Abstract
Although the antimicrobial effects of silver salts were noticed long ago, the molecular mechanism of the bactericidal action of Ag+ in low concentrations has not been elucidated. Here, we show that low concentrations of Ag+ induce a massive proton leakage through the Vibrio cholerae membrane, which results in complete deenergization and, with a high degree of probability, cell death.
The antibacterial effects of silver salts have been noticed since ancient times (for a review, see references 5, 11, and 12), and today, silver is used to control bacterial growth in a variety of applications, including dental work, catheters, and burn wounds. Added at high (i.e., millimolar) concentrations, Ag+ ions inhibit a number of enzymatic activities, reacting with electron donor groups, especially sulfhydryl groups (12). However, the molecular mechanism of the bactericidal effect of much lower, i.e., micromolar, concentrations of Ag+ ions remains somewhat controversial.
The Na+-translocating NADH:ubiquinone oxidoreductase (NQR) has been recognized as one of the primary targets for Ag+ ions. In two independent studies, submicromolar concentrations of Ag+ ions were shown to inhibit energy-dependent Na+ transport in membrane vesicles of the NQR-possessing alkalophilic Bacillus sp. strain FTU (10) and to inhibit purified NQR of Vibrio alginolyticus (4). These observations suggested that the specific binding to NQR may be responsible for the bactericidal effect of low concentrations of Ag+. However, we found that, like in V. alginolyticus (13), the NQR enzyme is not crucial for the survival of Vibrio cholerae (3). Indeed, a mutant of the V. cholerae wild-type strain O395N1, carrying a deletion of the entire nqr operon, was able to grow in Luria broth and in mineral medium supplemented with glucose at a neutral pH (data not shown). Nevertheless, growth in both strains was completely arrested by the addition of 1.25 μM AgNO3 to the minimal growth medium (Table 1). Therefore, the action of low concentrations of silver on the growth of V. cholerae could not be attributed to a specific binding to NQR.
TABLE 1.
Inhibition of bacterial growth by silver in M9 mineral medium
| AgNO3 (μM) | OD600a
|
|
|---|---|---|
| O395N1 | O395N1Δnqr | |
| 0 | 0.814 | 0.713 |
| 0.625 | 0.218 | 0.121 |
| 1.25 | 0.017 | 0.017 |
| 2.5 | 0.017 | 0.016 |
| 5 | 0.018 | 0.019 |
| 10 | 0.014 | 0.015 |
OD600, optical density at 600 nm.
In a study published almost 50 years ago, the uncoupler-like effects (stimulation of respiration and ATPase activity) of micromolar concentrations of Ag+ added to isolated mitochondria were documented by Chappell and Greville (2). This was done well before P. Mitchell formulated the chemiosmotic hypothesis revealing the role of the proton motive force in oxidative phosphorylation (7, 8). In the context of Mitchell's concept, the observation by Chappell and Greville suggests an ability of Ag+ ions to collapse the proton motive force on the membrane. Paradoxically, to our knowledge, no direct experimental evidence for the effect of Ag+ on the proton motive force has been published since then. In 1982, Schreurs and Rosenberg mentioned (as an unpublished observation) that Ag+ collapses the proton motive force on the membrane (9). However, neither the effective concentration of Ag+ nor the experimental model used was specified in that communication.
To investigate this long-standing issue, we decided to measure directly the effect of Ag+ ions on the proton motive force on the membranes of the wild-type V. cholerae strain O395N1 (6) and its isogenic ΔNQR derivative (1). The formation and dissipation of the respiration-generated transmembrane pH gradient (ΔpH) was measured by acridine orange (AO) fluorescence quenching and dequenching in inside-out membrane vesicles prepared from both strains. Vesicles were obtained as described in reference 14 with some modifications. Cells were grown aerobically in standard Luria broth medium at 37°C to mid-log phase, cooled in an ice bath for 30 min, and harvested by centrifugation. Cells were washed once and resuspended in buffer containing 10 mM MOPS (morpholinepropanesulfonic acid)-Tris (pH 7.5), 10% (wt/vol) glycerol, 0.2 M K2SO4, 25 mM MgSO4, 0.5 mM phenylmethylsulfonyl fluoride, 1 mM dithiothreitol, and 0.2 μg of pepstatin A/ml. The obtained suspension was passed twice through a French press at 1,000 kg/m2. After removal of the cell debris by low-speed centrifugation (25,000 × g for 10 min), vesicles were collected by ultracentrifugation (250,000 × g for 90 min) and washed once with and resuspended in the same buffer. Aliquots of vesicles were resuspended in the above-mentioned buffer supplemented with 0.5 μM AO.
