Abstract
Described here are the development and validation of a novel approach to identify genes encoding drug targets in Streptococcus pneumoniae. The method relies on the use of an ordered genomic library composed of PCR amplicons that were generated under error-prone conditions so as to introduce random mutations into the DNA. Since some of the mutations occur in drug target-encoding genes and subsequently affect the binding of the drug to its respective cellular target, amplicons containing drug targets can be identified as those producing drug-resistant colonies when transformed into S. pneumoniae. Examination of the genetic content of the amplicon giving resistance coupled with bioinformatics and additional genetic approaches could be used to rapidly identify candidate drug target genes. The utility of this approach was verified by using a number of known antibiotics. For drugs with single protein targets, amplicons were identified that rendered S. pneumoniae drug resistant. Assessment of amplicon composition revealed that each of the relevant amplicons contained the gene encoding the known target for the particular drug tested. Fusidic acid-resistant mutants that resulted from the transformation of S. pneumoniae with amplicons containing fusA were further characterized by sequence analysis. A single mutation was found to occur in a region of the S. pneumoniae elongation factor G protein that is analogous to that already implicated in other bacteria as being associated with fusidic acid resistance. Thus, in addition to facilitating the identification of genes encoding drug targets, this method could provide strains that aid future mechanistic studies.
Globally, millions die each year from infections caused by the gram-positive bacterium Streptococcus pneumoniae (6, 12). The effective treatment of pneumococcal pneumonia, otitis media, and meningitis has been compromised by the emergence of strains resistant to currently available antibiotics (2, 25). The development of new treatments for S. pneumoniae infections should be facilitated by the recently determined genome sequences of three strains of the organism (4, 8, 23).
One area of discovery research that could benefit from this new wealth of genetic information is drug target identification. In the drug development process, potential antibiotics may be identified as compounds that inhibit bacterial growth or the activity of a specific enzyme in vitro. In either case, the advancement of a promising compound can be accelerated by identifying the cellular target of the drug. Historically, many cellular drug targets have been identified in bacteria by genetic methods involving the screening of chromosomal libraries for genes that confer resistance to an otherwise susceptible host. This approach relies on the fact that drug resistance can arise when a resistant allele of the drug target is expressed or when the wild-type allele is overexpressed. There are several notable disadvantages to using such genetically based methods: drug targets may be unidentifiable when multiple targets exist in the cell, and not all of the genes identified as giving resistance encode the drug target. Other disadvantages include the laboriousness of library generation, the potential genetic bias of the resulting product, the need to construct multiple libraries when resistance alleles are examined, and the inability to identify genes encoding drug targets because the gene product is toxic at elevated concentrations.
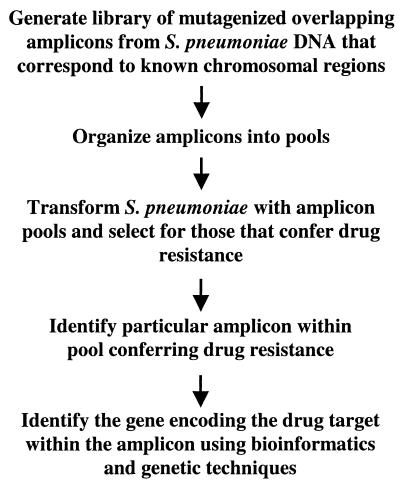
An advantage to performing genetic studies with S. pneumoniae is the natural competence of the organism. In particular, PCR fragments are readily taken up by the cell and then recombined into the chromosome when sufficient homology exists between the resident DNA and the incoming fragment (13). This is such an efficient process in S. pneumoniae that genetic manipulation of the organism is best achieved by this route. We realized that the natural transformation of pneumococci and the availability of the genomic sequence of S. pneumoniae R6 (8), a transformable avirulent laboratory strain, could be used to develop a new approach to drug target identification in this organism (Fig. 1). This novel strategy entails the generation of an ordered library of overlapping PCR amplicons that represents the entire chromosome. The PCR fragments are amplified under error-prone conditions such that random mutations are introduced into the amplicon. Presumably, some of these mutations would occur in drug target-encoding genes, resulting in resistance, e.g., by affecting the binding of the drug to its respective target. The strategy also relies on the premise that when S. pneumoniae R6 is transformed with a mutagenized amplicon containing the gene encoding the drug target, there should be a significant increase in the number of drug-resistant mutants over the background spontaneous resistance rate. Candidate drug target-encoding genes would be identified by first determining which of the ordered amplicons yields resistant transformants and then examining the genetic content of the amplicon in question. We describe here the development and validation of a method that uses PCR-based error-prone libraries for drug target identification in S. pneumoniae.
FIG. 1.
Proposed new method for drug target identification in S. pneumoniae.
MATERIALS AND METHODS
Bacteriology.
