Abstract
In a previous study, moxifloxacin was shown to ameliorate immunosuppression and enhance cytokine production in several tissues, including the lungs of cyclophosphamide-injected mice. We examined here the effects of moxifloxacin on Candida albicans lung infection in cyclophosphamide-injected mice. Mice were injected on day 0 with 250 mg of cyclophosphamide/kg, and on days 1 to 4 they were given moxifloxacin at 22.5 mg/kg/day compared to controls given ceftazidime at 75 mg/kg/day or saline. On day 6, C. albicans (10 7 CFU/mouse) was inoculated intratracheally, and animals were observed for the development of bronchopneumonia, weight loss, mortality, the presence of C. albicans, and lung cytokine production. Histopathology on day 10 postinoculation revealed bronchopneumonia in 50, 67, and 0% of saline-, ceftazidime-, and moxifloxacin-treated mice, respectively (P < 0.05). The mortality rates were 28, 17, and 5%, respectively (P < 0.05), and weight loss occurred at 20, 32, and 0%, respectively (P < 0.05). By day 15, C. albicans was eliminated from all moxifloxacin-treated mice but was still isolated from lung homogenates of 50 to 60% of the saline- and ceftazidime-treated groups. Among the cytokines tested on days 0 to 15, we found an increased production of tumor necrosis factor alpha, KC (functional interleukin-8), and gamma interferon in the lungs of ceftazidime- and saline-treated controls compared to the moxifloxacin pretreatment that abolished their secretion. In conclusion, moxifloxacin protected cyclophosphamide-injected mice from C. albicans-induced lung infection and significantly reduced pneumonia, weight loss, and mortality despite the lack of direct antifungal activity. This is most likely due to an immunomodulating activity conferred by moxifloxacin, as shown in this model and in our previous studies. Its potential protective role should be studied in patients undergoing chemotherapy and immune suppression.
Fluoroquinolones are synthetic, broad-spectrum antimicrobial agents in common use for a variety of infections, including systemic infections in immune compromised hosts (6, 8). Certain quinolones were shown to have, in addition to their antimicrobial properties, immunomodulating activity in animal models and humans (3, 13, 19). Quinolones such as ciprofloxacin and moxifloxacin induced enhanced production of certain cytokines in triggered human peripheral blood lymphocytes in vitro and in several models of immunosuppressed animals in vivo (13, 17, 21). The main cytokines induced by the above quinolones included interleukin-2 (IL-2), IL-3, granulocyte-macrophage colony-stimulating factor (GM-CSF) and gamma interferon (IFN-γ) (13, 20, 21). The effect on cytokine production is both quinolone and dose dependent (22). Structure-activity relationship analyses revealed that only quinolones with a cyclopropyl moiety at position 1 of the quinolone ring (i.e., ciprofloxacin, sparfloxacin, clinafloxacin, and moxifloxacin) were associated with the immunity-enhancing phenomena described above (20). In addition, these effects were dependent upon drug concentrations and were apparent only at clinically attainable levels. Higher concentrations of the same quinolones (i.e., ≥50 mg/liter or 100 mg/kg/day) led to inhibitory effects in vitro and in vivo, but these high concentrations are far beyond the therapeutic range (13, 20).
In sublethally irradiated mice, enhanced hematopoiesis and increased survival were demonstrated after a short course of treatment with ciprofloxacin compared to a nonquinolone antimicrobial agent or saline control. This effect was associated with increased production of IL-3 and GM-CSF in hematopoietic organs and was attained at clinically relevant concentrations (20). Based on these observations, we have recently studied the effect of moxifloxacin in the well-established model of cyclophosphamide (Cy)-induced neutropenia in mice. In our study, we found that 4 days of treatment with moxifloxacin ameliorated the immunosuppressive effects of Cy, as measured by various hematopoietic parameters, and led to enhanced cytokine production in several tissues, including the lungs, compared to animals treated with a nonquinolone antibiotic or to saline-treated controls (21). It is well known that Cy treatment is associated with distinct lung toxicity and an increased tendency to pulmonary infection with various organisms, including Candida albicans, and other fungal infections (14, 23).
Based on the reversal of some of the immunosuppressive effects in lungs of Cy-injected mice conferred by moxifloxacin, we decided to investigate the potential role of this quinolone in modulating the susceptibility of Cy-injected mice to an intra-tracheal challenge with C. albicans. Since moxifloxacin has no innate activity against candida, any such effect would most likely be attributed to immunomodulation rather than to a direct antifungal effect. In the present study, we compared the effect of short-term moxifloxacin treatment to that with ceftazidime and saline in Cy-injected mice challenged with intratracheal C. albicans. The study endpoints included the development of bronchopneumonia, morbidity parameters, mortality rate, and expanded analysis of various cytokines produced in the various treatment and control animal groups. (This study was presented in part at the 41st Interscience Conference on Antimicrobial Agents and Chemotherapy, Chicago, Ill., in December 2001.)
MATERIALS AND METHODS
Drugs.