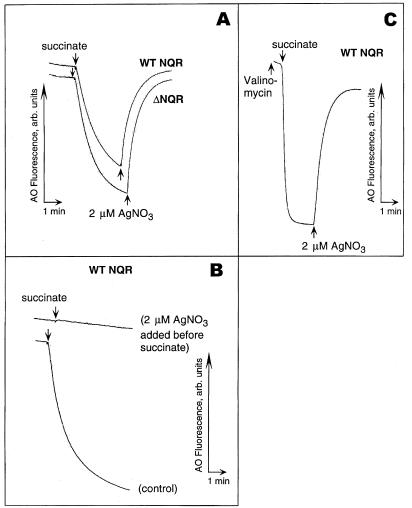
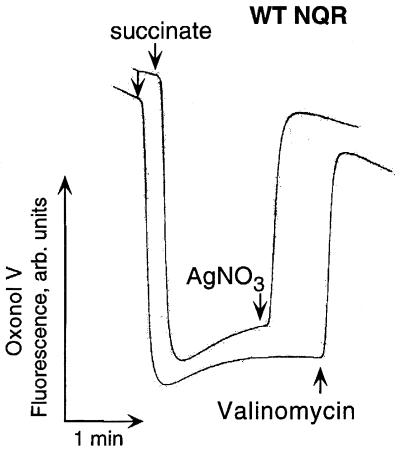
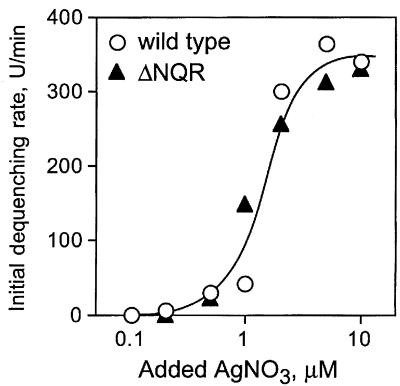
Here, we show that the addition of submicromolar to low micromolar concentrations of Ag+ to inside-out membrane vesicles of V. cholerae induced a total collapse of ΔpH irrespective of the presence of NQR in the membrane (Fig. 1A). Preincubation with Ag+ completely prevented the formation of the ΔpH in vesicles (Fig. 2B). It is worth noting that, in the presence of valinomycin, when the respiration-generated membrane electric potential (Δψ) did not limit the transmembrane ion flow, added Ag+ provoked the very fast dissipation of ΔpH, indicating that the resulting H+ leakage is massive (Fig. 1C). Figure 2 shows that the addition of Ag+ also collapses Δψ in the absence of added Na+ ions. These data clearly demonstrate that the Ag+-modified membrane is indeed leaky for protons and that the loss of NQR does not alter the sensitivity of the mutant V. cholerae membrane to Ag+ ions compared to that of the wild type. To demonstrate that NQR does not contribute significantly to the overall H+ leakage induced by Ag+ ions, we measured the initial rate of ΔpH dissipation at different concentrations of added Ag+ in vesicles isolated from either wild-type or ΔNQR cells (Fig. 3). We found that, in accordance with our growth experiments, the presence of NQR in the membrane is not required for the effect of Ag+ ions (Fig. 3).
FIG. 1.
Effects of Ag+ on the H+ permeability of the membrane measured in the inside-out membrane vesicles prepared from V. cholerae. Respiration-dependent formation of ΔpH was initiated by the addition of 10 mM Tris-succinate (down arrows). Where indicated, 2.0 μM AgNO3 was added to the vesicles (up arrows). (A) Addition of Ag+ after succinate collapses ΔpH generated in wild-type (WT) NQR (upper trek) as well as ΔNQR (lower trek) membranes. (B) Ag+ prevents the formation of the respiration-dependent ΔpH. (C) Ag+-induced ΔpH collapse is very fast in the presence of 0.2 μM valinomycin. In each case, typical treks from five independent experiments are shown.
FIG. 2.
Effects of Ag+ on Δψ in subbacterial vesicles of V. cholerae. The Δψ-sensitive dye Oxonol V (1.0 μM) was used instead of AO. Ag+ (4.0 μM) completely dissipates Δψ generated by respiration in vesicles derived from the strain possessing the wild-type (WT) NQR (upper trek). Nearly identical results were obtained with ΔNQR vesicles (data not shown). In the control experiment (lower trek), 0.2 μM valinomycin was added instead of AgNO3. Shown are typical treks from five independent experiments.
FIG. 3.
The initial rate of ΔpH dissipation in the wild-type and ΔNQR membrane vesicles as a function of the AgNO3 added. A transmembrane pH gradient was generated by the addition of 10 mM Tris-succinate to the vesicles at pH 7.5, and varying concentrations of AgNO3 were added after a steady-state ΔpH was reached. In each case, changes in AO fluorescence were monitored for 20 s after Ag+ addition. Initial dequenching rates are expressed in arbitrary fluorescence units per minute.
