Abstract
We have developed a method for visualizing Escherichia coli cells that are exposed to tetracycline in a biofilm, based on a previous report that liposomes containing the E. coli TetR(B) protein fluoresce when exposed to this antibiotic. By our method, cells devoid of TetR(B) also exhibited tetracycline-dependent fluorescence. At 50 μg of tetracycline ml−1, planktonic cells of a uropathogenic E. coli (UPEC) strain developed maximal fluorescence after 7.5 to 10 min of exposure. A similar behavior was exhibited by cells in a 24- or 48-h UPEC biofilm, as examined by confocal laser microscopy, regardless of whether they lined empty spaces or occupied densely packed regions. Further, a comparison of phase-contrast and fluorescent images of corresponding biofilm zones showed that all the cells fluoresced. Thus, all the biofilm cells were exposed to tetracycline and there were no pockets within the biofilm where the antibiotic failed to reach. It also appeared unlikely that niches of reduced exposure to the antibiotic existed within the biofilms.
Diseases involving bacterial biofilms are generally chronic and difficult to treat, because bacteria in biofilms are more resistant to antimicrobial agents than their planktonic (free-living) counterparts. Biofilms contribute to several serious diseases, such as endocarditis, cystitis, cystic fibrosis, and infections of prosthetic devices (4-6a).
It is not known why biofilms possess greater resistance. The possibility that this might be due to a lack of penetration of antibiotics has received considerable attention. Although several studies suggest that biofilms permit antibiotic penetration (1, 6, 8, 9, 10, 12, 14), it was not ascertained in these studies if this involved exposure of all constituent cells to the antibiotic.
We have developed a method to directly visualize cells coming in contact with tetracycline, based on the report that in the presence of the TetR(B) protein in liposomes (see http://biosafety.ihe.be/AR/Tetracycline/Menu_Tet for tetracycline resistance determinant nomenclature), this antibiotic fluoresces when activated by UV light (13). TetR(B) is constitutively synthesized in bacteria containing Tn10 and regulates the expression of the tetA(B) gene that encodes the tetracycline efflux pump (2). We report here that the biofilms of uropathogenic Escherichia coli (UPEC) and K-12 strains of E. coli (11) are readily permeable to tetracycline and permit exposure of all the constituent cells to this antibiotic.
MATERIALS AND METHODS
Bacterial strains and plasmids.
A clinical isolate of uropathogenic E. coli (AMG1), obtained from the Microbiology Section of the Infectious Diseases Department of the Stanford Medical Center, and our laboratory E. coli strain, AMS6 (11), were employed. A tetracycline-resistant strain of AMG1, referred to as AMG2, containing the TetR(B) protein was generated using the Tn10 transposon, and the “λ hop” technique; the latter employs a temperature-sensitive phage (λ561) as the Tn10 vehicle. Transposition was achieved as described previously (15), using λym plates supplemented with 15 μg of tetracycline ml−1. The selected mutant (AMG2) exhibited normal morphological and growth characteristics.
A recombinant pUC18 plasmid was used for TetR(B) overexpression. The plasmid was cleaved with BamHI and KpnI and ligated to a PCR product containing the full tetR(B) gene, a truncated tetA(B) gene encoding the first six amino acids of TetA(B), and the complete intergenic region (2). The PCR product was generated using DNA from the Tn10-bearing λ561 phage and the following primers: 5′-GCCGGGTACCTTAAGACCCACTTTCACATTT-3′ and 5′-GCCGTCTAGAAGCGATCTTTGTCGAACTATT-3′. The resulting recombinant plasmid was electroporated into AMG1, generating the strain AMG3, which overproduced TetR(B). Luria-Bertani (LB) broth, with or without ampicillin (50 μg ml−1), was used for cell growth (37°C); tetracycline was purchased from Sigma.
Fluorescence microscopy.