The study drugs included moxifloxacin (Bayer AG, Wuppertal, Germany), ceftazidime (Glaxo Group, Greenford, United Kingdom), and Cy (Taro Pharmaceutical Industries, Haifa Bay, Israel).
Mice.
Inbred male BALB/c mice aged 6 to 8 weeks were used. All mice were kept in conventional animal facilities. The mice received humane care.
Induction of neutropenia and treatment regimen.
Mice were injected intraperitoneally with 250 mg of Cy/kg on day zero. At 24 h after Cy injection mice were injected intraperitoneally with moxifloxacin at 7.5 mg/kg/dose thrice daily or with ceftazidime at 25 mg/kg/dose thrice daily. Control mice were injected with pyrogen-free saline. The drugs and saline were administered for 4 consecutive days.
Yeast growth and intratracheal challenge.
C. albicans was cultured on Sabouraud dextrose agar (Difco Laboratories, Detroit, Mich.) for 18 h at 28°C and then harvested into pyrogen-free saline. The number of yeast cells in a suspension was determined by a hemocytometer count and adjusted to a suitable concentration in saline. On day 6 after Cy injection, mice were lightly anesthetized with ketamine HCl (1.5 mg/mouse). A 16-gauge blunted needle was passed through the oral pharynx into the trachea. Mice were inoculated with 107 candidas/50 μl/mouse.
Body weight monitoring.
The body weight of mice was monitored from day 0 and then every 3 days until day 23 after Cy injection.
Survival.
The percentage of survivors relative to the total number of C. albicans-infected mice was monitored daily until day 22 postinoculation in each group.
Sample collection.
On days 1, 2, 4, 7, 10, and 15 after C. albicans inoculation, whole-lung specimens (both lungs) were removed. The right lung was homogenized in 1 ml of normal saline. The homogenates were centrifuged at 2,000 rpm for 10 min, and the supernatants were kept at −70°C until they were assayed for cytokines as described below. The left lung was examined for gross pathology and fixed in 10% neutral buffered formalin, dehydrated in ethanol, and embedded in paraffin for sectioning. For enumeration of viable candida in lungs, spleens, and kidneys, the organs were removed at various times after challenge with C. albicans and extracted aseptically and homogenized in normal saline. The homogenates were inoculated on Sabouraud dextrose agar, culture plates were incubated for 48 h at 28°C, and the numbers of viable C. albicans were determined.
Histopathology.
Sections were prepared and stained with hematoxylin and eosin (H&E), periodic acid-Schiff (PAS), and silver according to standard protocols. Lungs were examined microscopically by a pathologist (I.S.) who was blinded to the experimental conditions of the animals. The extent, nature, and distribution of lung pathology were recorded. Sections were carefully examined at high power for the presence of C. albicans or other microorganisms.
Cytokine assay.
The levels of murine GM-CSF, IL-2, KC (active IL-8), IL-10, IFN-γ, and tumor necrosis factor alpha (TNF-α) were determined by enzyme-linked immunosorbent assay (R&D Systems, Inc., Minneapolis, Minn.). The sensitivities of the assays are >1 pg/ml for GM-CSF, >3 pg/ml for IL-2, >2 pg/ml for KC (active IL-8), >4 pg/ml for IL-10, >2 pg/ml for IFN-γ, and >5.1 pg/ml for TNF-α.
Statistical differences between means were tested by Student t test and Fisher exact test.
RESULTS
The results represent a compilation of data from two to six separate experiments, depending on the specific parameter.
Clinical results.
The fur of the mice developed a ruffled appearance 2 to 3 days postinoculation of C. albicans in saline- and ceftazidime-treated mice. This ruffled appearance persisted for ca. 13 days. There was no visible difference between the moxifloxacin-treated mice and normal mice.
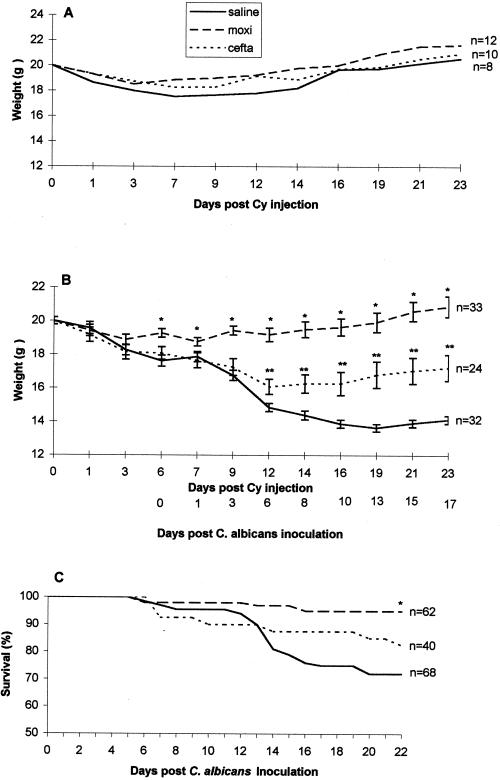
Effect of the drugs on the body weight and on the survival of Cy-injected mice inoculated with C. albicans
In order to evaluate the impact of C. albicans inoculation on the body weight of mice, we first monitored the body weight of Cy-injected mice treated with moxifloxacin, ceftazidime, or saline. The mean weight of mice prior to Cy injection was 21.2 ± 0.3 g.
