Abstract
Approximately 150,000 small-molecule compounds were tested by a robotic screening assay for their ability to inhibit nucleoside triphosphate hydrolase (NTPase), a novel enzyme of the tachyzoite form of Toxoplasma gondii. Five unrelated species of compounds were found to inhibit the activities of both NTPase isoforms (NTPase isoform I [NTPase-I] and NTPase-II). The 50% inhibitory concentrations (IC50s) ranged from 0.1 to 20 μM, and in general, the IC50s were similar for both NTPase isoforms. However, the activity of NTPase-I was 20 times more sensitive than the activity of NTPase-II to one of the inhibitors: 9-hydroxy-10-(pentachlorophenoxy)stearic acid. The five compounds identified also prevented tachyzoite replication in vitro, with IC50s ranging from ∼7 to ≥50 μM. The most effective of these initial compounds, 2-phenylthio-indole, was used to identify six additional, structurally related compounds, which were tested for their inhibitory effects on enzyme activities and tachyzoite replication. Surprisingly, these compounds were competitive inhibitors of NTPase-I but noncompetitive inhibitors of NTPase-II. Modifications to the indole and phenol rings resulted in alterations of activity, thus providing insight into the structural features that are important for inhibition of T. gondii NTPases.
Toxoplasma gondii is an obligate intracellular protozoan parasite that commonly infects many warm-blooded animals, including humans (8). Toxoplasmosis is a significant problem among patients with AIDS, organ transplant recipients, pregnant women who are infected during the early stage of gestation, and individuals with ocular toxoplasmosis (13). Although acute toxoplasmosis can be effectively treated with a variety of antibiotics, the drugs commonly cause side effects and treatment does not eradicate the infection (4, 6, 7). Therefore, new drugs, including those which act against the proliferative tachyzoite stage of the parasite, are highly desirable for the treatment of toxoplasmosis.
The rapidly multiplying tachyzoite form of T. gondii has a potent nucleoside triphosphate hydrolase (NTPase; EC 3.6.1.3) that has a number of unusual properties (3). Treatment with a dithiol compound such as dithiothreitol (DTT) is essential to activate the enzyme in vitro. NTPase has two isoforms, termed NTPase isoform I (NTPase-I) and NTPase-II, which differ in their kinetic properties. While both enzymes hydrolyze a variety of nucleoside triphosphates, NTPase-I is only minimally active against diphosphate nucleosides such as ADP, while NTPase-II has roughly equal activities against tri- and diphosphate nucleosides (2). These enzymatic differences are presumably the result of a small number of differences that exist between their respective genes. These differences result in 15 amino acid changes among the 603 residues of the mature enzymes (2, 5). The gene encoding NTPase-II is found in all strains of T. gondii, while the gene encoding NTPase-I is confined to virulent strains (2, 5). Several properties of the T. gondii NTPase, such as substrate specificity and divalent cation requirements, are most similar to those of E (ecto)-type ATPases (12). E-type ATPases are insensitive to known inhibitors of P-, F-, and V-type ATPases; however, the T. gondii NTPases are sensitive to quercitin (50% inhibitory concentration [IC50], ∼100 μM), an inhibitor of P-type ATPases (T. Asai, unpublished data). Furthermore, DTT-dependent NTPases have not been found in other organisms except Neospora caninum, which is closely related to T. gondii (1). Although the physiological roles of the T. gondii NTPases have not been identified, the enzymes are released into the parasite-containing vacuole (14), where their function appears to be essential for tachyzoite replication within the host cell (11). These observations suggest that NTPase may be an excellent target for new chemotherapeutic strategies against toxoplasmosis. Therefore, we searched for inhibitors of NTPase activity by robotic screening of approximately 150,000 small-molecule compounds and tested whether the compounds identified also inhibited tachyzoite replication in vitro.
In this paper, we report on the chemical structures, anti-NTPase activities, and antiproliferative activities of these compounds.
MATERIALS AND METHODS
Parasite and cell culture.