Planktonic cell suspensions (4.5 μl; 0.1 A660) were mixed with 0.5 μl of phosphate-buffered saline (PBS) solution of tetracycline at room temperature on a glass slide to give a tetracycline concentration of 5, 10, 50, or 100 μg ml−1, as specified. The suspension was overlaid with a no. 1 coverslip, and observed immediately in an Olympus BX-60 upright fluorescent microscope (100× oil immersion phase-contrast lens; total magnification, ×1,000). Conditions for optimal tetracycline-dependent fluorescence were determined empirically. For each time point, the cell-bound tetracycline was photoactivated by a short pulse of 360-nm light, followed by excitation of the resulting fluorophore at 490 nm and measurement of the emission at 510 nm; 360- and 490-nm lights were generated using a mercury arc lamp and DAPI (4′,6′-diamidino-2-phenylindole) and fluorescein isothiocyanate filters, respectively. Images were acquired with a Hamamatsu C4742CCD digital camera and Image Pro Plus software (version 4.1.0.0), using a 1-s capture time and a gain value of 70. Phase-contrast images were obtained under identical conditions. Some 300 cells were observed for each experimental condition.
Confocal microscopy.
Biofilms were grown at 37°C in Plexiglas flow cells (16), with interior dimensions of 4 by 36 by 1 mm. These were sterilized with 70% ethanol, filled with LB broth or (for strain AMG2) LB broth plus ampicillin, and inoculated with overnight cultures to an initial A660 of 0.1. After 1 h of incubation, the medium was pumped at a flow rate of 2.8 ml h−1 for 24 or 48 h. Biofilms (24 h) attained a thickness of 15 μm.
To determine tetracycline diffusion through the biofilms, the flow cell was detached from the medium feed assembly and mounted on a Zeiss 510 confocal microscope. Tetracycline in PBS at room temperature was added to the specified concentration, and the bacterial cells were imaged at a magnification of ×1,000 with an inverted (Pan Neofluar) 100× oil immersion differential interference contrast lens. Photoactivation of the cell-bound tetracycline and the excitation and emission light settings were as above; a scanning argon laser was used to generate the excitation light. Sagittal sections (Z series; vertical slices) were obtained using a software positioning system (Zeiss 510 LSM) to mark the top (biofilm-medium interface) and bottom (biofilm-coverslip interface) of the biofilm. A distance of 0.5 μm between each x-y plane was used. Reconstruction of fluorescence along the z axis necessitated some compromise on the resolution of individual cells; nevertheless, the approximate location of the individual could be discerned.
A 1- and 5-s 360-nm light exposure before each imaging of the planktonic and biofilm cells, respectively, permitted a snapshot of the cell-complexed tetracycline at a given time. Exposure of the planktonic cells for over 1 s (up to 5 s) of this light did not expedite fluorescence development, but it caused cell bleaching. Such bleaching was not observed upon a 5-s exposure of the biofilm cells (presumably because of the biofilm matrix and higher cell density) and permitted faster development of fluorescence in the upper layers of the biofilm (see Results). Fluorescence was seen only when both the cells and tetracycline were present, and not with either alone.
The image files were opened in Adobe Photoshop (version 6.01) and, after analyzing the histograms, an adjustment level correction was created and saved. This adjustment, which enhanced the contrast, was applied to each file with the exception of Fig. 2C, to which a lower-level correction was applied to prevent excessive brightness.
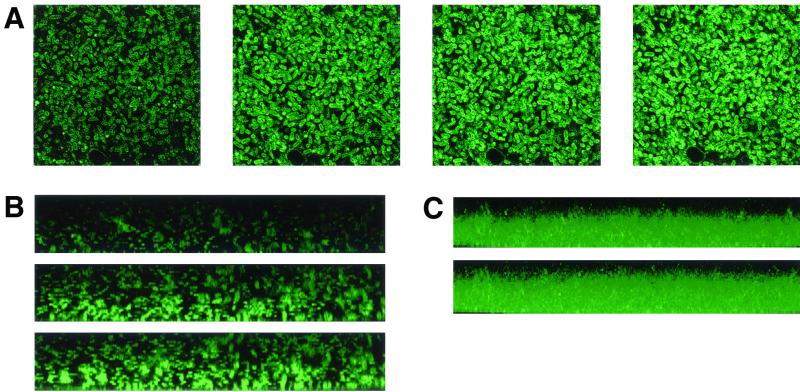
FIG. 2.