Figure 1A shows a slight nonsignificant decrease in the body weight of saline-treated mice on days 3 to 14 after Cy injection.
FIG. 1.
Body weight (A and B) and survival (C) of mice injected with Cy. Animals were treated for 4 days with moxifloxacin, ceftazidime, or saline (A) and then inoculated intratracheally 2 days later with C. albicans (107 CFU/mouse) (B and C). ∗, P < 0.05 moxifloxacin versus ceftazidime and saline; ∗∗, P < 0.05 ceftazidime versus saline.
Figure 1B shows that, after inoculation with C. albicans, a significant decrease in the body weights of ceftazidime- and saline-treated mice occurred compared to the moxifloxacin-treated mice. On days 10 to 23 after Cy injection (6 to 17 days after C. albicans inoculation), there were 20 and 32% decreases in the body weights in ceftazidime- and saline-treated mice, respectively. In contrast, moxifloxacin-treated mice showed no change in body weight throughout the experiment.
The cumulative survival of Cy-injected, C. albicans-inoculated mice in the three treatment groups is shown in Fig. 1C. By day 22 after C. albicans inoculation, 72% (49 of 68) of the saline-treated mice and 83% (33 of 40) of the ceftazidime-treated mice survived compared to 95% (59 of 62) of the moxifloxacin-treated mice (P < 0.05).
Gross pathology and histopathology of lungs.
On days 1 and 2 after C. albicans inoculation, the lungs from all groups were grossly and microscopically normal.
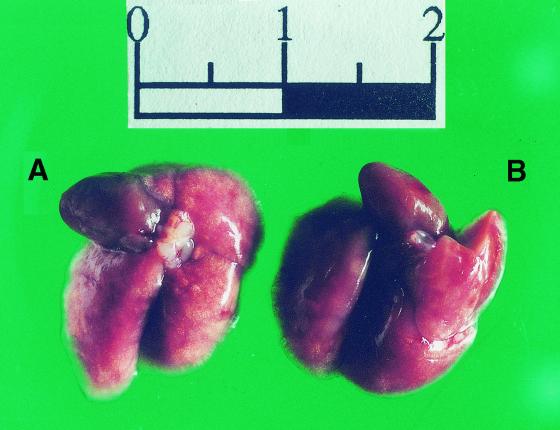
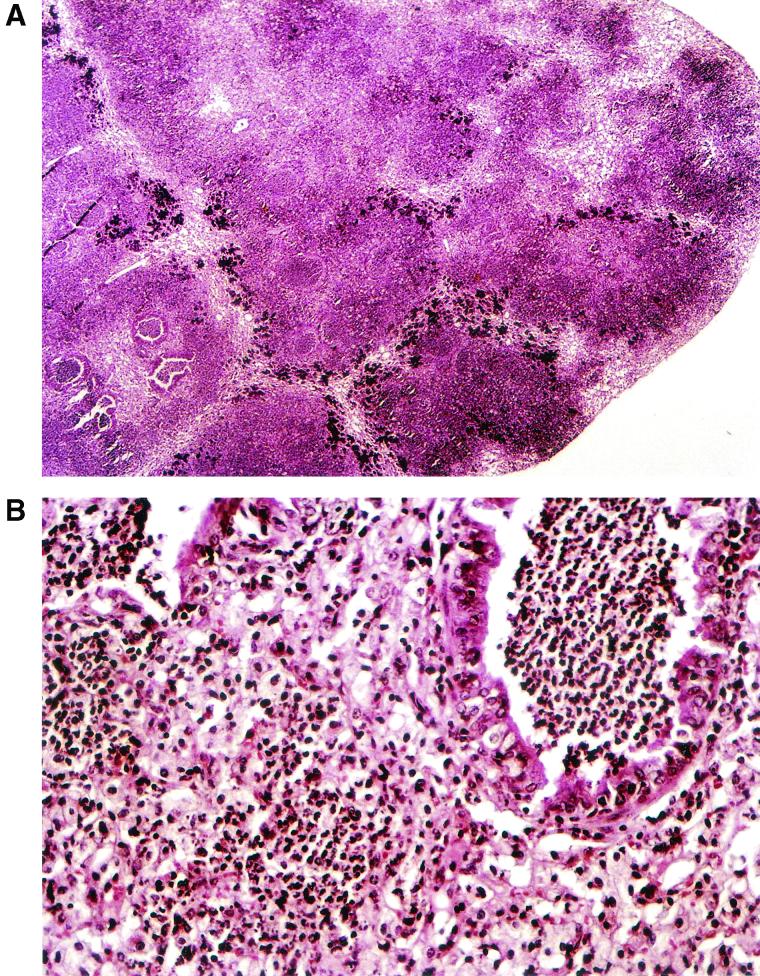
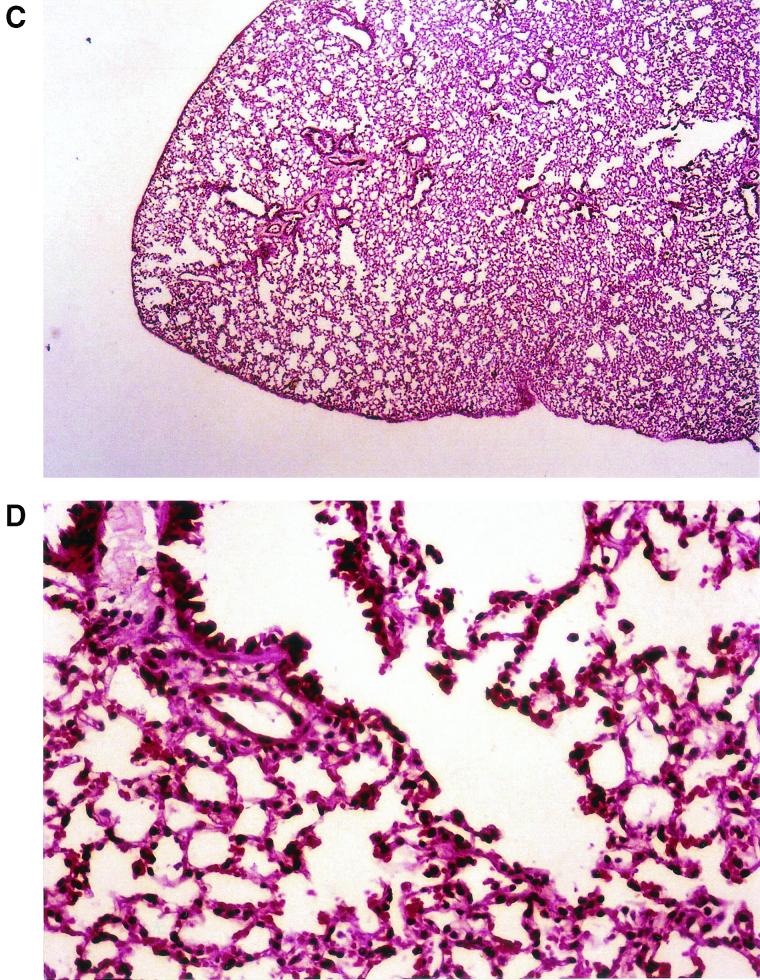
Upon gross pathological examination at 4, 7, and 10 days postinoculation, the lungs of mice treated with saline or ceftazidime had an edematous, coarse surface and macroscopic lesions resembling multiple abscesses. These patchy yellow-gray areas, 1 to 5 mm in diameter, involved all of the lobes. In marked contrast, no abnormal lesions were observed in the moxifloxacin-treated mice. Representative photographs of the gross appearance of the lungs of mice treated with saline or moxifloxacin at 10 days after C. albicans inoculation are shown in Fig. 2A and B, respectively. The lungs were then sliced longitudinally to obtain a maximal cut surface