Tachyzoites of the RH strain of T. gondii were propagated in ICR mice, and the NTPase-I and NTPase-II enzymes were purified to homogeneity as described previously (2). T. gondii clone 2F tachyzoites expressing bacterial β-galactosidase was maintained in vitro in human foreskin fibroblasts (HFFs; HS68; American Type Culture Collection) grown in Dulbecco's modified Eagle's medium (DMEM; Gibco BRL, Grand Island, N.Y.) containing 5 μg of gentamicin per ml and heat-inactivated fetal bovine serum (Gibco BRL). Toxicity for HFFs was tested by incubation with compounds overnight and staining with 0.02% trypan blue in DMEM. The percentage of positive cells was assessed by microscopic examination.
Automated screening of compounds.
Chemicals for testing were obtained from the compound collection at Merck Research Laboratories (Rahway, N.J.) and were screened for inhibition of NTPases by automated robotic screening in a 96-well plate format. The compounds were dissolved in dimethyl sulfoxide (DMSO) and dispensed into individual wells of a 96-well plate for screening at an initial concentration of 50 μM. The 96-well plate assay contained 10 U (1 U = 1 nmol ATP/min) of the isozyme NTPase-II and ADP substrate at a concentration of 0.5 mM. Compounds that caused >50% inhibition were further diluted and tested to establish IC50s. The reaction mixture (0.1 ml) contained 50 mM HEPES-NaOH (pH 7.5), 6 mM magnesium acetate, 0.2 mM ATP (for NTPase-I) or 1 mM ATP (for NTPase-II), 5% DMSO, and 2 ng of NTPase-I (3.2 U) or NTPase-II (0.9 U). The reaction was started by addition of 5 mM DTT, and the mixture was then incubated at 37°C for 10 min and terminated by adding 50 μl of 0.1 M HCl. Inorganic orthophosphate derived from cleavage of ATP was detected colorimetrically with a Fiske & Subbarow reducer (Sigma, St. Louis, Mo.) according to the instructions of the manufacturer. IC50s were determined by graphing NTPase activity versus compound concentration, determining the best-fit curve by linear regression, and calculating the concentration that resulted in 50% inhibition of activity. Regression coefficients were ≥0.88 for all compounds except compound 9, which failed to inhibit the enzymes in a dose-dependent manner.
To determine the inhibition profile, the enzymes were incubated with different concentrations of substrate (0.1 to 1 mM) in the presence or absence of a standard amount of each inhibitor (5 μM), and the mixtures were incubated at 37°C for 10 min. DTT was then added to a concentration of 5 mM to activate the enzyme. Alternatively, mixtures containing substrate, inhibitors, and DTT were incubated for 10 min at 37°C. The reaction was started by adding the enzyme and was continued for 10 min at 37°C. Inhibitory constants (Ki) were calculated, and the values were plotted as double-reciprocal Lineweaver-Burk plots with SigmaPlot 2000 software (SPSS, Inc., Chicago, Ill.).
Measurement of hexokinase activity.
The standard reaction mixture consisted of 0.5 mM glucose, 2 mM ATP, 7 mM MgCl2, 0.2 mM NADP+, 0.005 U of Toxoplasma recombinant hexokinase (T. Saito et al., unpublished data), 0.2 U of yeast glucose-6-phosphate dehydrogenase (type VII; Sigma), and 100 mM Tris-HCl (pH 7.0) in a volume of 1 ml. NADPH formation by dehydrogenation of glucose-6-phosphate was monitored at 340 nm with a UV-1600 spectrophotometer with a TCC-240A temperature-controlled cell holder unit (Schimazu Co., Kyoto, Japan).
Inhibition of in vitro parasite replication.
HFF monolayers grown in 96-well plates were inoculated with 103 tachyzoites of the 2F clone, which expresses β-galactosidase. The compounds were dissolved in DMSO and diluted in DMEM to a final concentration of 1% DMSO. Cultures infected four times were inoculated with either DMSO as a control or diluted compounds, and the mixtures were incubated for 48 h at 37°C. The monolayers were centrifuged at 2,000 ×g for 10 min, the culture supernatants were discarded, and the monolayer and parasites were lysed in phosphate-buffered saline containing 1% Triton X-100. β-Galactosidase activity was quantified with the substrate chlorophenol red β-d-galactopyranoside as described previously (9). Tachyzoite numbers were estimated from a standard curve that related cell numbers to enzyme activity. IC50s were determined by plotting the resulting data, fitting a best-fit curve by linear regression, and calculating the concentration that resulted in 50% inhibition. Values are reported as the means ± standard deviations for three experiments.