(A) Time-dependent development of tetracycline-induced fluorescence in a 24-h AMG1 biofilm cells in the x-y plane; panels from left to right represent 3, 7.5, 10, and 15 min of exposure, respectively. The images reproduced here are from about the middle of the biofilm but represent the situation throughout the biofilm, except in the uppermost layers (see Results). (B) Biofilm fluorescence in reconstructed z axis; panels from top to bottom represent 3, 7.5, and 10 min of exposure to the antibiotic, respectively. The bottom of the figure represents the biofilm base (coverslip end of the biofilm through which light was beamed). (C) Biofilm fluorescence in reconstructed composite z axis; panels from top to bottom represent 7.5 and 10 min of exposure to the antibiotic, respectively. The bottom of the figure represents the biofilm base, as in Fig. 2B.
Tetracycline sensitivity.
Overnight planktonic cultures were diluted to an A660 of 0.1 in LB medium, allowed to grow to an A660 of 0.8, and mixed with tetracycline (50 μg ml−1); controls did not receive the antibiotic. At indicated intervals, samples were removed and subjected to serial dilution and viability determination, as described previously (10a). Biofilms (24 h old) also received 50 μg of tetracycline ml−1. At indicated intervals biofilms were recovered by scraping and vortexing at high speed in 400 μl of 0.1 M phosphate buffer (pH 7.0); a Maxi Mix II Vortex mixer (Barnstead/Thermolyne, Dubuque, Iowa) (1) was used. The suspensions were serially diluted, and 50 μl of appropriate dilutions were grown on plates with 30 ml of LB agar for viability determination. As this entailed a 106 or greater dilution, the antibiotic carryover effect was negligible. Controls were treated identically except that they were not exposed to the antibiotic. In planktonic and biofilm control samples, the cell count continued to increase.
RESULTS
Tetracycline sensitivity.
At 50 μg of tetracycline ml−1, the biofilm-grown cells of the UPEC strain (initial CFU count, 3.2 × 108 ml−1) were more resistant, with nearly 90% survival after 1 h of exposure compared to 2% survival for the planktonic counterparts (initial CFU count, 5.9 × 109 ml−1). At subsequent time points also, the biofilm cells showed greater survival: for example, there was 18 and 0.01% survival for biofilm and planktonic cells, respectively, after 4 h of exposure. These data represent an average of two independent experiments; variation for individual time points was within 3%. The MIC of tetracycline at which 50% of isolates tested were inhibited for planktonic and biofilms cells of this strain were 100 and 400 ng ml−1, as determined by the method of Das et al. (7).
Tetracycline-induced fluorescence of planktonic cells.