area. H&E-stained sections of whole lungs were examined microscopically. At a low magnification, sections from mice treated with saline revealed edematous interlobular septae and infiltrates occupying between 45 and 60% of the lung (Fig. 3A ). At a higher magnification (Fig. 3B) the infiltrate consisted of neutrophils entirely filling alveolar and bronchial spaces, with intervening areas of uninvolved lung tissue. These pathological features are consistent with acute bronchopneumonia. In marked contrast, no gross or histological abnormalities were observed in the lungs of most of the moxifloxacin-treated mice (Fig. 3C). There were occasional isolated foci of macrophage accumulation within the alveolar spaces, but no neutrophils were observed (Fig. 3D).
FIG. 2.
Lung pathology observed in Cy-injected mice 10 days after intratracheal challenge with C. albicans. The images are representative of the gross appearance of the lungs of mice treated with saline (A) or moxifloxacin (B).
FIG. 3.
(A and B) Lung histopathology of Cy-injected mice treated with saline at 10 days after intratracheal inoculated with C. albicans. (C and D) Lung histopathology of Cy-injected mice treated with moxifloxacin 10 days after intratracheal inoculation with C. albicans. Magnifications: A, ×100; B, ×400; C, ×100; D, ×400. All sections were stained with H&E.
On day 10 postinoculation, 50 and 67% of the lungs of saline- and ceftazidime-treated mice, respectively, had acute bronchopneumonia, whereas none of the moxifloxacin-treated mice had bronchopneumonia (P < 0.05). Viable candidas could be grown from the lungs of animals in the three investigated groups on days 1 to 10 without a significant difference between the groups; however, by day 15, C. albicans was eliminated from all moxifloxacin-treated mice, whereas in 50 to 60% of the saline- and ceftazidime-treated animals C. albicans could still be isolated from the lungs. There was no evidence of C. albicans upon either silver or PAS staining, despite careful examination at high-power magnification. The spleens and kidneys were also examined by PAS and silver staining. There was no evidence of inflammation or candidas in these organs in any of the mice.