RESULTS
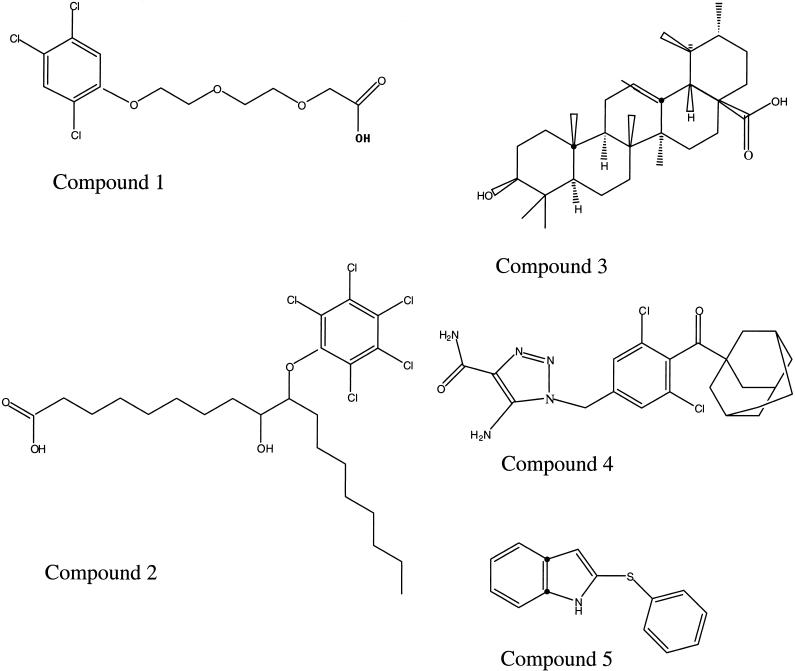
Analysis of a large collection of compounds by robotic screening identified five compounds that inhibited both the NTPase-I and the NTPase-II activities of T. gondii. The molecular structures of these compounds are shown in Fig. 1. The IC50s for the NTPase-I and NTPase-II activities of all compounds except compound 2 were very similar (Table 1). NTPase-I activity was highly sensitive to compound 2, and consequently, the inhibitory concentration was 20 times lower for NTPase-I activity than for NTPase-II activity (Table 1). All five inhibitors of NTPases identified in this study also inhibited parasite (tachyzoite) replication in vitro, with IC50s that ranged from 7 to ≥50 μM (Table 1). These compounds did not influence the activation of NTPases by DTT, and their inhibitory properties were not dependent on the presence of 5% DMSO in the reaction mixture (data not shown). At the concentrations tested, these five compounds did not cause a loss of integrity of the HFF monolayer, based on staining for trypan blue exclusion (≤5% of cells were positively stained).
FIG. 1.
Chemical structures of five species of compounds that where initially identified on the basis of inhibition of NTPase-II activity. The compounds are 2-{2-[2-(2,4,5-trichlorophenoxy)ethoxy]ethoxy}acetic acid (compound 1), 9-hydroxy-10-(pentachlorophenoxy)stearic acid (compound 2), urosolic acid (compound 3), 1H-1,2,3-triazole-4-carboxamide, 5-amino-1-({3,5-dichloro-4-[tricyclo(3.3.1.13,7)dec-1-ylcarbony]phenyl}methyl) (compound 4), and 2-phenylthio-indole (compound 5).
TABLE 1.