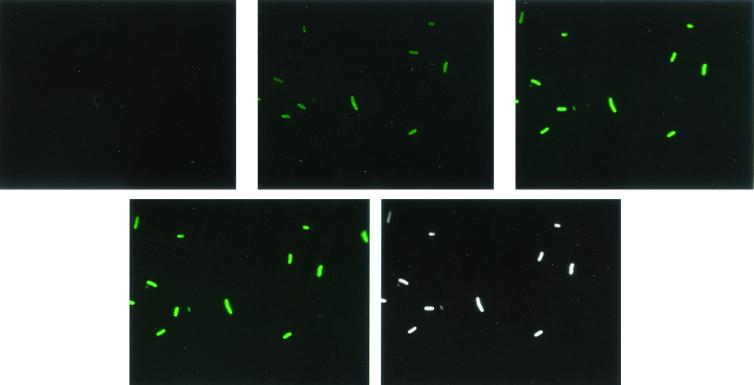
We determined optimal conditions for detecting cell contact-dependent tetracycline fluorescence by using planktonic cells of strain AMG2 [TetR(B) positive]. Using the range of tetracycline concentrations specified above, we found that a concentration of 50 μg ml−1 produced optimal results. Fluorescence was dim below this concentration and not enhanced above it (data not shown). Fluorescence development was time dependent at this concentration, being detectable at 3 min and maximal at 7.5 to 10 min (Fig. 1). Phase-contrast images of the same samples indicated that all cells fluoresced. While the fluorescence was more intense at the cell membrane, the cell interior also appeared to fluoresce (Fig. 1 and 2A). Exponential- and stationary-phase cells produced about equal fluorescence (not shown).
FIG. 1.
Time-dependent development of tetracycline-induced fluorescence in AMG2 planktonic cells. Panels from left to right represent fluorescence after 1, 3, 7.5, or 10 min of exposure to tetracycline, respectively; the last panel is a phase-contrast image of the corresponding region to visualize all the cells in the field (and not the fluorescent ones only). Magnification, ×2,000.
Effect of the absence of TetR(B).
As stated in the introduction, the presence of TetR(B) is required for tetracycline-induced fluorescence of liposomes (13). We found, however, that the planktonic cells of strains AMG1 and AMS6, which are devoid of TetR(B) (data not shown), exhibited a fluorescence pattern identical to that of AMG2 (Fig. 1); moreover, strain AMG3, which overproduces TetR(B), also generated similar fluorescence (not shown).
Tetracycline diffusion in biofilms.
Time-lapse imaging of tetracycline diffusion through the biofilms was performed by monitoring fluorescence development, using confocal microscopy. When tetracycline was added in 400 μl of PBS at 50 μg ml−1 to 24- or 48-h biofilms, fluorescence developed rapidly as shown by cross (x-y plane) sections. Within 3 min, fluorescent cells could be seen throughout the biofilms (except the uppermost layers; see below), indicating thorough penetration (Fig. 2A). As was the case with the planktonic cells, maximal fluorescence developed in ca. 10 min, with no further increase at 15 or 20 min (not shown). Similar results were seen when tetracycline was added at 500 μg ml−1 in 30 or 50 μl of PBS at the inflow end, except for a ca. 1-min lag in fluorescence development at biofilm distal end. When cells were exposed to PBS without tetracycline, no fluorescence developed.
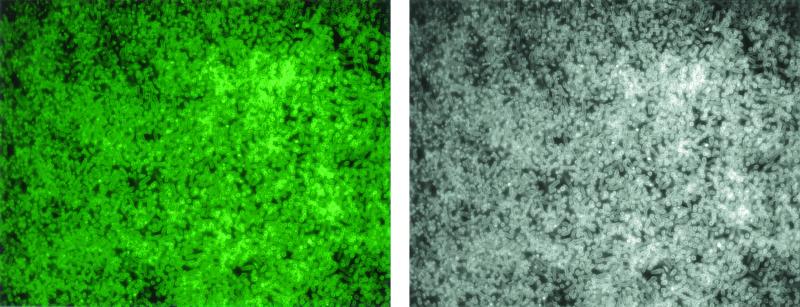
x-y plane images from the same biofilm regions were used to construct sagittal (x-z plane) images showing fluorescence along the z axis (Fig. 2B); from several z sections taken at constant intervals along a horizontal plane, a composite image of fluorescence along the z axis was obtained (Fig 2C). The biofilm cells adjacent to the glass coverslip (biofilm base) fluoresced more rapidly than those located at the top, where the antibiotic was added. We suspect that this effect arose from the inability of the 360-nm light, which is essential for the development of the fluorophore and is beamed through the coverslip end, to adequately penetrate the entire biofilm. That shorter exposures slowed and longer ones expedited fluorescence development in the upper layers is consistent with this interpretation and with the conclusion that tetracycline rapidly penetrated the entire biofilm. Figure 3 is a representative micrograph of comparison of the phase-contrast and fluorescent images of the above biofilms at corresponding regions, and shows that all the cells, regardless of their density, fluoresced and were exposed to the antibiotic.