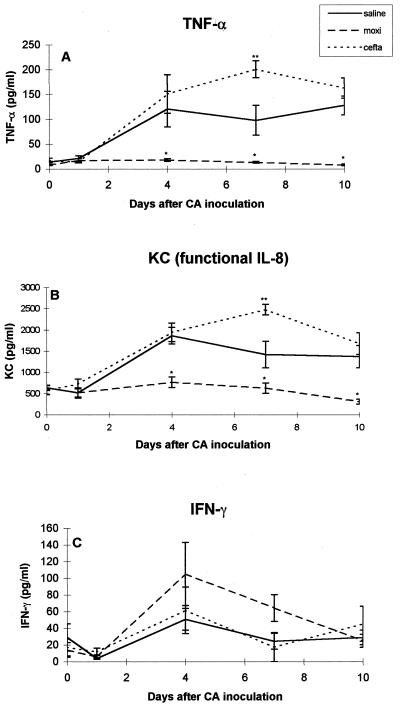
Cytokines levels in lung homogenates. (i) TNF-α.
Lung homogenates of normal, untreated mice had no detectable levels of TNF-α. Figure 4A shows that on day 6 after Cy injection (prior to C. albicans inoculation) there was a slight increase in TNF-α levels in saline- and ceftazidime-treated mice (13.6 ± 1.6 and 17 ± 4.7 pg/lung, respectively) compared to moxifloxacin-treated mice (8.1 ± 1.2 pg/lung). A marked increase in TNF-α levels was observed in saline- and ceftazidime-treated mice on days 4, 7, and 10 after C. albicans inoculation (up to 128 ± 19 and 201 ± 16.9 pg/lung, respectively). In contrast, TNF-α levels remained low in moxifloxacin-treated mice for the entire 10 days.
FIG. 4.
Cytokine levels in lung homogenates on days 0, 1, 4, 7, and 10 after C. albicans inoculation. (A) TNF-α; (B) KC (active IL-8); (C) IFN-γ. ✽, P < 0.05 moxifloxacin versus ceftazidime and saline; ✽✽, P < 0.05 ceftazidime versus saline.
(ii) KC (functional IL-8).
The level of KC (functional IL-8) in lung homogenates of normal, untreated mice was 77 ± 14 pg/lung. Figure 4B shows that on days 0 and 1 after C. albicans inoculation there was a 10-fold increase in KC levels in lung homogenates of the three investigated groups (compared to normal, untreated mice). A marked and significant increase in KC levels was observed in ceftazidime- and saline-treated mice on days 4, 7, and 10 after C. albicans inoculation (up to 1,942 ± 220 and 1,864 ± 196 pg/lung, respectively). In contrast, in moxifloxacin-treated mice, C. albicans inoculation did not affect KC levels up to day 7 postinfection, and a decline was observed on day 10 after C. albicans inoculation.
(iii) IFN-γ.
No significant difference in IFN-γ levels was observed among the three investigated groups on days 0 and 1 after C. albicans inoculation (Fig. 4C). An increase in IFN-γ levels was observed in moxifloxacin-treated versus saline-treated mice on days 4 and 7 postinoculation, but these results did not reach statistical significance.
(iv) IL-2, IL-10, and GM-CSF.
On day 0, IL-2 levels in moxifloxacin-, ceftazidime-, and saline-treated mice were 7.5 ± 2, 7.3 ± 2, and 12.6 ± 2.5 pg/lung, respectively. Between days 1 to 10 after C. albicans inoculation IL-2 levels in moxifloxacin-treated mice fluctuated between 5.2 ± 0.6 and 7.9 ± 1.8 pg/lung. No significant differences in the pattern of IL-2 levels were observed between the moxifloxacin-, ceftazidime-, or saline-treated groups.
On day 0, the IL-10 levels in moxifloxacin-, ceftazidime-, and saline-treated mice were 13.7 ± 2.8, 16.1 ± 5.7, and 11.7 ± 1.7, respectively. Between days 1 and 10 after C. albicans inoculation, the IL-10 levels in moxifloxacin-treated mice fluctuated between 15 ± 1.5 and 32.9 ± 6 pg/lung. No significant differences in the pattern of IL-10 levels were observed between the moxifloxacin-, ceftazidime-, or saline-treated groups.
On day 0, the GM-CSF levels in moxifloxacin-, ceftazidime-, and saline-treated mice were 5.2 ± 0.5, 2.4 ± 1, and 5.5 ± 1.8, respectively. Between days 1 and 10 after C. albicans inoculation, the GM-CSF levels in moxifloxacin-treated mice fluctuated between 5.7 ± 1.5 and 7.7 ± 3 pg/lung. No significant differences in the pattern of GM-CSF levels were observed between the moxifloxacin-, ceftazidime-, or saline-treated groups.