Activities of compounds identified by random screening for inhibition of NTPases
| Compound | Molecular mass (Da) | IC50 (μM) for enzyme activitya
|
IC50 (μM) for parasite growtha | |
|---|---|---|---|---|
| NTPase-I | NTPase-II | |||
| 1 | 343 | 1.0 | 0.6 | 21.0 |
| 2 | 1,129 | 0.1 | 2.0 | 29.0 |
| 3 | 456 | 0.6 | 2.0 | ≥50.0b |
| 4 | 448 | 20.0 | 15.0 | 37.0 |
| 5 | 225 | 4.5 | 2.0 | 7.0 |
Values were from a single representative experiment and one based on calculation of the IC50 by linear regression analysis.
No discernible effect at the highest dose tested.
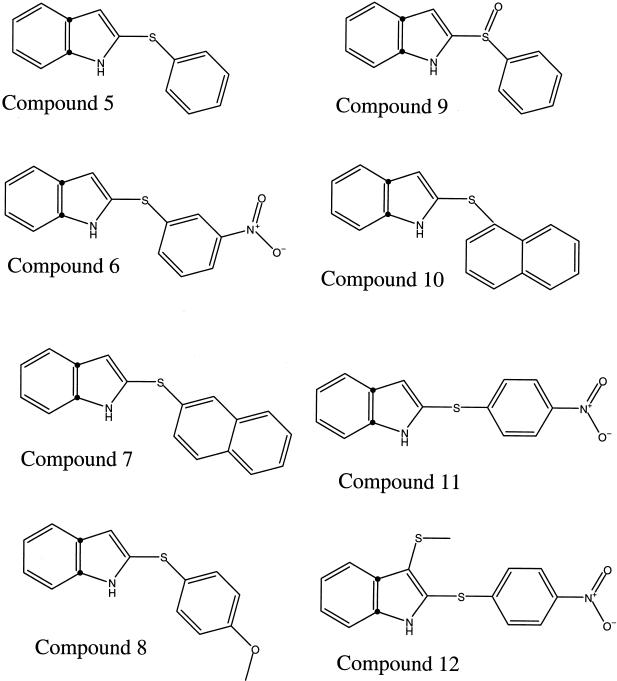
Of the initial five compounds tested, the compound most effective in inhibiting tachyzoite replication was 2-phenylthio-indole (compound 5). Consequently, we tested additional, structurally related indole-containing compounds. The molecular structures of these indole-containing compounds are shown in Fig. 2, and their concentrations for inhibition of NTPase and tachyzoite replication are shown in Table 2. The compound 2-[(3-nitrophenyl)thio]-1H-indole (compound 6), derived from 2-phenylthio-indole by the addition of a negatively charged nitro residue at the C-3 position of the phenol ring, was also highly inhibitory for the NTPases in vitro. However, the inhibitory concentration of compound 6 for tachyzoite replication was more than six times higher than that of 2-phenylthio-indole. Addition of a nonreactive oxygen to the sulfur residue of 2-phenylthio-indole (compound 5) to generate 3-phenylsulfenyl-indole (compound 9) resulted in a complete loss of all activity against both enzyme activity and tachyzoite growth. Addition of a nitro group to the C-4 position of the phenol ring of compound 5 to generate 2-(4-nitrophenylthio)indole (compound 11) reduced the levels of inhibition of NTPase activity and tachyzoite replication. Similarly, placement of a reactive oxygen moiety at this position in the compound 2-[(4-methoxyphenyl)thio]-1H-indole (compound 8) resulted in decreased levels of inhibition of both NTPase activity and tachyzoite replication. Addition of reactive sulfur to the indole ring of compound 11 to generate 3-methylthio-2-(4-nitrophenylthio)indole (compound 12) also decreased the levels of inhibition of enzyme activity and parasite growth.
FIG. 2.
Chemical structures of 2-phenylthio-indole and derivatives. The compounds are 2-phenylthio-indole (compound 5), 2-[(3-nitrophenyl)thio]-1H-indole (compound 6), 2-(2-naphthalenylthio)-1H-indole (compound 7), 2-[(4-methoxyphenyl)thio]-1H-indole (compound 8), 3-phenylsulfenyl indole (compound 9), 2-(1-naphthalenylthio)-1H-indole (compound 10), 2-(4-nitrophenylthio) indole (compound 11), and 3-metylthio-2-(4-nitrophenylthio) indole (compound 12)
TABLE 2.