FIG. 3.
Representative micrograph of comparison of the phase-contrast and fluorescent images of the above biofilms at corresponding regions. The images reproduced here are from about the middle of the biofilm but represent the situation throughout the biofilm, except the uppermost layers (see Results).
The data presented are for a 24-h biofilm of strain AMG1. Identical results were obtained for a 48-h biofilm of this strain and 24- or 48-h biofilms of the other strains studied.
DISCUSSION
The UPEC biofilm cells were more resistant to tetracycline than their planktonic counterparts. We determined whether this increased resistance was due to the inability of tetracycline to reach all the cells within the biofilm. Although biofilms as a whole have been shown to allow certain antibiotics to penetrate (1, 6, 8, 12, 14), it remains to be determined if this results in exposure of all the cells to the antibiotic. As the polymeric substance that surrounds biofilms is likely to be heterogeneous, it may be impenetrable in some regions while permitting passage in others. Furthermore, the channels contained in a biofilm (6) may also transport antibiotics, confining exposure mainly to cells lining the channels.
We developed a method for direct visualization of cells exposed to tetracycline. Although the original report of UV-induced tetracycline-dependent fluorescence suggested that the TetR(B) protein is required for this phenomenon (13), we found that E. coli strains devoid of TetR(B) also fluoresced upon tetracycline exposure. We could thus examine tetracycline penetration in the wild-type, tetracycline-sensitive, UPEC strain. The observed fluorescence of TetR(B)-deficient strains could be (i) because our method differed from that of Sigler et al. (13), (ii) because the strains we used contain homologues of TetR(B) (3), (iii) because the fluorescence is due to tetracycline interaction with the cell membrane, and/or (iv) because the tetracycline-30 S ribosomal complex fluoresces.
At 50 μg ml−1 tetracycline, both the planktonic and biofilm cells developed maximal fluorescence within 10 min; in the case of the biofilms, this was irrespective of whether the cells lined empty spaces or occupied dense regions. A comparison of phase contrast and fluorescent images of corresponding regions indicated that all the cells fluoresced. Thus, there were no pockets within the biofilms where the antibiotic failed to reach. The fact that the time-dependence of fluorescence development was the same in planktonic and biofilm cells may suggest that at 50 μg ml−1 tetracycline, the biofilm and the planktonic cells were exposed to the antibiotic to an equal degree, and that the increased biofilm resistance is not due to niches of decreased exposure. But firm conclusions in this latter regard require a more quantitative method.
Acknowledgments
This work was supported by a NASA grant (NAG2-1); G.F. and P.W. were supported by an NIH training grant (NRSA 5T32 AI0732801) and a Dean's fellowship from Stanford University School of Medicine, respectively.
We thank MaryAnn Wijtman for help with image processing.
REFERENCES
- 1.Anderl, J. N., M. J. Franklin, and P. S. Stewart. 2000. Role of antibiotic penetration limitation in Klebsiella pneumoniae biofilm resistance to ampicillin and ciprofloxacin. Antimicrob. Agents Chemother. 44:1818-1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chalmers, R., S. Sewitz, K. Lipkow, and P. Crellin. 2000. Complete nucleotide sequence of Tn10. J. Bacteriol. 182:2970-2972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cohen, S. P., H. Hachler, and S. Levy. 1993. Genetic and functional analysis of the multiple antibiotic resistance (mar) locus in Escherichia coli. J. Bacteriol. 175:1484-1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Conte, J. E. 1995. Manual of antibiotics and infectious diseases. Williams and Wilkins, Baltimore, Md.
