inhibition of viral replication than patients treated with lamivudine alone (13). These results suggest that the combination of lamivudine with adefovir, entecavir, and MCC-478 are advantageous because their drug resistance profiles differ from that for lamivudine. Our study indicates that MCC-478 is a potential antiviral agent for hepatitis B treatment and deserves further investigation.
Acknowledgments
This study was supported by Health Sciences Research Grants for Medical Frontier Strategy Research from the Ministry of Health, Labor, and Welfare of Japan and by grants-in-aid for scientific research from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
We thank the Mitsubishi Pharma Corporation for the donation of MCC-478. We also thank Mitsuko Tsubouchi for technical assistance.
REFERENCES
- 1.Bartholomew, M. M., R. W. Jansen, L. J. Jeffers, K. R. Reddy, L. C. Johnson, H. Bunzendahl, L. D. Condreay, A. G. Tzakis, E. R. Schiff, and N. A. Brown. 1997. Hepatitis-B-virus resistance to lamivudine given for recurrent infection after orthotopic liver transplantation. Lancet 349:20-22. [DOI] [PubMed] [Google Scholar]
- 2.Ben-Ari, Z., O. Pappo, R. Zemel, E. Mor, and R. Tur-Kaspa. 1999. Association of lamivudine resistance in recurrent hepatitis B after liver transplantation with advanced hepatic fibrosis. Transplantation 68:232-236. [DOI] [PubMed] [Google Scholar]
- 3.Bessesen, M., D. Ives, L. Condreay, S. Lawrence, and K. E. Sherman. 1999. Chronic active hepatitis B exacerbations in human immunodeficiency virus-infected patients following development of resistance to or withdrawal of lamivudine. Clin. Infect. Dis. 28:1032-1035. [DOI] [PubMed] [Google Scholar]
- 4.Colledge, D., S. Locarnini, and T. Shaw. 1997. Synergistic inhibition of hepadnaviral replication by lamivudine in combination with penciclovir in vitro. Hepatology 26:216-225. [DOI] [PubMed] [Google Scholar]
- 5.Gilson, R. J., K. B. Chopra, A. M. Newell, I. M. Murray-Lyon, M. R. Nelson, S. J. Rice, R. S. Tedder, J. Toole, H. S. Jaffe, and I. V. Weller. 1999. A placebo-controlled phase I/II study of adefovir dipivoxil in patients with chronic hepatitis B virus infection. J. Viral Hepatol. 6:387-395. [DOI] [PubMed] [Google Scholar]
- 6.Gordon, D., and J. H. Walsh. 1998. Hepatitis drugs win approval. Gastroenterology 116:235-236. [Google Scholar]
- 7.Günther, S., B. C. Li, S. Miska, D. H. Kruger, H. Meisel, and H. Will. 1995. A novel method for efficient amplification of whole hepatitis B virus genomes permits rapid functional analysis and reveals deletion mutants in immunosuppressed patients. J. Virol. 69:5437-5444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Honkoop, P., R. A. de Man, H. R. Scholte, P. E. Zondervan, J. W. Van Den Berg, L. H. Rademakers, and S. W. Schalm. 1997. Effect of lamivudine on morphology and function of mitochondria in patients with chronic hepatitis B. Hepatology 26:211-215. [DOI] [PubMed] [Google Scholar]
- 9.Hoofnagle, J. H., and A. M. di Bisceglie. 1997. The treatment of chronic viral hepatitis. N. Engl. J. Med. 336:347-356. [DOI] [PubMed] [Google Scholar]
- 10.Kahn, J., S. Lagakos, M. Wulfsohn, D. Cherng, M. Miller, J. Cherrington, D. Hardy, G. Beall, R. Cooper, R. Murphy, N. Basgoz, E. Ng, S. Deeks, D. Winslow, J. J. Toole, and D. Coakley. 1999. Efficacy and safety of adefovir dipivoxil with antiretroviral therapy: a randomized controlled trial. JAMA 282:2305-2312. [DOI] [PubMed] [Google Scholar]
- 11.Korba, B. E. 1996. In vitro evaluation of combination therapies against hepatitis B virus replication. Antivir. Res. 29:49-51. [DOI] [PubMed] [Google Scholar]
- 12.Lai, C. L., R. N. Chien, N. W. Leung, T. T. Chang, R. Guan, D. I. Tai, K. Y. Ng, P. C. Wu, J. C. Dent, J. Barber, S. L. Stephenson, and D. F. Gray. 1998. A one-year trial of lamivudine for chronic hepatitis B. N. Engl. J. Med. 339:61-68. [DOI] [PubMed] [Google Scholar]
- 13.Lau, G. K., M. Tsiang, J. Hou, S. T. Yuen, W. F. Carman, L. Zhang, C. S. Gibbs, and S. K. Lam. 2000. Combination therapy with lamivudine and famciclovir for chronic hepatitis B-infected Chinese patients: a viral dynamics study. Hepatology 32:394-399. [DOI] [PubMed] [Google Scholar]