S. pneumoniae R6, an avirulent, highly transformable derivative of S. pneumoniae D39 (8), was used in these studies. The strain was routinely propagated in either Todd-Hewitt broth (Difco, Detroit, Mich.) supplemented with 0.5% yeast extract (Difco) or brain heart infusion broth (Difco) at 37°C with 5% CO2. Transformants were selected on TSA II plus 5% sheep blood agar (BBL, Cockeysville, Md.) by using soft agar overlays made from Nutrient Broth (Difco) containing 1% Bacto Agar (Difco). The drug concentrations used for selection of resistant transformants were determined by plating mock transformations on media containing twofold drug dilutions. The drug concentration chosen for use was 1 dilution higher than that at which no background growth occurred. The antibiotic concentrations used in these studies were as follows: ciprofloxacin, 4 μg/ml; fusidic acid, 64 μg/ml; penicillin G, 0.05 μg/ml; rifampin (RIF), 0.1 μg/ml; spectinomycin, 250 μg/ml; streptomycin (STR), 100 μg/ml; trimethoprim (TMP), 16 μg/ml. Rates of spontaneous mutation to drug resistance were determined as follows. A log phase culture of S. pneumoniae R6 grown in Todd-Hewitt broth containing 0.5% yeast extract was concentrated by centrifugation. Approximately 109 bacteria were spread onto antibiotic-impregnated blood agar plates. Following incubation, drug-resistant colonies were enumerated. Fusidic acid MICs were considered to be the lowest dilution of the drug at which no growth was evident. MICs were determined by serially diluting fusidic acid twofold in 100 μl of 2× Todd-Hewitt broth (Difco) and then adding 100 μl of an S. pneumoniae R6 or fusidic acid-resistant mutant culture with an optical density at 600 nm of 0.1. The samples were incubated overnight at 37°C with 5% CO2 and then examined for growth.
DNA manipulations.
Genomic DNA was isolated from S. pneumoniae R6 and its derivatives by the following protocol. The bacterial pellet resulting from the centrifugation of a 10-ml stationary-phase culture was resuspended in 370 μl of a solution containing 6.7% sucrose, 50 mM Tris-HCl (pH 8.0), and 1 mM EDTA. After addition of 97 μl of 10 mg of lysozyme per ml and 2.5 μl of mutanolysin at 5 U/ml, the cell mixture was incubated at 37°C for 30 min. A 24-μl volume of 0.5 M EDTA and a 27-μl volume of 20% sodium dodecyl sulfate was then added, and the cells were lysed by gentle inversion. A 50-μl volume of 2 mg of RNase A per ml of Tris-EDTA, pH 8.0, was added, and then the lysed cell mixture was incubated at 37°C for 1 h. The mixture was cooled to room temperature, and 200 μl of 7.5 M ammonium acetate was added. Following 20 s of vortexing, the samples were centrifuged at top speed (16,000 × g) in a microcentrifuge for 10 min. The supernatant was transferred to a fresh tube, and then the DNA was precipitated with 1 volume of isopropanol. The DNA was pelleted by centrifugation as before. The DNA was washed with 70% ethanol and then resuspended in 100 μl of Tris-EDTA, pH 8.0. S. pneumoniae R6 was routinely transformed with 100 ng of DNA by an established protocol (18), except that a 1-ml volume of cells was used and the incubation time for expression was increased to 3 h.
PCR and library generation.
PCR primers were designed by using Vector NTI Suite (InforMax, North Bethesda, Md.) with a target length of 25 to 30 nucleotides and a desired melting temperature (Tm) of 60 to 65°C; default settings were used for all other parameters. Matched forward and reverse primer pairs were designed to yield a PCR product of approximately 4 kb. The primer sequences used for these studies may be found at http://www.streppneumoniae.com/belanger.asp. Primers were synthesized by Sigma Genosys (The Woodlands, Tex.). High-fidelity PCRs were performed by using Platinum Taq High Fidelity polymerase (Invitrogen, Carlsbad, Calif.) in accordance with the manufacturer's specifications. Error-prone PCR was performed by using condition 1 of the Diversify PCR Random Mutagenesis Kit (Clontech, Palo Alto, Calif.). Touchdown PCR (3, 21) was performed for all samples with a starting annealing temperature of 62°C that was decreased by 2°C for four more cycles. All of the other PCR parameters used were those recommended by the manufacturer of the particular DNA polymerase used. PCRs were run in duplicate and then combined prior to pool generation. To create library pools, the PCR products of six sequential reactions were combined such that pool 1 (P1) contained amplicons 1 to 6 (A1 to A6) and pool 2 (P2) contained amplicons 7 to 12 (A7 to A12), etc. Thus, each pool contained six contiguous amplicons that span approximately 24 kb of the S. pneumoniae R6 genome. The amplicon pools were purified by using the Qiagen PCR purification kit (Qiagen, Valencia, Calif.) prior to use in transformation.
RESULTS
Error-prone PCR of known drug targets.