- 5.Costerton, J. W., P. S. Stewart, and E. P. Greenberg. 1999. Bacterial biofilms: a common cause of persistent infections. Science 284:1318-1322. [DOI] [PubMed] [Google Scholar]
- 6.Costerton, J. W., K.-J. Cheng, G. G. Geesey, T. J. Ladd, J. C. Nickel, M. Dasgupta, and T. J. Marie. 1987. Bacterial biofilms in nature and disease. Annu. Rev. Microbiol. 41:435-464. [DOI] [PubMed] [Google Scholar]
- 6a.Darouiche, R. O., A. Dhir, A. J. Miller, G. C. Landon, I. I. Raad, and D. M. Musher. 1994. Vancomycin penetration into biofilm covering infected prostheses and effect on bacteria. J. Infect. Dis. 170:720-723. [DOI] [PubMed] [Google Scholar]
- 7.Das, J. R., M. Bhakoo, M. V. Jones, and P. Gilbert. 1998. Changes in the biocide susceptibility of Staphylococcus epidermidis and Escherichia coli cells associated with rapid attachment to plastic surfaces. J. Appl. Microbiol. 84:852-858. [DOI] [PubMed] [Google Scholar]
- 8.Dunne, W. M., Jr., E. O. Mason, Jr., and S. L. Kaplan. 1993. Diffusion of rifampin and vancomycin through a Staphylococcus epidermidis biofilm. Antimicrob. Agents Chemother. 37:2522-2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ghigo, J. M. 2001. Natural conjugative plasmids induce bacterial biofilm development. Nature 412:442-445. [DOI] [PubMed] [Google Scholar]
- 10.Hvidberg, H., C. Struve, K. A. Krogfelt, N. Christensen, S. N. Rasmussen, and N. Frimodt-M/oller. 2000. Development of a long-term ascending urinary tract infection mouse model for antibiotic treatment studies. Antimicrob. Agents Chemother. 44:156-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10a.Pandza, S., M. Baetens, C.-H. Park, T. Au, M. Keyhan, and A. Matin. 2000. The putative G-protein FLHF has a role in polar flagellar placement and general stress response induction in Pseudomonas putida. Mol. Microbiol. 36:414-423. [DOI] [PubMed] [Google Scholar]
- 11.Schweder, T., K.-H. Lee, O. Lomovskaya, and A. Matin. 1996. Regulation of Escherichia coli starvation sigma factor (σs) by ClpXP protease. J. Bacteriol. 178:470-476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shigeta, M., G. Tanaka, H. Komatsuzawa, M. Sugai, H. Suginaka, and T. Usui. 1997. Permeation of antimicrobial agents through Pseudomonas aeruginosa biofilms: a simple method. Chemotherapy (Basel) 43:340-345. [DOI] [PubMed] [Google Scholar]
- 13.Sigler, A., P. Schubert, W. Hillen, and M. Niederweis. 2000. Permeation of tetracyclines through membranes of liposomes and Escherichia coli. Eur. J. Biochem. 267:527-534. [DOI] [PubMed] [Google Scholar]
- 14.Suci, P. A., M. W. Mitterman, F. P. Yu, and G. G. Geesey. 1994. Investigation of ciprofloxacin penetration into Pseudomonas aeruginosa biofilms. Antimicrob. Agents Chemother. 38:2125-2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Way, J. C., M. A. Davis, D. Morisato, D. E. Roberts, and N. Kleckner. 1984. New Tn10 derivatives for transposon mutagenesis and for construction of lacZ operon fusions by transposition. Gene 32:369-379. [DOI] [PubMed] [Google Scholar]
- 16.Wolfaardt, G. M., J. R. Lawrence, R. D. Robarts, S. J. Caldwell, and D. E. Caldwell. 1994. Multicellular organization in a degradative biofilm community. Appl. Environ. Microbiol. 60:434-446. [DOI] [PMC free article] [PubMed] [Google Scholar]