Abstract
The synergistic potential of a range of biguanides, their triazine metabolites, tetracyclines, and pyrimethamine in combination with atovaquone has been assessed. All five biguanides tested interacted synergistically with atovaquone against Plasmodium falciparum in vitro. All of the other compounds tested were either additive or antagonistic.
The antimalarial Malarone is a novel combination of the napthoquinone atovaquone plus the biguanide proguanil. This drug combination, which is highly active even against multidrug resistant parasites (14), has recently been introduced into clinical practice for the treatment and prophylaxis of falciparum malaria (9).
Atovaquone was originally developed as an antimalarial monotherapy (2, 5, 18). As early as the 1940s it was recognized that the hydroxynaphthoquinones inhibited mitochondrial respiration (1, 24). Atovaquone and related compounds share structural similarities with ubiquinone or coenzyme Q. Subsequently, it was demonstrated that the drug can selectively inhibit the mitochondrial electron transport chain of protozoa through its action at the bc1 complex (complex III) of the respiratory chain (12). The existence of a functional citric acid cycle in malaria parasites is controversial, and it was originally proposed that the mechanism of action of atovaquone was due to inhibition of pyrimidine biosynthesis at the level of dihydroorotate dehydrogenase, an enzyme requiring a functional electron transport chain (13). Recent studies have demonstrated that at therapeutically relevant drug concentrations, atovaquone results in the depolarization of malarial mitochondrial membranes. It is unclear if this is a primary drug effect or the consequence of a related action. However the loss of mitochondrial membrane potential will cause wide-ranging disruption of essential parasite processes, resulting in cellular damage and cell death (22).
Despite this novel mechanism of action, initial clinical studies with atovaquone monotherapy resulted in unacceptable failure rates (8, 15, 16). Moreover, analysis of paired parasites from recrudescing infections demonstrated a >1,000-fold reduction in in vitro sensitivity compared to that of the parasites at the start of treatment (15). This rapid development and selection of resistance to atovaquone monotherapy prompted the search for a partner compound with the potential to reduce resistance selection. The result of this search was the identification of proguanil as a drug that interacted synergistically with atovaquone in vitro (6).
The biguanide proguanil is a well-established antimalarial prodrug which has been in clinical use for more than 50 years. The conventional view is that proguanil is metabolically converted to the cyclic metabolite cycloguanil, a potent inhibitor of parasite dihydrofolate reductase (7). Thus, through the action of this cyclic metabolite, proguanil inhibits folate biosynthesis. Proguanil itself has very weak inherent antimalarial activity (several-thousandfold weaker than cycloguanil). It rapidly became apparent that the synergy between proguanil and atovaquone was totally independent from the activity of its cyclic metabolite and inhibition of dihydrofolate reductase. A recent study suggested that proguanil exerts its effects, directly or indirectly, by sensitizing the parasite to the mitochondrial depolarizing effects of atovaquone without affecting mitochondrial electron transport (23).
In this study we investigated the potential for a range of biguanides, their cyclic metabolites, and related structures to synergize with atovaquone in vitro. In particular, we were interested in establishing whether the sensitizing effects of proguanil were specific to this drug or a class effect.
Proguanil, cycloguanil, chlorproguanil, and chlorcycloguanil were gifts from AstraZeneca (Macclesfield, United Kingdom). WR99210 was a gift from SmithKline Beecham (Brentford, United Kingdom). Clociguanil, WR158122, and WR159412 were gifts from the Walter Reed Army Institute of Research (Washington, D.C.). Atovaquone was a gift from the Wellcome Foundation. PS-15 was a gift from Jacobus Pharmaceuticals. Pyrimethamine, metformin, phenformin, tetracycline, and doxycycline were purchased from Sigma Chemicals (Poole, United Kingdom). Synthalin was purchased from Tocris Cookson Ltd. (Avonmouth, United Kingdom).