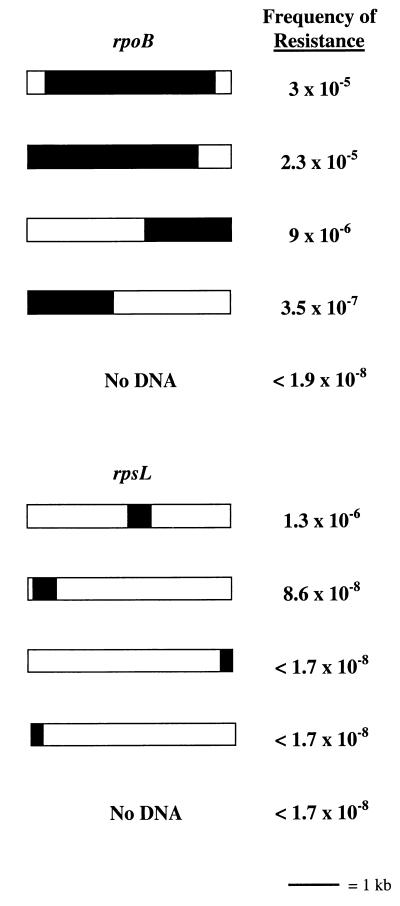
The cornerstone of our method is that the number of drug-resistant transformants increases significantly over the background when S. pneumoniae R6 is transformed with mutagenized amplicons containing the gene encoding the drug target. This hypothesis was tested by using amplicons containing genes encoding known S. pneumoniae drug targets. Since the polymerase error rate is a function of the total number of nucleotides incorporated, we theorized that the size of the gene would be one factor that influences the mutational frequency. That is, the bigger the gene, the more likely it is that a mutation that influences drug resistance will occur in it during error-prone PCR. To ensure that this method would work for genes encoding drug targets, irrespective of size, we used rpoB, the gene encoding the target of RIF, and rpsL, the gene encoding the target of STR. These genes represent the upper and lower size limits of genes encoding drug targets; rpoB is 3,351 bp, and rpsL is 427 bp. The system that we chose to use for error-prone PCR has been validated for fragments of up to 4 kb (Diversify PCR Random Mutagenesis Kit User Manual [Clontech]). The ideal situation would be to make even larger PCR fragments so as to minimize the number of amplicons needed to represent the S. pneumoniae R6 genome. Pilot studies were performed with primers designed to amplify 10 5-kb PCR products that span approximately the first 50 kb of the S. pneumoniae genome. Under mutagenic amplification conditions, more than half of the primer pairs generated multiple spurious products in addition to the correct-size product. These same primer pairs did not produce spurious products when DNA was amplified under high-fidelity conditions (data not shown). New primer pairs were designed that amplify nine 4-kb PCR products that span approximately the first 36 kb of the S. pneumoniae genome. In the 4-kb PCR product size range, the problem of amplifying spurious PCR products under mutagenic amplification conditions was eliminated (data not shown). Therefore, all subsequent primers were designed such that the resulting PCR products were all approximately 4 kb. In our initial experiments, the amplicons were also designed so that rpoB and rpsL were centrally located within the PCR product (Fig. 2). PCR products were amplified under both high-fidelity and error-prone conditions. The high-fidelity polymerase used is six times more faithful than Taq polymerase (Platinum Taq DNA Polymerase High Fidelity User Manual [Invitrogen]) (1, 11, 16, 24) and, thus, has an error rate of approximately 2 × 10−5 errors per bp. The error rate for the error-prone conditions used is approximately 2 × 10−3 errors per bp (Diversify PCR Random Mutagenesis Kit User Manual). S. pneumoniae R6 was transformed with the various amplicons and then selected on media containing the appropriate antibiotic. The resulting drug-resistant colonies were enumerated, and the mutational frequency was determined. STR-resistant R6 transformants were not obtained with rpsL-containing amplicons generated under high-fidelity conditions, and thus, the mutational frequency fell below the limit of detection by our assay (<1.7 × 10−8). When S. pneumoniae R6 was transformed with rpsL-containing amplicons generated under error-prone conditions, STR-resistant transformants were obtained (Fig. 2). The mutational frequency was approximately 3 orders of magnitude greater than the spontaneous mutation frequency, which we determined to be 2.2 × 10−9. Amplicons containing rpoB that were generated under high-fidelity conditions gave an appreciable frequency of RIF resistance, 4.9 × 10−5. However, the frequency of RIF resistance increased by nearly a log when S. pneumoniae R6 was transformed with rpoB-containing amplicons made under error-prone conditions (Fig. 2). The frequency of RIF resistance when high-fidelity and error-prone amplicons were used increased 4 to 5 orders of magnitude, respectively, over our measured rate of spontaneous mutation of 7.4 × 10−9. Since a significant intrinsic error rate is already associated with the high-fidelity polymerase and the size of rpoB is large, it is perhaps not surprising that a large number of RIF-resistant transformants were obtained by using both types of amplicons. Although the numbers of RIF- and STR-resistant transformants increased considerably only when rpoB- and rpsL-containing amplicons were used, it was possible that the resistance phenotype was not associated with the particular genetic region represented by the amplicon but, rather, was due to a mutation(s) located elsewhere in the chromosome. To demonstrate that the drug resistance phenotype was specifically associated with the genetic region represented by the amplicon, genomic DNA was isolated from three drug-resistant mutants and then used to reamplify the amplicon in question under high-fidelity conditions. If either the RIF or the STR resistance observed in the mutants was due to a mutation(s) in the region of DNA represented by the rpoB- or rpsL-containing amplicons, then these newly generated amplicons should confer drug resistance when transformed into S. pneumoniae R6. Indeed, when used in transformation experiments, the amplicons generated from the RIF- and STR-resistant mutants conferred RIF and STR resistance, respectively, to 100% of the transformable population (∼1% of the total number of cells present). The results described validated our assumption that the number of drug-resistant transformants will increase when S. pneumoniae R6 is transformed with mutagenized amplicons containing the drug target. The data also suggest that while the size of the gene encoding the drug target appears to contribute to the frequency at which drug resistance occurs, size alone does not preclude us from using a PCR-based error-prone library to find genes encoding drug targets.