In summary, the addition of low micromolar concentrations of Ag+ to inside-out membrane vesicles of V. cholerae induced a total collapse of both ΔpH and Δψ irrespective of the presence of Na+ ions. This effect of Ag+ was independent of the presence of the Na+-translocating NQR, known as a specific target for submicromolar Ag+, suggesting that the other Ag+-modified membrane proteins (or perhaps the Ag+-modified phospholipid bilayer itself) can cause the H+ leakage, thus explaining the broad spectrum of the antimicrobial activity of Ag+ ions. The two most significant results of this study are (i) the first (to our knowledge) direct experimental demonstration of the ability of Ag+ ions to collapse the proton motive force and (ii) the irrelevance of NQR as a specific target for such a protonophore-like action of low micromolar concentrations of Ag+. It is conceivable that the bactericidal action of these concentrations of Ag+ in V. cholerae is not mediated by a specific target but is due to the H+ leakage occurring through virtually any Ag+-modified membrane protein or perhaps through the Ag+-modified phospholipid bilayer itself. In the absence of Ag+ resistance determinants (encoding pumps capable of efficient expelling of the Ag+ ion), this would result in a complete deenergization of the membrane. Taking into account the well-documented crucial importance of the transmembrane proton gradient in overall microbial metabolism, it seems inevitable that the protonophore-like effect of Ag+ described here should result in cell death. Thus, finally, the controversy over the mechanism of the bactericidal activity of low concentrations of Ag+ ions has been clarified.
Acknowledgments
We acknowledge support by operating grant no. 34021 from the Manitoba Health Research Council and by operating grant no. 227414-00 from the Natural Sciences and Engineering Research Council of Canada (to P.D. and J.D.). This work was supported in part by a Cancer Center support grant (CA 21765) and ALSAC (American Lebanese Syrian Associated Charities).
REFERENCES
- 1.Barquera, B., P. Hellwig, W. Zhou, J. E. Morgan, C. C. Häse, K. K. Gosink, M. Nilges, P. J. Bruesehoff, A. Roth, C. R. D. Lancaster, and R. B. Gennis. 2002. Purification and characterization of the recombinant Na+-translocating NADH:quinone oxidoreductase from Vibrio cholerae. Biochemistry 41:3781-3791. [DOI] [PubMed] [Google Scholar]
- 2.Chappell, J. B., and G. D. Greville. 1954. Effect of silver ions on mitochondrial adenosine triphosphatase. Nature 174:930-931. [DOI] [PubMed] [Google Scholar]
- 3.Häse, C. C., and J. J. Mekalanos. 1998. TcpP protein is a positive regulator of virulence gene expression in Vibrio cholerae. Proc. Natl. Acad. Sci. USA 95:730-734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hayashi, M., T. Miyoshi, M. Sato, and T. Unemoto. 1992. Properties of respiratory chain-linked Na+-independent NADH-quinone reductase in a marine Vibrio alginolyticus. Biochim. Biophys. Acta 1099:145-151. [PubMed] [Google Scholar]
- 5.Klasen, H. J. 2000. A historical review of the use of silver in the treatment of burns. II. Renewed interest for silver. Burns 26:131-138. [DOI] [PubMed] [Google Scholar]
- 6.Mekalanos, J. J., S. J. Schwartz, G. D. N. Pearson, N. Hardford, F. Groyne, and M. de Wilde. 1983. Cholera toxin genes: nucleotide sequences, deletion analysis and vaccine development. Nature 306:551-557. [DOI] [PubMed] [Google Scholar]
- 7.Mitchell, P. 1966. Chemiosmotic coupling in oxidative and photosynthetic phosphorylation. Biol. Rev. 41:445-502. [DOI] [PubMed] [Google Scholar]
- 8.Mitchell, P. 1961. Coupling of phosphorylation to electron and hydrogen transfer by a chemiosmotic type of mechanism. Nature 191:144-148. [DOI] [PubMed] [Google Scholar]
- 9.Schreurs, W. J., and H. Rosenberg. 1982. Effect of silver ions on transport and retention of phosphate by Escherichia coli. J. Bacteriol. 152:7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Semeykina, A. L., and V. P. Skulachev. 1990. Submicromolar Ag+ increases passive Na+ permeability and inhibits the respiration-supported formation of Na+ gradient in Bacillus FTU vesicles. FEBS Lett. 269:69-72. [DOI] [PubMed] [Google Scholar]
- 11.Silver, S., and L. T. Phung. 1996. Bacterial heavy metal resistance: new surprises. Annu. Rev. Microbiol. 50:753-789. [DOI] [PubMed] [Google Scholar]
- 12.Slawson, R. M., M. I. Van Dyke, H. Lee, and J. T. Trevors. 1992. Germanium and silver resistance, accumulation, and toxicity in microorganisms. Plasmid 27:72-79. [DOI] [PubMed] [Google Scholar]
- 13.Tokuda, H., M. Asano, Y. Shimamura, T. Unemoto, S. Sugiyama, and Y. Imae. 1988. Roles of the respiratory Na+ pump in bioenergetics of Vibrio alginolyticus. J. Biochem. 103:650-655. [DOI] [PubMed] [Google Scholar]
- 14.Unemoto, T., A. Akagawa, M. Mizugaki, and M. Hayashi. 1992. Distribution of Na+-dependent respiration and respiration-driven electrogenic Na+ pump in moderately halophilic bacteria. J. Gen. Microbiol. 138:1999-2005. [Google Scholar]