Abstract
We determined whether an antifungal 14-kDa protein trypsin inhibitor isolated from corn is able to cross the blood-brain barrier. We found that it completely crossed the blood-brain barrier by means of a saturable mechanism at a rate of 0.153 μl/g · min, with about 0.082% of the intravenously injected dose being taken up per gram of brain.
Central nervous system (CNS) fungal infections are increasing in number, with few useful agents for their treatment (9, 24, 28). Aspergillus, Fusarium, and Candida commonly cause disease, and newer genera are emerging (16). Antifungal agents which penetrate the blood-brain barrier (BBB) are needed, and plants may be a source of such agents. Plants lack an immune system and so rely on other mechanisms to protect themselves (21), including production of antimicrobial compounds (15, 20, 22, 25). Corn genotypes resistant to A. flavus express high levels of a 14-kDa trypsin inhibitor (TI) (10-13). TI inhibits the growth of Aspergillus and Fusarium spp., affecting conidium germination, hyphal extension (11), and fungal α-amylases (19). Here, we determined whether TI can cross the BBB.
TI isolated from the corn population GT-MAS:gk was radioactively labeled with 131I (I-TI) by using chloramine-T and purified on a column of Sephadex G-10 (Sigma Chemical, St. Louis, Mo.) as previously described (3). Specific activity was about 186 Ci/g.
All animal research adhered to the Principles of Laboratory Animal Care (22) and was conducted under approved protocols in a facility approved by the Association for Accreditation of Laboratory Animal Care. Multiple-time regression analysis was used to measure BBB transport (8, 23). ICR mice from our in-house colony were anesthetized with urethane, and the right carotid artery and left jugular vein were exposed. Lactated ringers (0.2 ml) (LR) containing 1% bovine serum albumin and I-TI (7 × 106 cpm/mouse) were injected into the jugular vein. Arterial blood and brain specimens (minus pituitary and pineal glands) were collected 2 to 30 min later. Radioactivity in the brain and arterial serum was measured with a gamma counter. The unidirectional influx rate (Ki, in microliters per gram per minute) and the apparent initial volume of distribution (at time zero) (Vi, in microliters per gram) were determined from the linear portion of the equation
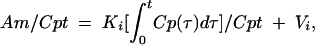 |
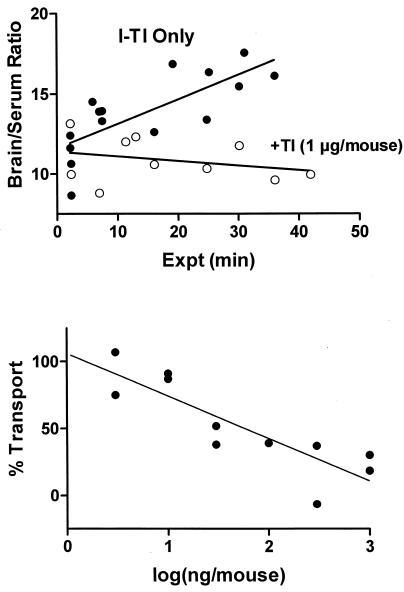
where Am is counts per minute per gram of brain, Cpt is counts per minute per milliliter of serum at time t, and Expt is exposure time in minutes, calculated by the formula 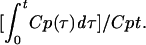 Figure 1 (upper panel) shows that the brain/serum ratio increased with time (r = 0.758, n = 15, P < 0.005). The Ki was 0.153 ± 0.037 μl/g · min, and the Vi was 11.6 ± 0.7 μl/g. This rate is somewhat lower than rates measured for leptin, interleukin 1, and tumor necrosis factor, all of which are transported across the BBB by unique transporters (3, 4, 6, 17).
Figure 1 (upper panel) shows that the brain/serum ratio increased with time (r = 0.758, n = 15, P < 0.005). The Ki was 0.153 ± 0.037 μl/g · min, and the Vi was 11.6 ± 0.7 μl/g. This rate is somewhat lower than rates measured for leptin, interleukin 1, and tumor necrosis factor, all of which are transported across the BBB by unique transporters (3, 4, 6, 17).
FIG. 1.
(Upper panel) Uptake of I-TI by brain. The slope of the linear relation between brain/serum ratios and Expt measures the unidirectional influx constant (Ki). For I-TI, Ki was 0.153 ± 0.037 μl/g ·min. Inclusion of unlabeled TI completely inhibited uptake, with there being no statistically significant correlation between brain/serum ratios and Expt. (Lower panel) Rate of transport (with 100% defined as the rate in mice receiving only I-TI) versus the log of the amount of unlabeled TI included in the injection. Each point represents a mean for three to four mice. The amount calculated to produce a 50% inhibition was 58 ng/mouse.