FIG. 2.
Frequencies of drug-resistant mutants obtained by using amplicons that differ with regard to drug target-encoding gene location and completeness. Shaded areas represent complete or partial coding sequences of either the rpoB or the rpsL gene. The area of the shaded region corresponds to the size of the coding sequence or partial coding sequence relative to the 4-kb amplicon.
Our method is contingent on the fact that homologous DNA is taken up during transformation and then recombined into the chromosome. Allelic replacement in S. pneumoniae can be hampered by a phenomenon termed end exclusion (14). Here, the closer a marker is to the end of the donor DNA molecule, the less likely it is to be acquired by the recipient. Presumably, double-crossover events are hindered by insufficient base pairing on one side of the molecule. Since our proposed strategy entailed the use of ordered amplicons designed only with regard to nucleotide position within the genome, the location and completeness of any drug target-encoding gene within the amplicon could vary. To assess the influence of such variables on the ability to detect drug-resistant transformants, several new amplicons were made. In one instance, both the rpsL and rpoB genes were moved from the center of the amplicon to the 5′ end of the amplicon (Fig 2). Shifting rpsL to the 5′ end of the amplicon resulted in a frequency of STR resistance in S. pneumoniae R6 transformants that was barely above the background (Fig. 2). The rpoB gene occupies most of the 4-kb amplicon, so shifting the gene slightly to one end should have less impact than shifting rpsL. Indeed, the frequency of RIF-resistant transformants remained the same (Fig. 2). In another instance, two additional amplicons were constructed in which the first half of the gene was located at the 3′ end of one amplicon and the last half was located at the 5′ end of the other amplicon (Fig. 2). Splitting the rpsL gene in half resulted in an inability to detect STR-resistant transformants. However, significant numbers of RIF-resistant transformants were still obtained by using the split rpoB amplicons although more transformants were obtained by using the amplicon containing the first half of the gene (Fig. 2). These results suggest that the completeness and location within the amplicon of the drug target-encoding gene influence the ability to obtain drug-resistant mutants. Thus, we would expect that a library of amplicons designed irrespective of content could be used to find most, but not all, drug target-encoding genes. Specifically, if the gene encoding a drug target is small and represented incompletely on an amplicon, drug-resistant mutants may not arise at a significant enough frequency to be detected.
Library generation and compound screening.
Once we had ascertained that the frequency of drug-resistant transformants could be used to predict whether an amplicon contained the gene encoding the drug target, we turned our attention to the logistics of screening a library of PCR products. Given the size restriction of the error-prone PCR products that we could generate, more than 500 ordered amplicons would be required to represent the entire S. pneumoniae R6 genome. Testing of each individual amplicon for the ability to give resistant transformants on a given drug is impractical, so we explored the idea of using amplicon pools. In these studies, we used the centrally located rpoB amplicon described above and an amplicon containing centrally located dfr. The dfr gene encodes dihydrofolate reductase, the cellular target for the drug TMP. Pools of amplicons were made that contained the dfr and rpoB amplicons, as well as eight other amplicons that correspond to the first 32 kb of the S. pneumoniae R6 genome. These eight amplicons were not expected to contain any genes that influence either RIF or TMP resistance. When S. pneumoniae R6 was transformed with either the dfr or the rpoB amplicon only and selected on the corresponding compound, drug-resistant transformants were readily obtained (Table 1). The frequency of RIF- and TMP-resistant transformants was diminished, but they were still detectable when S. pneumoniae R6 was transformed with a constant amount of DNA containing the 10 amplicons (Table 1). The number of drug-resistant transformants obtained with the amplicon pool was 2 to 3 logs higher than that obtained when R6 was transformed with a pool containing only the eight unrelated amplicons or no DNA (Table 1). These results suggested that pools of amplicons could be used to detect drug-resistant transformants instead of individual amplicons. Such an arrangement increases the feasibility of screening the genome for drug target-encoding genes by using amplicons.