Parasites were maintained in continuous culture in RPMI 1640 medium supplemented with 25 mM HEPES, 25 mM bicarbonate, and 10% human serum of the AB type. Parasitemia was monitored daily by staining thin blood smears with Giemsa stain. Ring-stage parasites were maintained at a maximum parasitemia level of 20%, and trophozoite-stage parasites were maintained at a maximum parasitemia level of 3%. In vitro drug sensitivities were assessed using the method of Desjardins (10). This is a microdilution technique measuring radiolabeled hypoxanthine incorporation. Drug stocks were serially diluted and added to a microtiter plate with parasites and uninfected erythrocytes to give a final hematocrit of 1%. Parasites were incubated (37°C, 4% CO2) for 24 h before the addition of [3H]hypoxanthine (1 μCi) and incubation for a further 24 h. The antimalarial activity of each compound was tested against the K1 isolate of Plasmodium falciparum. A drug's 50% inhibitory concentration (IC50) was determined as the concentration of drug required to inhibit 50% of the [3H]hypoxanthine uptake relative to that of control parasites. Based on these initial data, the interaction between atovaquone and each of the remaining compounds was determined by isobologram analysis as described by Berenbaum (3, 4) and Bloland et al. (5).
Briefly, fixed-ratio drug combinations of drug A to drug B (1:0, 9:1, 7:3, 5:5, 3:7, 1:9, and 0:1) were prepared such that the IC50 for each drug when used alone would fall around the fourth serial dilution. Each fixed-ratio combination was then serially diluted down the microtiter plate, and parasite sensitivity was determined as described above. The fractional inhibitory concentration (FIC) of each drug was determined (FIC of drug A [IC50 of drug A in combination/IC50 of drug A alone] and FIC of drug B [IC50 of drug B in combination/IC50 of drug B alone]), and the results were plotted as an isobol.
The inherent antimalarial activities of all the compounds studied against the K1 parasite isolate are shown in Table 1 (data are the means and standard deviations of results from six separate sensitivity assays). The data are consistent with earlier reports. As established previously, atovaquone exhibits activity in the low nanomolar range compared to proguanil, which is active only in the micromolar range. The poor susceptibility to pyrimethamine is typical of a dihydrofolate reductase inhibitor-resistant isolate such as K1, which carries mutations at positions 59 (arginine to cysteine) and 108 (asparagine to threonine) (21).
TABLE 1.
In vitro antimalarial activities of drugs tested
| Class | Drug | Mean IC50 ± SDa |
|---|---|---|
| Atovaquone | 0.9 nM ± 0.1 | |
| Pyrimethamine | 26.6 μM ± 1.7 | |
| Biguanides | Synthalin | 15.9 nM ± 3.0 |
| PS-15 | 6.7 μM ± 0.9 | |
| Chlorproguanil | 8.6 μM ± 1.0 | |
| Proguanil | 36.2 μM ± 10.8 | |
| Phenformin | 0.1 mM ± 0.02 | |
| Triazines | WR99210 | 0.2 nM ± 0.1 |
| Chlorcycloguanil | 1.6 μM ± 0.4 | |
| Cycloguanil | 3.7 μM ± 1.0 | |
| Dihydrotriazines | Clociguanil | 0.1 μM ± 0.08 |
| WR159412 | 0.4 μM ± 0.07 | |
| WR158122 | 1.0 μM ± 0.3 | |
| Tetracyclines | Doxycycline | 18.1 μM ± 3.4 |
| Tetracycline | 0.1 mM ± 0.02 |
The results shown are the means ± standard deviations of results of six separate experiments.