I-TI uptake by brain was saturable. Unlabeled TI (1 μg/mouse) totally inhibited the transport of I-TI (Fig. 1, upper panel). The mean brain/serum ratio determined from values combined across time was 10.9 ± 0.4, which is about the size of the vascular space of the brain. I-TI plus unlabeled TI (3 to 1,000 ng/mouse; three to four mice per determination) was injected intravenously (i.v.) in other mice and brain/serum ratios were determined 30 min later. The lower panel of Fig. 1 shows a dose-dependent inhibition, with 58 ng/mouse producing a 50% inhibition (r = 0.858, n = 11, P < 0.001; slope, −31.67 ± 6.310; y intercept, 10.95 ± 9.87).
The percentage of an i.v. dose of I-TI taken up per gram of brain (%Inj/g) was calculated from the equation
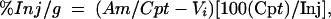 |
where Inj is the counts per minute injected i.v. Vi corrects for I-TI in the vascular space. After i.v. injection, I-TI was cleared from blood in a linear fashion, with a half-life of 26 min and Vi of 5.4 ml. The peak %Inj/g value of 0.082, reached 5 min after i.v. injection, is similar to those for leptin, interleukin-1, and tumor necrosis factor (4, 6, 17) but lower than that for morphine (0.02) (2). Enkephalin analogs (14), pituitary adenylate cyclase-activating peptide (5, 27), and a neurotensin analog (1, 7, 18) have effects on the CNS at these levels. More encouraging, the %Inj/g for I-TI was sustained at about 0.05 for the duration of the study. Sustained levels in the CNS are critical for antifungal activity.
To determine whether I-TI crossed the BBB intact, radioactivity appearing in brain and serum after i.v. injection of I-TI (3 × 106 cpm/mouse) was analyzed. Brains homogenized with a glass tissue grinder in 2 ml of buffer (0.03 M bicarbonate, 10 mM EDTA, 10 mM l-thyroxine) were centrifuged at 14,000 rpm for 20 min. To determine the degradation of I-TI that occurred during processing, 100 μl of I-TI in LR-bovine serum albumin was placed on the surface of a nonradioactive brain or in a tube used to collect carotid blood, and the samples were processed as described above. The radioactivity recovered was characterized by acid precipitation or by high-pressure liquid chromatography (HPLC). For acid precipitation, brain homogenate or serum was mixed with 30% trichloroacetic acid and centrifuged at 5,000 × g for 10 min. The percentage of radioactivity precipitated by the acid was calculated as the percentage of total counts per minute in the pellet. The results were expressed as a percentage of values for processing controls. For brain HPLC samples, the supernatant was again centrifuged at 14,000 rpm for 20 min and then analyzed by reversed-phase HPLC on a C4 column (Vydac, Hesperia, Calif.). The mobile phase increased linearly from 30 to 90% acetonitrile in water over a 30-min interval, with 0.1% trifluoroacetic acid being used for ion pairing. Fractions of the eluent were collected at 1 ml/min and counted on the gamma counter.
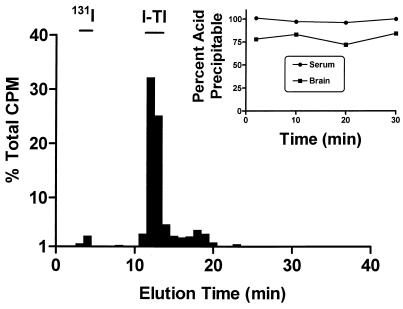
I-TI levels were very stable in blood and brain after i.v. injection. Acid precipitation of radioactivity recovered from serum averaged 98% ± 1% during the 30 min of the study (Fig. 2, inset). About 70% of the radioactivity in brain was extracted into the supernatant. The majority of this radioactivity was identified as intact I-TI by both acid precipitation (Fig. 2, inset) and HPLC (Fig. 2).
FIG. 2.
Identification of radioactivity in brain after i.v. injection of I-TI. The bar graph shows that the majority of radioactivity recovered from brain 30 min after the i.v. injection of I-TI elutes in the position of intact I-TI. (Inset) Acid precipitation of radioactivity recovered from serum and brain at various times after i.v. injection of I-TI.
The modified (17) capillary depletion method (26) was used to determine whether the I-TI completely crossed the BBB to enter the brain's parenchymal space. Anesthetized mice (n = 5) received 105 cpm/mouse of I-TI i.v. Thirty minutes later, blood was collected from the abdominal aorta. The vascular contents of the brain were washed free of blood by perfusing 20 ml of LR through the heart. The cerebral cortex was removed, weighed, homogenized in a dextran gradient, and centrifuged at 5,400 × g for 15 min at 4°C in a swing bucket rotor. The pellet (brain vasculature) and supernatant (parenchyma) were carefully separated, and their radioactivity was measured. The parenchyma/serum and capillary/serum ratios (microliters per gram) were calculated with the following equation: ratio = (cpm Fr)/(w)(cpm/μl), where cpm Fr is the counts per minute in the parenchyma or supernatant fraction, w is the cortical weight, and cpm/μl is the counts per minute per milliliter of serum. Capillary depletion showed that I-TI taken up by brain completely crossed the BBB. Since the brain was washed free of vascular contents, only extravascular radioactivity remained. Of the 4.93 ± 074 μl of I-TI per gram in the cortex 30 min after i.v. injection, the parenchyma contained 4.65 ± 0.85 μl/g (94%) and the capillaries contained 0.28 ± 0.02 μl/g (n = 5).