TABLE 1.
Frequency of drug-resistant transformants obtained with pooled amplicons
| DNA samplea | TMP-resistant transformants | RIF-resistant transformants |
|---|---|---|
| dfr amplicon | 3 × 10−5 | NDb |
| rpoB amplicon | NDb | 2.7 × 10−3 |
| Pool of 10 amplicons | 4.7 × 10−6 | 5 × 10−5 |
| Pool of 8 amplicons | <3.7 × 10−8 | 7.5 × 10−8 |
| No DNA | <3.7 × 10−8 | 7.5 × 10−8 |
Pool of 10 amplicons: dfr amplicon, rpoB amplicon, and 8 unrelated amplicons; pool of 8 amplicons contains the unrelated amplicons only.
ND, not done.
To initiate library generation, a total of 521 amplicons were designed that cover the entire genome of S. pneumoniae R6. The amplicons have an average size of 4 kb and an average overlap of 70 bp. A total of 1,042 primers were originally synthesized and validated by using high-fidelity PCR amplification conditions. Approximately 10% (n = 116) of the primer pairs gave either no product or spurious products and were redesigned and resynthesized. All of these new primer pairs except one gave the correct PCR product when retested. The validated primer pairs were subsequently used to amplify S. pneumoniae R6 DNA under error-prone PCR conditions. Of the 521 primer pairs, 498 gave the correct-size PCR product. For the remaining 43 primers that did not work, amplicons were generated under high-fidelity conditions and then the resulting DNA was used as the template in the error-prone PCR. This approach was successful for amplifying 39 of the 43 troublesome amplicons under error-prone conditions. All told, 517 of the 521 amplicons, representing 99% of the total number of genes in the chromosome, were generated. Although our studies described above indicated that 10 amplicons could be combined per pool, we opted to add only six sequential amplicons per pool. This particular number was more compatible with the 96-well format of the PCR plates used; each plate would be used to make 16 pools. Since there were originally 521 amplicons, 87 pools were designed, of which the first 86 pools contained 6 amplicons and the last pool contained only 5 amplicons. However, because of the unsuccessful amplification of four error-prone PCR products, two additional pools contained only five amplicons and one pool contained only four amplicons.
The ability to use a PCR-based ordered genomic library made under error-prone conditions to identify genes encoding potential drug targets was confirmed by using seven known antibacterial compounds. Five of these compounds, namely, fusidic acid, RIF, spectinomycin, STR, and TMP, are known to have a single protein target. Our method, as designed, is not suitable for identifying the targets of drugs for which multiple cellular targets exist because only a single round of transformation is performed. One exception would be if the multiple targets were found in a single pool. Therefore, to define the useful parameters of our system, we also included in our studies as negative controls two drugs with multiple targets: penicillin G and ciprofloxacin. For the five drugs with only one protein target, a single pool in the library was found to give readily detectable amounts of drug-resistant transformants in S. pneumoniae R6 (Table 2). To determine which amplicon in the pool was specifically associated with drug resistance, chromosomal DNA was isolated from several drug-resistant transformants and then used as the template to reamplify all of the amplicons within a positive pool. Transformation of S. pneumoniae R6 with these new amplicons resulted in the identification of a single amplicon that conferred drug resistance on 100% of the transformable population (Table 2). Examination of the genetic content of the amplicons deemed positive revealed that each contained the proposed protein target for the drug (Table 2) (5, 10, 15, 17, 19, 20, 22). While the genes encoding the targets for fusidic acid and RIF were split between two amplicons, only the amplicon containing the C-terminal portion of the fusA gene and the N-terminal portion of the rpoB gene conferred resistance (Table 2). As expected, no penicillin- or ciprofloxacin-resistant transformants were obtained. In the case of penicillin G, there are multiple penicillin-binding protein targets in the cell and resistance requires more extensive alterations in the penicillin-binding protein pool than mere single-point mutations (7). Ciprofloxacin-resistant mutants have been isolated in vitro that sustain mutations in both gyrA and parE (9), but in the S. pneumoniae amplicon library, these genes are not found within the same pool.
TABLE 2.