DISCUSSION
The present study demonstrates that pretreatment with moxifloxacin in Cy-injected mice prevents C. albicans-induced bronchopneumonia despite the absence of direct antifungal activity by quinolones. This protection includes decreased mortality, preservation of body weight, and a marked reduction in the histopathological findings of bronchopneumonia. Moxifloxacin, ceftazidime, or saline was given to the animals for 4 days after Cy injection, while C. albicans was administered intratracheally 2 days after stopping this treatment (6 days after Cy injection). Therefore, moxifloxacin is unlikely to have had a direct antimicrobial effect.
In the two control groups, TNF-α levels were increased even before candida challenge (day 6 after Cy injection) and showed a further >10-fold increase on days 4, 7, and 10 after the candida challenge. Similarly, KC (functional IL-8) increased three- to fivefold after the candida challenge, a finding correlating remarkably well with weight loss, massive bronchopneumonia, and widespread neutrophilic lung infiltrates. In contrast, animals pretreated with moxifloxacin had lower levels of TNF-α on day 6, even prior to candida challenge, with no increase in either TNF-α or IL-8 during the entire 10 days after infection. These animals did not lose weight or develop bronchopneumonia. It thus appears likely that inhibition of TNF-α and KC secretion is the mechanism whereby moxifloxacin protected the immunosuppressed mice.
Several investigators have looked into the role of TNF-α and KC (functional IL-8) in candida infections in vitro. Yamamoto et al. (28) and Kim et al. (11) found that C. albicans induced gene expression of KC (functional IL-8) in murine peritoneal macrophages. Torosantucci et al. (25, 26) demonstrated increased IL-8 production by human monocytes exposed to yeast and hyphal forms of C. albicans and increased production of TNF-α in PMN obtained from patients infected with human immunodeficiency virus that were stimulated in vitro by the mannoprotein fraction of C. albicans. Castro et al. (4) showed an increased production of IL-1β, IL-6, IL-8, and TNF by human monocytes stimulated by C. albicans. Human oral mucosal fibroblasts exposed in vitro to C. albicans showed enhanced secretion of IL-8 and IL-6 (5). In an additional model, Orozco et al. (15) demonstrated that C. albicans stimulated human endothelial cells to synthesize TNF-α and IL-8 among other cytokines, chemokines, and adhesion molecules. Rosseau et al. (18) showed C. albicans enhanced TNF-α and MCP-1 production by human alveolar macrophages.
Several in vivo observations are of note. Allendoerfer et al. (1) found that a high-dose intravenous injection of C. albicans was associated with increased levels of TNF-α and an increased mortality in mice. In another model of lipopolysaccharide (LPS)-induced pulmonary inflammation, Huang et al. (9) demonstrated a marked, rapid increase in functional IL-8 mRNA in bronchoalveolar lavage macrophages, highlighting the role of this chemokine in pulmonary inflammation. Waage et al. (27) anecdotally demonstrated increased IL-8 levels in serum of a febrile neutropenic patient during C. albicans infection and a gradual decline of IL-8 during recovery. The most relevant study, however, was reported by Futenma et al. (7). Pulmonary candidiasis was established in Cy-injected, neutropenic mice, resulting in increased levels of TNF-α in serum and bronchoalveolar lavage fluid, as well as significant mortality. In contrast, TNF-α levels in candida-infected normal mice were undetectable in sera and very low in bronchoalveolar lavage fluid. Treatment of the neutropenic, infected mice with G-CSF prolonged survival time, enhanced candida clearance, and strongly inhibited levels of TNF-α in serum and bronchoalveolar lavage fluid. The present study supports our observation regarding the role of TNF-α in the development of pulmonary candida infection in Cy-injected mice and the protection conferred by inhibiting TNF production, as demonstrated in our model.
Bailly et al. (2) first demonstrated a dose-dependent decrease in TNF-α production by LPS-stimulated human monocytes exposed in vitro to quinolones. The kinetics suggested that quinolones affected a very early step of intracellular TNF-α synthesis. Kakinuma et al. (10) examined the novel quinolone compounds quinolactines A, B, and C in LPS-stimulated murine peritoneal macrophages, finding that compound A inhibited TNF-α production. Kim et al. (12) showed that the quinolone rebamipide inhibited IL-8 production in Helicobacter pylori-stimulated gastric epithelial cells, thus possibly explaining the protection by that drug against H. pylori-associated gastric inflammation.