In conclusion, we showed that I-TI is able to cross the BBB by using a saturable transporter. The radioactivity taken up by the brain represents intact I-TI; it therefore completely crosses the BBB to reach the brain parenchyma. The rate of transport and the %Inj/g of brain are similar to those of similar-sized proteins transported across the BBB by saturable systems, with levels being sustained for at least 30 min. TI may be a useful antifungal in the treatment of CNS disease.
Acknowledgments
This work was supported by the USDA-ARS-SRRC, the VA Merit Review, and NIH grant R01 NS41863.
REFERENCES
- 1.Akunne, H. C., S. B. Demattos, S. Z. Whetzel, D. J. Wustrow, M. D. Davis, L. D. Wise, W. L. Cody, T. A. Pugsley, and T. G. Heffner. 1995. Agonist properties of a stable hexapeptide analog of neurotensin, N′γMeArg-Lys-Pro-Trp-tLeu-Leu [NT1]. Biochem. Pharmacol. 49:1147-1154. [DOI] [PubMed] [Google Scholar]
- 2.Banks, W. A., and A. J. Kastin. 1994. Opposite direction of transport across the blood-brain barrier for Tyr-MIF-1 and MIF-1: comparison with morphine. Peptides 15:23-29. [DOI] [PubMed] [Google Scholar]
- 3.Banks, W. A., A. J. Kastin, and D. A. Durham. 1989. Bidirectional transport of interleukin-1 alpha across the blood-brain barrier. Brain Res Bull. 23:433-437. [DOI] [PubMed] [Google Scholar]
- 4.Banks, W. A., A. J. Kastin, W. Huang, J. B. Jaspan, and L. M. Maness. 1996. Leptin enters the brain by a saturable system independent of insulin. Peptides 17:305-311. [DOI] [PubMed] [Google Scholar]
- 5.Banks, W. A., A. J. Kastin, G. Komaki, and A. Arimura. 1993. Passage of pituitary adenylate cyclase activating polypeptide1-27 and pituitary adenylate cyclase activating polypeptide1-38 across the blood-brain barrier. J. Pharmacol. Exp. Ther. 267:690-696. [PubMed] [Google Scholar]
- 6.Banks, W. A., L. Ortiz, S. R. Plotkin, and A. J. Kastin. 1991. Human interleukin (IL) 1α, murine IL-1α and murine IL-1β are transported from blood to brain in the mouse by a shared saturable mechanism. J. Pharmacol. Exp. Ther. 259:988-996. [PubMed] [Google Scholar]
- 7.Banks, W. A., D. J. Wustrow, W. L. Cody, M. D. Davis, and A. J. Kastin. 1995. Permeability of the blood-brain barrier to the neurotensin8-13 analog NT1. Brain Res. 695:59-63. [DOI] [PubMed] [Google Scholar]
- 8.Blasberg, R. G., J. D. Fenstermacher, and C. S. Patlak. 1983. Transport of α-aminoisobutyric acid across brain capillary and cellular membranes. J. Cereb. Blood Flow Metab. 3:8-32. [DOI] [PubMed] [Google Scholar]
- 9.Boon, A. P., D. H. Adams, U. Buckels, and P. McMaster. 1983. Cerebral aspergillosis in liver transplantation. J. Clin. Pathol. 43:114-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brown, R. L., Z.-Y. Chen, T. E. Cleveland, and J. S. Russin. 1999. Advances in the development of host resistance in corn to aflatoxin contamination by Aspergillus flavus. Phytopathology 89:113-117. [DOI] [PubMed] [Google Scholar]
- 11.Chen, Z.-Y., R. L. Brown, A. R. Lax, T. E. Cleveland, and J. S. Russin. 1999. Inhibition of plant-pathogenic fungi by a corn trypsin inhibitor overexpressed in Escherichia coli. Appl. Environ. Microbiol. 65:1320-1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen, Z.-Y., R. L. Brown, A. R. Lax, B. Z. Gou, T. E. Cleveland, and J. S. Russin. 1998. Resistance to Aspergillus flavus in corn kernels is associated with a 14-kDa protein. Phytopathology 88:276-281. [DOI] [PubMed] [Google Scholar]
- 13.Chen, Z.-Y., R. L. Brown, J. S. Russin, A. R. Lax, and T. E. Cleveland. 1999. A corn trypsin inhibitor with antifungal activity inhibits Aspergillus flavus α-amylase. Phytopathology 89:902-907. [DOI] [PubMed] [Google Scholar]