Activities of indole-containing compounds against NTPase isoforms and T. gondii replication
| Compound | Molecular mass (Da) | IC50 (μM) for enzyme activitya
|
Ki for NTPase-Ib | IC50 (μM) for parasite growthc | |
|---|---|---|---|---|---|
| NTPase-I | NTPase-II | ||||
| 5 | 225 | 3.6 | 1.4 | 1.5 | 7.0 ± 1.8 |
| 6 | 270 | 3.2 | 2.3 | 1.4 | 44.0 ± 5.6 |
| 7 | 275 | 7.2 | 14.5 | 3.1 | 3.6 ± 0.5 |
| 8 | 255 | 26.2 | 26.9 | 11.2 | 17.2 ± 2.2 |
| 9 | 241 | >50.0d | >50.0d | >50d | >50.0d |
| 10 | 275 | 1.3 | 1.3 | 0.6 | 3.2 ± 0.6 |
| 11 | 270 | 23.2 | 17.7 | 10.0 | 13.7 ± 1.2 |
| 12 | 316 | 27.6 | 25.4 | 11.9 | 16.4 ± 0.8 |
Values are based on ATP substrate concentrations of 0.2 mM for NTPase-I and 1 mM for NTPase-II. Values were calculated on the basis of a best-fit curve by linear regression analysis, where r was ≥0.88.
Inhibition profiles for NTPase-I where competitive; hence, Kis differ from the IC50s, whereas the inhibition profile for NTPase-II is noncompetitive and the Kis are equal to the IC50s.
The results of parasite growth assays are the averages of three experiments and are presented as means ± standard deviations.
No discernible effect at the highest does tested.
Replacement of the phenyl residue of 2-phenylthio-indole (compound 5) with a naphthalene residue to generate 2-(1-naphthalenylthio)-1H-indole (compound 10) resulted in slightly increased the levels of inhibition of NTPase activity, and a corresponding increase in the level of inhibition of tachyzoite replication was observed. Changing the position at which the naphthalene residue was attached to indole to generate 2-(2-naphthalenylthio)-1H-indole (compound 7) increased the IC50s for NTPase activity and yet, surprisingly, also increased the antiproliferative effects.
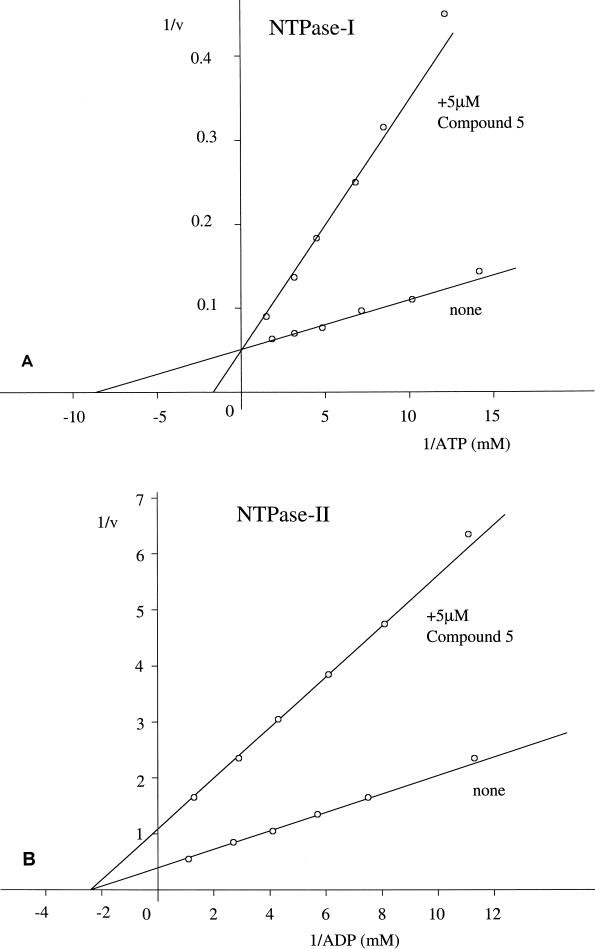
Analysis of the inhibition profiles of the compounds listed in Table 2 revealed a surprising difference between the two enzymes. Inhibition of NTPase-II activity required preincubation with the enzyme prior to activation, while inhibition of NTPase-I activity did not. Additionally, double-reciprocal plots of the inhibition profiles with different substrate concentrations revealed that the inhibition of NTPase-II activity resulted in a change in Vmax but not a change in Km, while inhibition of NTPase-I activity resulted in a change in Km but not a change in Vmax (shown in Fig. 3 for compound 5).