Screening of the amplicon library for genes encoding drug targets
| Drug tested | Positive pool | Frequency of transformantsa | Positive ampliconb | Genes in amplicon/drug targetc (reference[s]) |
|---|---|---|---|---|
| Ciprofloxacin | None | None | ||
| Fusidic acid | P11 | 2.6 × 10−6 | A64 | fusA*, polC* (10, 15) |
| Penicillin G | None | |||
| RIF | P75 | 4.5 × 10−6 | A449 | rpoB*, tRNA-cys, hylX* (5, 17) |
| Spectinomycin | P9 | 1.8 × 10−5 | A52 | rplE*, rpsN, rpsH, rplF, rplR, rpsE, rpmD, rplO, secY* (20) |
| STR | P11 | 7.8 × 10−7 | A63 | pulA*, rpsL, rpsG, fusA* (22) |
| TMP | P61 | 1.3 × 10−6 | A362 | clpX*, hypo, dfr, dpr, boxCA, lytC* (19) |
Frequency of drug-resistant transformants obtained with transformations containing an average of 4 × 107 CFU/ml.
Amplicon giving drug-resistant colonies at a frequency of 10−2 when transformed into S. pneumoniae R6.
Asterisks denote partial coding sequences. hypo encodes a hypothetical protein. Underlined genes are known drug targets.
To ensure that the drug resistance described above resulted from a mutation(s) in the target gene, three fusidic acid-resistant mutants that were obtained by the transformation of S. pneumoniae R6 with amplicon pool 11 were further characterized. Drug susceptibility determinations revealed that fusidic acid has an MIC for S. pneumoniae R6 of 8 μg/ml while for each of the mutants, the MIC was 256 μg/ml, a 32-fold increase in resistance. The fusA gene (2,709 bp) and the upstream region likely to contain the promoter (428 bp) were sequenced for each of the mutants and the wild type. A single T→G mutation was found at nucleotide 1,379 of fusA of all of the mutants. The presence of only a single-base change in the 3,137 bp sequenced falls short of the predicted error rate of two mutations per kilobase of DNA for the Diversify PCR Random Mutagenesis Kit. The latter mutation rate was determined by direct sequencing of mutagenized libraries (Diversify PCR Random Mutagenesis Kit User Manual). One explanation for the disparity between our error rate and that predicted for the Diversify PCR Random Mutagenesis Kit is that selection on fusidic acid produces a bias for the number and types of mutations that can be obtained. The isolation of three transformants with identical mutations might be attributed to the PCR-based nature of our assay or to clonal expansion during the transformation portion of the procedure. The T→G mutation in the fusidic acid-resistant S. pneumoniae R6 mutants causes an amino acid substitution of leucine to arginine at position 460 of elongation factor G (EF-G). This amino acid change does not correspond to any of the other known fusidic acid-resistant mutations in the previously studied Staphylococcus aureus or Salmonella enterica serovar Typhimurium EF-G proteins (10, 15). However, the L460R mutation is still located in a region of the protein corresponding to domain III of the Thermus thermophilus EF-G protein (15). The analogous domain in S. aureus and S. enterica serovar Typhimurium is where numerous other fusidic acid resistance mutations are also located (15). The data described here suggest that an additional benefit to using this new method is that drug target identification and mutagenesis can be combined in a single step. Thus, the transformants resulting from the initial library screening procedure can be used for subsequent protein function studies.
DISCUSSION
The work presented here is a proof-of-concept study that employed PCR-based ordered genomic libraries to identify known targets of known antibiotics. The real potential of this method is identification of the cellular targets of antibacterial compounds with unknown targets. PCR-based ordered genomic libraries could be a boon to drug discovery programs with research arms that identify potential antibiotics on the basis of antibacterial activity against whole cells and that require target identification for further drug development to occur. In this application, the utilization of PCR-based ordered genomic libraries to identify target genes will require work beyond what was described here. For example, once an amplicon yielding mutants resistant to a compound is identified, the actual gene contained within the amplicon that is responsible for the phenotype must be determined. We envision using a combination of bioinformatics and newly designed overlapping amplicons to identify the specific gene associated with drug resistance.
One of the motivations for developing a PCR-based method for drug target identification was that the genetic manipulation of S. pneumoniae is best achieved by using this type of DNA. Such a method does not overcome all of the problems associated with conventional plasmid-based genetic methods, but it is still advantageous in many ways. Above all, the use of ordered amplicons would allow potential drug target-encoding genes to be identified more rapidly than conventional methods. Our method may appear to be unnecessarily complex. However, once the PCR-based library has been generated, candidate target genes can be identified for many different antibacterial compounds within a 2-day turnaround. Yet another advantage is that a PCR-based error-prone library represents a single renewable and unbiased resource that can be used to screen for the targets of many compounds. Also, this method efficiently combines drug target identification with mutagenesis and, therefore, should facilitate downstream work such as mechanistic studies
The ability to identify drug targets by using PCR-based ordered genomic libraries is influenced by how the gene in question is represented on the amplicon. If a gene is located at the end of an amplicon or is incompletely represented on an amplicon, drug-resistant mutants may not occur at a detectable frequency. One solution to this problem would be to make a second library of amplicons offset from the first library by 2 kb. Thus, genes bisected into two amplicons in the first library would likely be complete and centrally located on a single amplicon in the second library. While two libraries might ensure that all of the drug targets that can be found will be found, both the expense and the labor associated with this method would increase. This is true even if the second library is only used when the first library fails to yield a result. Since a single PCR-based ordered genomic library has the potential to find most drug target-encoding genes, the added cost of generating a second library may outweigh the advantages of its utilization.