Of most relevance, Purswani et al. (16) examined the effect of trovafloxacin, ciprofloxacin, and ceftriaxone on human mononuclear cells exposed to LPS, lipoteichoic acid, and encapsulated bacteria (Streptococcus pneumoniae, Haemophilus influenzae). Trovafloxacin, which shares the same cyclopropyl moiety at position 1 of the quinolone ring as moxifloxacin, inhibited the production of TNF-α, IL-1, IL-6, and IL-8 by stimulated monocytes. In another in vivo model of LPS-injected mice, the levels of TNF-α and IL-6 in serum were 95% less than in mice not treated with trovafloxacin, whereas mRNA levels were similar to those seen in unstimulated cells. Quinolones do not affect intact, unstimulated cells or normal animals, but they do modulate either activated cells (using LPS, phytohemagglutinin, pokeweed mitogen, candida, etc.) or challenged animals (after sublethal irradiation, Cy injection, LPS injection, candida sepsis, etc.). We speculate that the basic mechanisms of moxifloxacin immune modulation may involve the known inhibitory effect of quinolones on topoisomerases in eukaryotic cells (24), leading to an accumulation of DNA fragments. A common pathway might involve modulation of the intracellular transcription factors required for cytokine expression. Further in vitro studies, as well as clinical trials, of moxifloxacin use to prevent candida infection after chemotherapy and immune suppression are strongly recommended.
Acknowledgments
This study was supported in part by a research grant from Bayer AG (Germany) and by the Gershon Meerbaum Fund.
REFERENCES
- 1.Allendoerfer, R., D. M. Magee, J. G. Smith, L. Bonewald, and J. R. Graybill. 1993. Induction of tumor necrosis factor-alpha in murine Candida albicans infection. J. Infect. Dis. 167:1168-1172. [DOI] [PubMed] [Google Scholar]
- 2.Bailly, S., M. Fay, Y. Roche, and M. A. Gougerot-Pocidalo. 1990. Effects of quinolones on tumor necrosis factor production by human monocytes. Int. J. Immunopharmacol. 12:31-36. [DOI] [PubMed] [Google Scholar]
- 3.Blank, M., J. George, P. Fishman, Y. Levy, V. Toder, S. Savion, V. Barak, T. Koike, and Y. Shoenfeld. 1998. Ciprofloxacin immunomodulation of experimental antiphospholipid syndrome associated with elevation of interleukin-3 and granulocyte-macrophage colony-stimulating factor expression. Arthritis Rheum. 41:224-232. [DOI] [PubMed] [Google Scholar]
- 4.Castro, M., J. A. Bjoraker, M. S. Rohrbach, and A. H. Limper. 1996. Candida albicans induces the release of inflammatory mediators from human peripheral blood monocytes. Inflammation 20:107-122. [DOI] [PubMed] [Google Scholar]
- 5.Dongari-Bagtzoglou, A., K. Wen, and I. B. Lamster. 1999. Candida albicans trigger interleukin-6 and interleukin-8 responses by oral fibroblasts in vitro. Oral Microbiol. Immunol. 14:364-370. [DOI] [PubMed] [Google Scholar]
- 6.Freifeld, A., D. Marchigiani, T. Walsh, S. Chanock, L. Lewis, J. Hiemenz, S. Hiemenz, J. E. Hicks, V. Gil, S. M. Steinberg, and P. A. Pizzo. 1999. A double-blind comparison of empirical oral and intravenous antibiotic therapy for low-risk febrile patients with neutropenia during cancer chemotherapy. N. Eng. J. Med. 341:305-311. [DOI] [PubMed] [Google Scholar]
- 7.Futenma, M., K. Kawakami, and A. Saito. 1995. Production of tumor necrosis factor-alpha in granulocytopenic mice with pulmonary candidiasis and its modification with granulocyte colony-stimulating factor. Microbiol. Immunol. 39:411-417. [DOI] [PubMed] [Google Scholar]
- 8.Hooper, D. C., and J. S. Wolfson. 1991. Fluoroquinolone antimicrobial agents. N. Engl. J. Med. 324:384-394. [DOI] [PubMed] [Google Scholar]
- 9.Huang, S., J. D. Paulauskis, J. J. Godleski, and L. Kobzik. 1992. Expression of macrophage inflammatory protein-2 and KC mRNA in pulmonary inflammation. Am. J. Pathol. 141:981-988. [PMC free article] [PubMed] [Google Scholar]
- 10.Kakinuma, N., H. Iwai, S. Takahashi, K. Hamano, T. Yanagisawa, K. Nagai, K. Tanaka, K. Suzuki, F. Kirikae, T. Kirikae, and A. Nakagawa. 2000. Quinolactacins A, B, and C: novel quinolone compounds from Penicillium sp. EPF-6. I. Taxonomy, production, isolation and biological properties. J. Antibiot. 53:1247-1251. [DOI] [PubMed] [Google Scholar]
- 11.Kim, H. S., D. H. Shin, and S. K. Kim. 1999. Effects of interleukin-10 on chemokine KC gene expression by mouse peritoneal macrophages in response to Candida albicans. J. Korean Med. Sci. 14:480-486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim, H., J. Y. Seo, and K. H. Kim. 2000. Inhibition of lipid peroxidation, NF-κB activation and IL-8 production by rebamipide in Helicobacter pylori-stimulated gastric epithelial cells. Dig. Dis. Sci. 45:631-638. [DOI] [PubMed] [Google Scholar]