FIG. 3.
Double-reciprocal Lineweaver-Burk plots of the activities of NTPase-I (A) and NTPase-II (B). Activities were determined with various substrate concentrations in the absence (none) or presence (5 μM compound 5) of the inhibitor. The inhibition profile for NTPase-I activity is consistent with a noncompetitive mechanism, while the inhibition profile for NTPase-I activity is consistent with a competitive mechanism.
The activity of an unrelated kinase, hexokinase, that phosphorylates glucose during the initial stages of glycolysis was not affected by the compounds shown in Table 2 at concentrations of 10 or 50 μM (data not shown).
DISCUSSION
We have identified five species of compounds with anti-NTPase activities by robotic screening of approximately 150,000 randomly chosen compounds. These compounds inhibited the activities of both isoforms of T. gondii NTPase in vitro and were also potent inhibitors of parasite replication. The five species of compounds identified in the present study have not previously been reported to be inhibitors of E-type ATPases or other types of ATPases. In addition to the originally identified compounds, examination of several indole-containing compounds related to one of the lead compounds (compound 5) revealed several important features about the structural motifs involved in inhibition of NTPase activity by this class of compound. Further analysis of structure-activity relationships may provide compounds with higher levels of activity that would be useful for addressing the biological roles of NTPases and possibly as therapeutic interventions in patients with toxoplasmosis.
The levels of inhibition of NTPase-I and NTPase-II activities were highly similar for all compounds except compound 2. NTPase-I activity was 20 times more sensitive to compound 2 than NTPase-II activity, suggesting that the chemical structure of compound 2 may mimic substrate recognition differences between NTPase-I and NTPase-II. The activity of NTPase-I is unusual due to its high specific activity for triphosphate nucleosides, and in this regard it behaves less like a classical apyrase than NTPase-II does. Analysis of chimeric constructs between the two isoforms of the T. gondii NTPases implicates a region in the C terminus in the mediation of substrate binding (11). However, the remaining residues involved in substrate binding have not been identified. One surprising finding is that the inhibition profiles of the two enzymes were fundamentally different. While the inhibition of NTPase-I activity was competitive, a noncompetitive profile was observed for NTPase-II activity. This result suggests that while the inhibitors bind competitively with the substrate to NTPase-I, they bind at another site to NTPase-II and indirectly influence its activity. Consequently, the differences in the primary sequences among these enzymes may be involved in molecular interactions other than substrate binding which indirectly influence activity.
Among the five classes of inhibitory compounds identified by random screening, chlorobenzene is a common structure in three of the compounds (compounds 1, 2, and 4). However, as the chlorobenzene of compound 4 is present in an internal position of the structure, the triazole-4-carboxamide group that is externally exposed may be more important for the inhibitory effect of compound 4.
Ursolic acid (compound 3) has previously been identified as an inhibitor of several enzymes, including adenosine deaminase, arachidonate lipoxygenase, aromatase, cyclooxygenase, DNA ligase I, elastase, protein kinases A and C, and RNA-directed DNA polymerase (15). Moreover, ursolic acid has been found to have biological activities that include anti-inflammatory, hepatoprotective, immunomodulatory, and anti-tumor cell proliferative effects (10). The precise mechanisms by which ursolic acid inhibits the activities of these enzyme and exerts biological functions are unknown at present (10). The enzyme reaction of NTPase is quite simple compared to those of the other enzymes listed above; therefore, kinetic studies of the inhibitory effect of ursolic acid on NTPase activity may provide useful information on the mechanism of action.