A number of variables beyond those identified in these studies likely influence the frequency at which drug-resistant transformants occur when S. pneumoniae R6 is transformed with mutagenized amplicons containing genes encoding drug targets. For example, the total number of amino acid mutations giving rise to drug resistance that any protein can sustain without compromising function should also contribute to the frequency at which drug-resistant transformants are obtained. The drug targets rpsL and rpoB are a case in point. For RpsL, a single amino acid substitution, K56T, has been associated with high-level STR resistance (22). For RpoB, two amino acids have been implicated in RIF resistance in S. pneumoniae: the aspartic acid at position 60 and the histidine at position 70 (5, 17). In the case of the histidine at position 70, the first nucleotide of the codon can be changed to any of the other three nucleotides, resulting in an asparagine, tyrosine, or aspartic acid substitution (5, 17). Thus, the increase in the number of RIF-resistant transformants relative to the STR-resistant transformants obtained in our studies could also be explained, in part, by the fact that more mutational options exist that give rise to RIF resistance.
Acknowledgments
We thank Linda N. Lee for helpful suggestions and the S. pneumoniae R6 genomic DNA and Thalia I. Nicas for carefully reading the manuscript.
This work was supported by Eli Lilly and Company.
REFERENCES
- 1.Cariello, N. F., J. A. Swenberg, A. De Bellis, and T. R. Skopek. 1991. Analysis of mutations using PCR and denaturing gradient gel electrophoresis. Environ. Mol. Mutagen. 18:249-254. [DOI] [PubMed] [Google Scholar]
- 2.Doern, G. V., A. B. Brueggemann, H. Huynh, and E. Wingert. 1999. Antimicrobial resistance with Streptococcus pneumoniae in the United States, 1997-98. Emerg. Infect. Dis. 5:757-765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Don, R. H., P. T. Cox, B. J. Wainwright, K. Baker, and J. S. Mattick. 1991. "Touchdown' PCR to circumvent spurious priming during gene amplification. Nucleic Acids Res. 19:4008.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dopazo, J., A. Mendoza, J. Herrero, F. Caldara, Y. Humbert, L. Friedli, M. Guerrier, E. Grand-Schenk, C. Gandin, M. De Francesco, A. Polissi, G. Buell, G. Feger, E. García, M. Peitsch, and J. F. García-Bustos. 2001. Annotated draft sequence from a Streptococcus pneumoniae type 19F clinical isolate. Microb. Drug Res. 7:99-125. [DOI] [PubMed] [Google Scholar]
- 5.Enright, M., P. Zawadski, P. Pickerill, and C. G. Dowdson. 2000. Molecular evolution of rifampicin resistance in Streptococcus pneumoniae, p. 427-432. In A. Tomasz (ed.), Streptococcus pneumoniae: molecular biology and mechanism of disease. Mary Ann Liebert, Inc., Larchmont, N.Y.
- 6.Greenwood, B. 1999. The epidemiology of pneumococcal infection in children in the developing world. Philos. Trans. R. Soc. Lond. B Biol. Sci. 354:777-785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hakenbeck, R., K. Kaminski, A. König, M. van der Linden, J. Paik, P. Reichmann, and D. Zähner. 1999. Penicillin-binding proteins in β-lactam-resistant Streptococcus pneumoniae. Microb. Drug Resist. 5:91-99. [DOI] [PubMed] [Google Scholar]
- 8.Hoskins, J., W. E. Alborn, Jr., J. Arnold, L. C. Blaszczak, S. Burgett, B. S. DeHoff, S. T. Estrem, L. Fritz, D.-J. Fu, W. Fuller, C. Geringer, R. Gilmour, J. S. Glass, H. Khoja, A. R. Kraft, R. E. Lagace, D. J. LeBlanc, L. N. Lee, E. J. Lefkowitz, J. Lu, P. Matsushima, S. M. McAhren, M. McHenney, K. McLeaster, C. W. Mundy, T. I. Nicas, F. H. Norris, M. O'Gara, R. B. Peery, G. T. Robertson, P. Rockey, P.-M. Sun, M. E. Winkler, Y. Yang, M. Young-Bellido, G. Zhao, C. A. Zook, R. H. Baltz, S. R. Jaskunas, P. R. Rosteck, Jr., P. L. Skatrud, and J. I. Glass. 2001. Genome of the bacterium Streptococcus pneumoniae strain R6. J. Bacteriol. 183:5709-5717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Janoir, C., E. Varon, M.-D. Kitzis, and L. Gutmann. 2001. New mutation in ParE in a pneumococcal in vitro mutant resistant to fluoroquinolones. Antimicrob. Agents Chemother. 45:952-955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johanson, U., and D. Hughes. 1994. Fusidic acid-resistant mutants define three regions in elongation factor G of Salmonella typhimurium. Gene 143:55-59. [DOI] [PubMed] [Google Scholar]
- 11.Keohavong, P., and W. G. Thilly. 1989. Fidelity of DNA polymerases in DNA amplification. Proc. Natl. Acad. Sci. USA 86:9253-9257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klein, D. L. 1999. Pneumococcal disease and the role of conjugate vaccines. Microb. Drug Resist. 5:147-157. [DOI] [PubMed] [Google Scholar]
- 13.Lacks, S. 2000. Cloning and expression of pneumococcal genes in Streptococcus pneumoniae, p. 67-77. In A. Tomasz (ed.), Streptococcus pneumoniae: molecular biology and mechanism of disease. Mary Ann Liebert, Inc., Larchmont, N.Y.