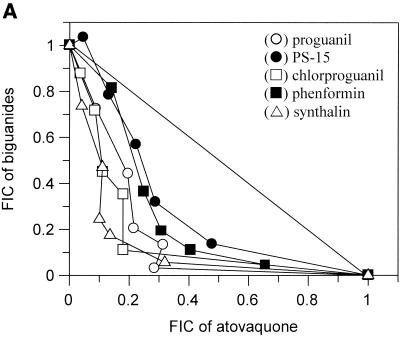
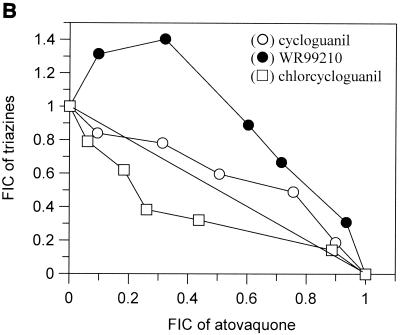
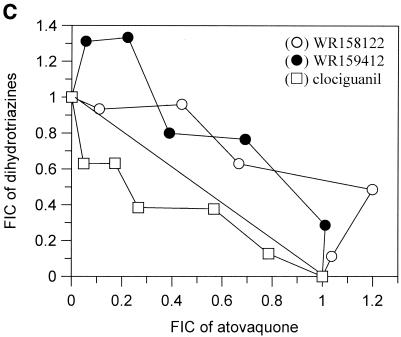
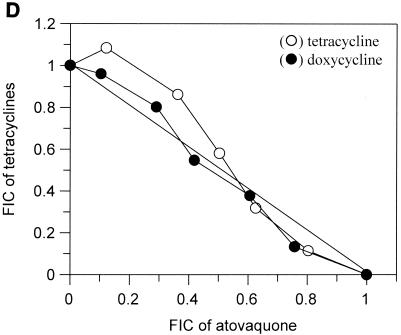
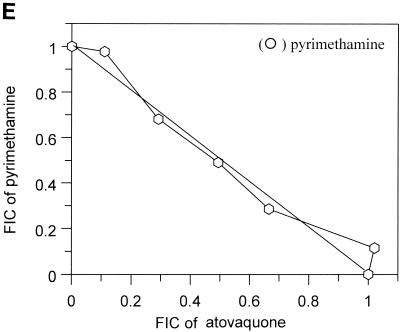
The interactions of atovaquone with five classes of potential antimalarial partners are presented in Fig. 1. All of the biguanides tested (i.e., proguanil, chlorproguanil, PS-15, synthalin, and metformin) demonstrated clear and marked synergy with atovaquone (Fig. 1A). In contrast, the cyclic metabolites derived from the biguanides in vivo were not synergistic in the cases of cycloguanil and chlorcycloguanil and were antagonistic in the case of WR99210 (Fig. 1B). The dihydrotriazines (Fig. 1C), tetracyclines (Fig. 1D), and pyrimethamine (Fig. 1E) did not display any synergy with atovaquone; in fact, WR158122 and WR159412 appeared to be antagonistic.
FIG. 1.
Antimalarial activities of atovaquone in combination with proguanil (○), PS-15 (•), chlorproguanil (□), phenformin (▪), or synthalin (▵) (A); cycloguanil (○), WR99210 (•), or chlorcycloguanil (□) (B); WR158122 (○), WR159412 (•), or clociguanil (□) (C); tetracycline (○) or doxycycline (•) (D); and pyrimethamine (○) (E). Data are plotted as FICs. Each isobol experiment was performed on six separate occasions. Data presented for each drug combination are from a single representative experiment.
The primary aim of this study was to establish whether the synergy observed between the combination of proguanil and atovaquone was a function of the biguanide class of compounds or a feature unique to proguanil. All of the biguanides tested here showed a clear synergistic action when they were used in combination with atovaquone against the K1 strain of P. falciparum. This observation confirms that synergy with atovaquone is a class effect.
The results obtained with pyrimethamine or the triazines in combination with atovaquone clearly demonstrate that this synergy is unrelated to any effect on the folate pathway per se. As described earlier, atovaquone binds to the bc1 complex in parasite mitochondria, inhibiting electron transport. This inhibition results in parasite death possibly by preventing pyrimidine synthesis (20) or by depolarizing mitochondrial membrane potential, resulting in cellular damage and death (13).