One of the most effective inhibitors found by random screening was 2-phenylthio- indole (compound 5), and several derivatives of this compound were tested to explore the relationship between structure and activity. Modifications of the indole and phenol rings influenced the effectiveness of these compounds. For example, addition of a nitro group to the C-4 position of the phenol ring or addition of oxygen to the thiol group or to the C-4 position of the phenol ring reduced the level of activity. Addition of a nitro group to the C-3 position of the phenol ring preserved the IC50 for the enzyme in vitro, yet this compound was less effective in blocking parasite replication. This result is not likely to be due simply to reduced levels of entry into the cell, owing to an increased polarity, as compound 11, which has a similar nitro group in a different position, retained its antiparasitic activity.
Substitution of a naphthlene group for the phenol increased the level of activity when it was linked as 2-(1-naphthalenylthio), and this was reflected by greater inhibition of enzyme activities and parasite growth. Somewhat surprisingly, the addition of this group linked as 2-(2-naphthalenylthio) slightly decreased the levels of activity against the enzymes yet resulted in enhanced activity against parasite growth. While the results obtained with these compounds provide some insight into the regions of the molecule important for activity, precise analysis of structure-activity relationships requires additional compounds that are not available in our collection.
The T. gondii NTPases belong to the GDA1/CD39 (Pfam 01150) conserved protein family of ecto ATPases with apyrase activities. Like the other members of this group, the NTPase enzymes contain a rudimentary ATP binding site in the N-terminal portion of the enzyme which is thought to be important in the binding of the β-phosphate group of the substrate (2). The original member of this family, the GDA1 gene of Saccharomyces cerevisiae, is found in the Golgi complex and participates in O- and N-linked glycosylation, while other members of this family are found in plants (in the genera Pisum, Arabidopsis, Solanum, and Glycine), and mammals (in the genera Rattus, Mus, and Homo), where they serve diverse roles. At present no structural data are available for any of the enzymes in this group, and elucidation of such a structure for the NTPase would aid in the interpretation of the differences in the levels of inhibition by the compounds studied here.
The T. gondii NTPases have several features that enhance their potential as therapeutic targets, including (i) the fact that they are unique to the parasite and are not found in the host and (ii) the fact that the enzyme activity appears to be essential for proliferation of the parasite. The experiments reported here indicate that there is a good correlation between the abilities of compounds to inhibit enzyme activity and their abilities to inhibit tachyzoite cell replication, suggesting that the basis of the antiproliferative effect is due to inhibition of NTPase. This conclusion is consistent with those from a previous report that indicated that NTPases play an important role in intracellular replication of the parasite, based on antisense interruption of expression (11). The compounds identified here have modest IC50s in the low micromolar range. While we did not detect any nonspecific inhibition against an unrelated ATPase (hexokinase), we cannot completely rule out the possibility that there are targets in addition to the NTPases within the parasite that are susceptible to these compounds. Nonetheless, these compounds provide useful leads for investigation of the roles of the NTPases in the biology of T. gondii. They may also provide useful leads for further chemical modifications designed to improve their antiparasitic activities while reducing their potential toxicities. The identification of new anti-T. gondii drugs remains an important priority for the patient populations that remain at risk of this opportunistic infection.
Acknowledgments
This work was supported in part by grant-in-aid 13670255 from the Ministry of Education, Culture, Sports, Science, and Technology of Japan and grants from the Project to Promote Development of Anti-HIV Pharmatherapeutics, Japan Health Science Foundation, Health Sciences Research Grants, Research on HIV/AIDS, Ministry of Health, Labour, and Welfare of Japan, and the Promotion of the Advancement of Education and Research in Graduate Schools.
We are grateful to Dennis Schmatz and Marc Feiglin for assistance in conducting the screens with the inhibitors and Helen Profous-Juchelka for assistance with obtaining compounds (Merck Research Laboratories, Inc.).