- 14.Delay-Goyet, P., M. Ruiz-Gayo, A. Baamonde, G. Gacel, J.-L. Morgat, and B. P. Roques. 1991. Brain passage of BUBU, a highly selective and potent agonist for δ opioid receptors: in vivo binding and μ versus δ receptors occupancy. Pharmacol. Biochem. Behav. 38:155-162. [DOI] [PubMed] [Google Scholar]
- 15.Guo, B. Z., J. S. Russin, T. E. Cleveland, R. L. Brown, and N. W. Widstrom. 1995. Wax and cutin layers in maize kernels associated with resistance to aflatoxin production by Aspergillus flavus. J. Food Prot. 58:296-300. [DOI] [PubMed] [Google Scholar]
- 16.Guppy, K. H., C. Thomas, K. Thomas, and D. Anderson. 1999. Cerebral fungal infections in the immunocompromised host: a literature review and a new pathogen—Chaetomium atrobrunneum: case report. Neurosurgery 43:1463-1469. [DOI] [PubMed] [Google Scholar]
- 17.Gutierrez, E. G., W. A. Banks, and A. J. Kastin. 1993. Murine tumor necrosis factor alpha is transported from blood to brain in the mouse. J. Neuroimmunol. 47:169-176. [DOI] [PubMed] [Google Scholar]
- 18.Heyl, D. L., A. M. Sefler, J. X. He, T. K. Sawyer, D. J. Wustrow, H. C. Akunne, M. D. Davis, T. A. Pugsley, T. G. Heffner, A. E. Corbin, and W. L. Cody. 1994. Structure-activity and conformational studies of a series of modified C-terminal hexapeptide neurotensin analogues. Int. J. Peptide Protein Res. 44:233-238. [DOI] [PubMed] [Google Scholar]
- 19.Hoima, Y., J. V. Pierce, and J. J. Pisano. 1980. Hageman factor fragment inhibitor in corn seeds: purification and characterization. Thromb. Res. Suppl. 20:149-162. [DOI] [PubMed] [Google Scholar]
- 20.Hutcheson, S. W. 1998. Current concepts of active defense in plants. Annu. Rev. Phytopathol. 36:59-90. [DOI] [PubMed] [Google Scholar]
- 21.Huynh, Q. K., C. M. Hironaka, E. B. Levine, C. E. Smieth, J. R. Borgmeyer, and D. M. Shah. 1992. Antifungal proteins from plants. Purification, molecular cloning, and antifungal properties of chitinases from maize seed. J. Biol. Chem. 267:6635-6640. [PubMed] [Google Scholar]
- 22.Jackson, A. O., and C. B. Taylor. 1996. Plant-microbe interactions: life and death at the interface. Plant Cell 8:1651-1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22a.National Institutes of Health. 1985. Principles of laboratory animal care. Publication 85-23. National Institute of Health, Bethesda, Md.
- 23.Patlak, C. S., R. G. Blasberg, and J. D. Fenstermacher. 1983. Graphical evaluation of blood-to-brain transfer constants from multiple-time uptake data. J. Cereb. Blood Flow Metab. 3:1-7. [DOI] [PubMed] [Google Scholar]
- 24.Peacock, J. E., Jr., M. R. McGinnis, and M. S. Cohen. 1984. Persistent neutrophilic meningitis: report of four cases and review of the literature. Medicine 63:379-395. [PubMed] [Google Scholar]
- 25.Russin, J. S., B. Z. Guo, K. M. Tubajika, R. L. Brown, T. E. Cleveland, and N. W. Widstrom. 1997. Comparison of kernel wax from corn genotypes resistant or susceptible to Aspergillus flavus. Phytopathology 87:529-533. [DOI] [PubMed] [Google Scholar]
- 26.Triguero, D., J. Buciak, and W. M. Pardridge. 1990. Capillary depletion method for quantification of blood-brain barrier transport of circulating peptides and plasma proteins. J. Neurochem. 54:1882-1888. [DOI] [PubMed] [Google Scholar]
- 27.Uchida, D., A. Arimura, A. Somogyvari-Vigh, S. Shioda, and W. A. Banks. 1996. Prevention of ischemia-induced death of hippocampal neurons by pituitary adenylate cyclase activating polypeptide. Brain Res. 736:280-286. [DOI] [PubMed] [Google Scholar]
- 28.Walsh, T. J., D. B. Hier, and L. R. Caplan. 1985. Aspergillosis of the central nervous system: clinicopathological analysis of 17 patients. Ann. Neurol. 18:574-585. [DOI] [PubMed] [Google Scholar]