The biguanides are an old class of compounds which were originally used for the treatment of non-insulin-dependent patients with diabetes mellitus. The most extensively used drugs were phenformin and synthalin, which were eventually withdrawn from use in several countries due to unacceptable toxicity in the form of lacticacidosis, making them unsuitable for long-term use (19). It is clear from early research in the antidiabetic field that the biguanides had effects on mammalian mitochondria. The positively charged biguanides accumulate within mitochondria driven by the mitochondrial membrane potential (17). It is unclear how this accumulation results in loss of normal mitochondrial function, but an interference with ion homeostasis seems a likely explanation. Although the mitochondria of malarial parasites are poorly understood, they share many similarities with mammalian mitochondria (20), and the maintenance of a transmembrane electrical potential is essential to viability (11). Based on this information, a role for the parasite mitochondria in the synergy of buiguanide and atovaquone appears logical. In support of this, Srivastava et al. (23) have demonstrated that proguanil enhances the atovaquone-mediated collapse of a parasite's mitochondrial membrane potential at micromolar concentrations. Proguanil had no effect on membrane potential when it was used alone. The molecular basis for this effect is still unresolved.
REFERENCES
- 1.Ball, E. G., C. B. Anfinsen, and O. Cooper. 1947. The inhibitory action of naphthoquinones on respiratory processes. J. Biol. Chem. 169:406-407. [PubMed] [Google Scholar]
- 2.Basco, L. K., P. E. Pecoulas, C. M. Wilson, J. Le Bras, and A. Mazabraud. 1995. Point mutations in the dihydrfolate reductase-thymidylate synthase gene and pyrimethamine and cycloguanil resistance in Plasmodium falciparum. Mol. Biochem. Parasitol. 69:135-138. [DOI] [PubMed] [Google Scholar]
- 3.Berenbaum, M. C. 1978. A method for testing synergy with any number of agents. J. Infect. Dis. 137: 122-130. [DOI] [PubMed] [Google Scholar]
- 4.Berenbaum, M. C. 1989. What is synergy? Pharmacol. Rev. 41:93-141. [PubMed] [Google Scholar]
- 5.Bloland, P. B., P. N. Kazembe, W. M. Watkins, O. K. Doumbo, O. C. Nwanyanwu, and T. K. Ruebush. 1997. Malarone donation programme in Africa. Lancet 350:1624-1625. [DOI] [PubMed] [Google Scholar]
- 6.Canfield, C. J., M. Pudney, and W. E. Gutteridge. 1995. Interactions of atovaquone with other antimalarial drugs against Plasmodium falciparum in vitro. Exp. Parasitol. 80:373-381. [DOI] [PubMed] [Google Scholar]
- 7.Carrington, H. C., A. F. Crowther, D. G. Davey, A. A. Levi, and F. L. Rose. 1951. A metabolite of “Paludrine” with high antimalarial activity. Nature 168:1080.. [DOI] [PubMed] [Google Scholar]
- 8.Chiodini, P. L., C. P. Conlon, D. B. Hutchinson, J. A. Farquhar, A. P. Hall, T. E. A. Peto, H. Birley, and D. A. Warrell. 1995. Evaluation of atovaquone in the treatment of patients with uncomplicated Plasmodium falciparum malaria. J. Antimicrob. Chemother. 36:1073-1078. [DOI] [PubMed] [Google Scholar]
- 9.De Alencar, F. E. C., C. Cerutti, R. R. Durlacher, M. Boulos, F. P. Alves, W. Milhous, and W. Pang. 1997. Atovaquone and proguanil for the treatment of malaria in Brazil. J. Infect. Dis. 175: 1544-1547. [DOI] [PubMed] [Google Scholar]