REFERENCES
- 1.Asai, T., D. K. Howe, K. Nakajima, T. Nozaki, T. Takeuchi, and L. D. Sibley. 1998. Neospora caninum: tachyzoites express a potent type-I nucleoside triphosphate hydrolase, but lack nucleoside diphosphate hydrolase activity. Exp. Parasitol. 90:277-285. [DOI] [PubMed] [Google Scholar]
- 2.Asai, T., S. Miura, L. D. Sibley, H. Okabayashi, and T. Takeuchi. 1995. Biochemical and molecular characterization of nucleoside triphosphate hydrolase isozymes from the parasitic protozoan Toxoplasma gondii. J. Biol. Chem. 270:11391-11397. [DOI] [PubMed] [Google Scholar]
- 3.Asai, T., W. J. O'Sullivan, and M. Tatibana. 1983. A potent nucleoside triphosphate hydrolase from the parasitic protozoan Toxoplasma gondii. J. Biol. Chem. 258:6816-6822. [PubMed] [Google Scholar]
- 4.Beaman, M. H., B. J. Luft, and J. S. Remington. 1992. Assessment of therapy for toxoplasmic encephalitis. Ann. Intern. Med. 117:163-164. [DOI] [PubMed] [Google Scholar]
- 5.Bermudes, D., K. R. Peck, M. A. Afifi, C. J. M. Beckers, and K. A. Joiner. 1994. Tandemly repeated genes encode nucleoside triphosphate hydrolase isoforms secreted into the parasitophorous vacuole of Toxoplasma gondii. J. Biol. Chem. 269:29252-29260. [PubMed] [Google Scholar]
- 6.Brooks, R. G., J. S. Remington, and B. J. Luft. 1987. Drugs used in the treatment of toxoplasmosis, p. 297-306. In P. K. Peterson and J. Verhoef (ed.), The antimicrobial agents manual II. Elsevier/North-Holland Publishing Co., Amsterdam, The Netherlands.
- 7.Dannemann, B., J. A. McCutchan, D. Israelski, et al. 1992. Treatment of toxoplasmic encephalitis in patients with AIDS: a randomized trial comparing pyrimethamine plus clindamycin to pyrimethamine plus sulfonamides. Ann. Intern. Med. 116:33-43. [DOI] [PubMed] [Google Scholar]
- 8.Dubey, J. P., and C. P. Beattie. 1988. Toxoplasmosis of animals and man. CRC Press, Inc., Boca Raton, Fla.
- 9.Eustice, D. C., P. A. Feldman, A. M. Colberg-Poley, R. M. Buckery, and R. H. Neubauer. 1991. A sensitive method for the detection of β-galactosidase in transfected mammalian cells. BioTechniques 11:739-742. [PubMed] [Google Scholar]
- 10.Hollosy, F., G. Meszaros, G. Bokonyi, M. Idei, A. Seprodi, B. Szende, and G. Keri. 2000. Cytostatic, cytotoxic and protein tyrosine kinase inhibitory activity of ursolic acid in A431 human tumor cells. Anticancer Res. 20:4563-4570. [PubMed] [Google Scholar]
- 11.Nakaar, V., B. U. Samuel, E. O. Ngo, and K. A. Joiner. 1999. Targeted reduction of nucleoside triphosphate hydrolase by antisense RNA inhibits Toxoplasma gondii proliferation. J. Biol. Chem. 274:5083-8087. [DOI] [PubMed] [Google Scholar]
- 12.Plesner, L. 1995. Ecto-ATPase: identities and function. Int. Rev. Cytol. 151:141-214. [DOI] [PubMed] [Google Scholar]
- 13.Remington, J. S., R. McLeod, and G. Desmonts. 1995. Toxoplasmosis. In J. S. Remington and J. O. Klein (ed.), Infectious diseases of the fetus and newborn infant. The W. B. Saunders Co., Philadelphia, Pa.
- 14.Sibley, L. D., I. R. Niesman, T. Asai, and T. Takeuchi. 1994. Toxoplasma gondii: secretion of a potent nucleoside triphosphate hydrolase into the parasitophorous vacuole. Exp. Parasitol. 79:301-311. [DOI] [PubMed] [Google Scholar]
- 15.Zollner, H. 1999. Handbook of enyzyme inhibitors. Wiley-VCH, Weinheim, Germany.