- 14.Lataste, H., J.-P. Claverys, and A. M. Sicard. 1981. Relation between the transforming activity of a marker and its proximity to the end of the DNA particle. Mol. Gen. Genet. 183:199-201. [DOI] [PubMed] [Google Scholar]
- 15.Laurberg, M., O. Kristensen, K. Martemyanov, A. T. Gudkov, I. Nagaev, D. Hughes, and A. Liljas. 2000. Structure of a mutant EF-G reveals domain III and possibly the fusidic acid binding site. J. Mol. Biol. 303:593-603. [DOI] [PubMed] [Google Scholar]
- 16.Ling, L. L., P. Keohavong, C. Dias, and W. G. Thilly. 1991. Optimization of the polymerase chain reaction with regard to fidelity: modified T7, Taq, and Vent DNA polymerases. PCR Methods Appl. 1:63-69. [DOI] [PubMed] [Google Scholar]
- 17.Padayachee, T., and K. P. Klugman. 1999. Molecular basis of rifampin resistance in Streptococcus pneumoniae. Antimicrob. Agents Chemother. 43:2361-2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pestova, E. L., L. S. Haverstein, and D. A. Morrison. 1996. Regulation of competence for genetic transformation in Streptococcus pneumoniae by an auto-induced peptide pheromone and a two-component regulatory system. Mol. Microbiol. 21:853-862. [DOI] [PubMed] [Google Scholar]
- 19.Pikis, A., J. A. Donkersloot, W. J. Rodriguez, and J. M. Keith. 1998. A conservative amino acid mutation in the chromosome-encoded dihydrofolate reductase confers trimethoprim resistance in Streptococcus pneumoniae. J. Infect. Dis. 178:700-706. [DOI] [PubMed] [Google Scholar]
- 20.Ramakrishnan, V., and S. W. White. 1992. The structure of ribosomal protein S5 reveals sites of interaction with 16S rRNA. Nature 358:768-771. [DOI] [PubMed] [Google Scholar]
- 21.Roux, K. H. 1995. Optimization and troubleshooting in PCR. PCR Methods Appl. 4:5185-5194. [DOI] [PubMed] [Google Scholar]
- 22.Salles, C., L. Créancier, J.-P. Claverys, and V. Méjean. 1992. The high level streptomycin resistance gene from Streptococcus pneumoniae is a homolog of the ribosomal protein S12 gene from Escherichia coli. Nucleic Acids Res. 20:6103.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tettelin, H., K. E. Nelson, I. T. Paulsen, J. A. Eisen, T. D. Read, S. Peterson, J. Heidelberg, R. T. DeBoy, D. H. Haft, R. J. Dodson, A. S. Durkin, M. Gwinn, J. F. Kolonay, W. C. Nelson, J. D. Peterson, L. A. Umayam, O. White, S. L. Salzberg, M. R. Lewis, D. Radune, E. Holtzapple, H. Khhouri, A. M. Wolf, T. R. Utterback, C. L. Hansen, L. A. McDonald, T. V. Feldblyum, S. Angiuoli, T. Dickinson, E. K. Hickey, I. E. Holt, B. J. Loftus, F. Yang, H. O. Smith, J. C. Venter, B. A. Dougherty, D. A. Morrison, S. K. Hollingshead, and C. Fraser. 2001. Complete genome sequence of a virulent isolate of Streptococcus pneumoniae. Science 293:498-506. [DOI] [PubMed] [Google Scholar]
- 24.Tindall, K. R., and T. A. Kunkel. 1988. Fidelity of DNA synthesis by the Thermus aquaticus DNA polymerase. Biochemistry 27:6008-6013. [DOI] [PubMed] [Google Scholar]
- 25.Tomasz, A. 1995. The pneumococcus at the gates. N. Engl. J. Med. 333:514-515. [DOI] [PubMed] [Google Scholar]