- 10.Desjardins, R. E., C. J. Canfield, J. D. Haynes, and J. D. Chulay. 1979. Quantitative assessment of antimalarial activity in vitro by a semiautomated microdilution technique. Antimicrob. Agents Chemother. 16:710-718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Divo, A. A., T. G. Geary, J. B. Jensen, and H. Ginsburg. 1985. The mitochondrion of Plasmodium falciparum visualised by rhodamine 123 fluorescence. J. Protozool. 32:442-446. [DOI] [PubMed] [Google Scholar]
- 12.Fry, M., and M. Pudney. 1992. Site of action of the antimalarial hydroxynaphthoquinone 2-[trans-4-(4′-chlorophenyl) cyclohexyl]-3-hydroxy-1,4-naphthoquinone (566C80). Biochem. Pharmacol. 43:1545-1553. [DOI] [PubMed] [Google Scholar]
- 13.Gutteridge, W. E., D. Dave, and W. H. G. Richards. 1979. Conversion of dihydroorotate to orotate in parasitic protozoa. Biochim. Biophys. Acta 582:390-401. [DOI] [PubMed] [Google Scholar]
- 14.Hudson, A. T., M. Dickins, C. D. Ginger, W. E. Gutteridge, T. Holdich, D. B. Hutchinson, M. Pudney, A. W. Randall, and V. S. Latter. 1991. 566C80: a broad spectrum anti-infective agent with activity against malaria and opportunistic infections in AIDS patients. Drugs Exp. Clin. Res. 17:427-435. [PubMed] [Google Scholar]
- 15.Looareesuwan, S., C. Viravan, H. K. Webster, D. E. Kyle, D. B. Hutchinson, and C. J. Canfield. 1996. Clinical studies of atovaquone, alone or in combination with other antimalarial drugs for treatment of acute uncomplicated malaria in Thailand. Am. J. Trop. Med. Hyg. 54:62-66. [DOI] [PubMed] [Google Scholar]
- 16.Looaresuwan, S., P. Wilairatana, K. Chalermarut, Y. Rattanapong, C. J. Canfield, and D. B. A. Hutchinson. 1999. Efficacy and safety of atovaquone/proguanil compared with mefloquine for treatment of acute Plasmodium falciparum malaria in Thailand. Am. J. Trop. Med. Hyg. 60:526-532. [DOI] [PubMed] [Google Scholar]
- 17.Owen, M. R., E. Doran, and A. P. Halestrap. 2000. Evidence that metformin exerts its anti-diabetic effects through inhibition of complex I of the mitochondrial respiratory chain. Biochem. J. 15:607-614. [PMC free article] [PubMed] [Google Scholar]
- 18.Radloff, P. D., J. Phillips, M. Nkeyi, D. Hutchinson, and P. D. Kremsner. 1996. Atovaquone and proguanil for Plasmodium falciparum malaria. Lancet 347:1511-1514. [DOI] [PubMed] [Google Scholar]
- 19.Schafer, G. 1983. Biguanides. A review of history, pharmacodynamics and therapy. Diabete Metab. 9:148-163. [PubMed] [Google Scholar]
- 20.Sherman, I. W. 1979. Biochemistry of Plasmodium (malarial parasites). Microbiol. Rev. 43:453-495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Snewin, V. A., S. M. England, P. F. Sims, and J. E. Hyde. 1989. Characterisation of the dihydrofolate reductase-thymidylate synthetase gene from human malaria parasites highly resistant to pyrimethamine. Gene 76:41-52. [DOI] [PubMed] [Google Scholar]
- 22.Srivastava, I. K., H. Rottenberg, and A. B. Vaidya. 1997. Atovaquone a broad spectrum antiparasitic drug collapses mitochondrial membrane potential in a malaria parasite. J. Biol. Chem. 272:3961-3966. [DOI] [PubMed] [Google Scholar]
- 23.Srivastava, L. K., and A. B. Vaidya. 1999. A mechanism for the synergistic antimalarial action of atovaquone and proguanil. Antimicrob. Agents Chemother. 43:1334-1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wendle, W. B. 1946. The influence of naphthoquinones upon the respiratory and carbohydrate metabolism of malarial parasites. Fed. Proc. 5:406-407. [PubMed] [Google Scholar]