- 13.Kletter, Y., S. Riklis, I. Shalit, and I. Fabian. 1991. Enhanced repopulation of murine hematopoietic organs in sublethally irradiated mice after treatment with ciprofloxacin. Blood 78:1685-1691. [PubMed] [Google Scholar]
- 14.Lechner, A. J., K. E. E. Lamprech, L. H. Potthoff, T. L. Tredway, and G. M. Matuschak. 1994. Recombinant GM-CSF reduces lung injury and mortality during neutropenic Candida sepsis. Am. J. Physiol. 266:L561-L568. [DOI] [PubMed] [Google Scholar]
- 15.Orozco, A. S., X. Zhou, and S. G. Filler. 2000. Mechanisms of the proinflammatory response of endothelial cells to Candida albicans infection. Infect. Immun. 68:1134-1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Purswani, M., S. Eckert, H. Arora, R. Johann-Liang, and G. J. Noel. 2000. The effect of three broad-spectrum antimicrobials on mononuclear cell responses to encapsulated bacteria: evidence for downregulation of cytokine mRNA transcription by trovafloxacin. J. Antimicrob. Chemother. 46:921-929. [DOI] [PubMed] [Google Scholar]
- 17.Riesbeck, K., and A. Forsgren. 1994. Increased interleukin 2 transcription in murine lymphocytes by ciprofloxacin. Immunopharmacology 27:155-164. [DOI] [PubMed] [Google Scholar]
- 18.Rosseau, S., P. Hammerl, U. Maus, A. Gunther, W. Seeger, F. Grimminger, and J. Lohmeyer. 1999. Surfactant protein A downregulates proinflammatory cytokine production evoked by Candida albicans in human alveolar macrophages and monocytes. J. Immunol. 163:4495-4502. [PubMed] [Google Scholar]
- 19.Rubinstein, E., and I. Shalit. 1993. Effect of the quinolones on the immune system, p. 519-526. In D. C. Hooper and J. D. Wolfson (ed.), Quinolone antimicrobial agents. ASM Press, Washington, D.C.
- 20.Shalit, I., Y. Kletter, K. Weiss, T. Gruss, and I. Fabian. 1997. Enhanced hematopoiesis in sublethally irradiated mice treated with various quinolones. Eur. J. Haematol. 58:92-98. [DOI] [PubMed] [Google Scholar]
- 21.Shalit, I., Y. Kletter, D. Halperin, D. Waldman, E. Vasserman, A. Nagler, and I. Fabian. 2000. Immunology effects of moxifloxacin in comparison to ciprofloxacin and G-CSF in a murine model of cyclophosphamide-induced leukopenia. Eur. J. Haematol. 66:287-296. [DOI] [PubMed] [Google Scholar]
- 22.Somekh, E., B. Lev, E. Schwartz, A. Barzilai, and E. Rubenstein. 1989. Effect of ciprofloxacin and pefloxacin on bone marrow engraftment in the spleen of mice. J. Antimicrob. Chemother. 23:247-251. [DOI] [PubMed] [Google Scholar]
- 23.Spector, J. I., H. Zimbler, and J. S. Ross. 1979. Early onset cyclophosphamide-induced interstitial pneumonitis. JAMA 242:2852-2854. [PubMed] [Google Scholar]
- 24.Takei, M., H. Fuda, T. Yasue, M. Hosaka, and Y. Oomori. 1998. Inhibitory activities of gatifloxacin (AM-1155), a newly developed fluoroquinolone, against bacterial and mammalian type II topoisomerases. Antimicrob. Agents Chemother. 42:2678-2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Torosantucci, A., P. Chiani, I. Quinti, C. M. Ausiello, I. Mezzaroma, and A. Cassone. 1997. Responsiveness of human polymorphonuclear cells (PMNL) to stimulation by a mannoprotein fraction (MP-F2) of Candida albicans: enhanced production of IL-6 and tumour necrosis factor-alpha (TNF-α) by MP-F2-stimulated PMNL from HIV-infected subjects. Clin. Exp. Immunol. 107:451-457. [DOI] [PubMed] [Google Scholar]
- 26.Torosantucci, A., P. Chiani, and A. Cassone. 2000. Differential chemokine response of human monocytes to yeast and hyphal forms of Candida albicans and its relation to the β-1,6 glucan of the fungal cell wall. J. Leukoc. Biol. 68:923-932. [PubMed] [Google Scholar]
- 27.Waage. A., D. Demick, S. Steinshamn, L. Deforge, and J. Lamvik. 1994. Interleukin 8 in serum in granulocytopenic patients with infections. Br. J. Haematol. 86:36-40. [DOI] [PubMed] [Google Scholar]
- 28.Yamamoto, Y., T. W. Klein, and H. Friedman. 1997. Involvement of mannose receptor in cytokine interleukin-1β (IL-1β), IL-6, and granulocyte-macrophage colony-stimulating factor responses, but not in chemokine macrophage inflammatory protein 1β (MIP-1β), MIP-2, and KC responses, caused by attachment of Candida albicans to macrophages. Infect. Immun. 65:1077-1082. [DOI] [PMC free article] [PubMed] [Google Scholar]