- 14.Liaw, Y. F., N. W. Y. Leung, T. T. Chang, R. Guan, D. I. Tai, K. Y. Ng, R. N. Chien, J. Dent, L. Roman, S. Edmundson, and C. L. Lai. 2000. Effects of extended lamivudine therapy in Asian patients with chronic hepatitis B. Gastroenterology 119:172-180. [DOI] [PubMed] [Google Scholar]
- 15.Liaw, Y. F., R. N. Chien, C. T. Yeh, S. L. Tsai, and C. M. Chu. 1999. Acute exacerbation and hepatitis B virus clearance after emergence of YMDD motif mutation during lamivudine therapy. Hepatology 30:567-572. [DOI] [PubMed] [Google Scholar]
- 16.Ling, R., D. Mutimer, M. Ahmed, E. H. Boxall, E. Elias, G. M. Dusheiko, and T. J. Harrison. 1996. Selection of mutations in the hepatitis B virus polymerase during therapy of transplant recipients with lamivudine. Hepatology 24:711-713. [DOI] [PubMed] [Google Scholar]
- 17.Melegari, M., P. P. Scaglioni, and J. R. Wands. 1998. Hepatitis B virus mutants associated with 3TC and famciclovir administration are replication defective. Hepatology 27:628-633. [DOI] [PubMed] [Google Scholar]
- 18.Nakabayashi, H., K. Taketa, K. Miyano, T. Yamane, and J. Sato. 1982. Growth of human hepatoma cells lines with differentiated functions in chemically defined medium. Cancer Res. 42:3858-3863. [PubMed] [Google Scholar]
- 19.Omata, M., F. Imazeki, O. Yokosuka, Y. Ito, K. Uchiumi, J. Mori, and K. Okuda. 1985. Recombinant leukocyte A interferon treatment in patients with chronic hepatitis B virus infection. Pharmacokinetics, tolerance, and biologic effects. Gastroenterology 88:870-880. [DOI] [PubMed] [Google Scholar]
- 20.Omata, M. 1998. Treatment of chronic hepatitis B infection. N. Engl. J. Med. 339:114-115. [DOI] [PubMed] [Google Scholar]
- 21.Ono, S. K., N. Kato, Y. Shiratori, J. Kato, T. Goto, R. F. Schinazi, F. J. Carrilho, and M. Omata. 2001. The polymerase L528M mutation cooperates with nucleotide binding-site mutations, increasing hepatitis B virus replication and drug resistance. J. Clin. Investig. 107:449-455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ono-Nita, S. K., N. Kato, Y. Shiratori, T. Masaki, K. H. Lan, F. J. Carrilho, and M. Omata. 1999. YMDD motif in hepatitis B virus DNA polymerase influences on replication and lamivudine resistance: a study by in vitro full-length viral DNA transfection. Hepatology 29:939-945. [DOI] [PubMed] [Google Scholar]
- 23.Ono-Nita, S. K., N. Kato, Y. Shiratori, K. H. Lan, H. Yoshida, F. J. Carrilho, and M. Omata. 1999. Susceptibility of lamivudine-resistant hepatitis B virus to other reverse transcriptase inhibitors. J. Clin. Investig. 103:1635-1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Perrillo, R., E. Schiff, E. Yoshida, A. Statler, K. Hirsch, T. Wright, K. Gutfreund, P. Lamy, and A. Murray. 2000. Adefovir dipivoxil for the treatment of lamivudine-resistant hepatitis B mutants. Hepatology 32:129-134. [DOI] [PubMed] [Google Scholar]
- 25.Pichoud, C., B. Seigneres, Z. Wang, C. Trepo, and F. Zoulim. 1999. Transient selection of a hepatitis B virus polymerase gene mutant associated with a decreased replication capacity and famciclovir resistance. Hepatology 29:230-237. [DOI] [PubMed] [Google Scholar]
- 26.Tipples, G. A., N. M. Ma, K. P. Fischer, V. G. Bain, N. M. Kneteman, and D. L. Tyrrell. 1996. Mutation in HBV RNA-dependent DNA polymerase confers resistance to lamivudine in vivo. Hepatology 24:714-717. [DOI] [PubMed] [Google Scholar]
- 27.Tsurimoto, T., A. Fujiyama, and K. Matsubara. 1987. Stable expression and replication of hepatitis B virus genome in an integrated state in a human hepatoma cell line transfected with the cloned viral DNA. Proc. Natl. Acad. Sci. USA 84:444-448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wong, D. K., A. M. Cheung, K. O'Rourke, C. D. Naylor, A. S. Detsky, and J. Heathcote. 1993. Effect of alpha-interferon treatment in patients with hepatitis B e antigen-positive chronic hepatitis B. A meta-analysis. Ann. Intern. Med. 119:312-323. [DOI] [PubMed] [Google Scholar]