Abstract
In this study, the safety, tolerability, and pharmacokinetics of intravenous (i.v.)- to oral-dose regimens of voriconazole were evaluated with a group of 42 healthy men, 41 of whom completed the study. Two cohorts of subjects participated in the study. Cohort 1 (n = 28) took part in two study periods, each consisting of 14 days separated by a minimum 7-day washout. In one of the periods, 14 subjects received 6 mg/kg i.v. twice a day (b.i.d.) on day 1 followed by 3 mg/kg i.v. b.i.d. on days 2 to 7 and were then switched to 200 mg orally b.i.d. for days 8 to 14. In the other period, subjects received 6 mg/kg i.v. b.i.d. on day 1 followed by 5 mg/kg i.v. b.i.d. on days 2 to 7and were then switched to 400 mg orally b.i.d. for days 8 to 14. The remaining 14 subjects in cohort 1 received a matching placebo throughout the study. In cohort 2 (n = 14), 7 subjects received 6 mg/kg i.v. b.i.d. on day 1 followed by 4 mg/kg i.v. b.i.d. on days 2 to 7 and were then switched to 300 mg orally b.i.d. for days 8 to 14. The remaining seven subjects in cohort 2 received a matching placebo. Blood samples were taken prior to dosing on days 1 to 6 and on days 8 to 13. Blood samples were drawn prior to dosing and at frequent intervals up to 12 h following the morning dose on days 7 and 14 of each study period. The samples were assayed for voriconazole by a high-performance liquid chromatography method. The maximum concentration in plasma (Cmax) occurred at the end of the 1-h i.v. infusion and between 1.4 and 1.8 h after oral administration. Voriconazole exhibited nonlinear pharmacokinetics, possibly due to saturable metabolism. For cohort 1, both Cmax and the area under the concentration-time curve within a dosage interval (AUCτ) increased disproportionately with dose for both i.v. and oral dosing. For i.v. dosing, a 1.7-fold increase in dose resulted in 2.4- and 3.1-fold increases in Cmax and AUCτ, respectively. Similarly, a 2-fold increase in oral dosing resulted in 2.8- and 3.9-fold increases in Cmax and AUCτ, respectively. The mean values for Cmax observed following oral dosing were lower than those obtained after i.v. administration, ranging from 62.7 to 89.6% of the i.v. value. After the switch from i.v. to oral dosing, most subjects achieved steady state by day 4, and mean minimum concentrations in plasma remained above clinically important MICs. The pharmacokinetic profiles for saliva followed a pattern similar to those observed for plasma; there was a highly significant correlation between plasma and saliva voriconazole concentrations (P < 0.0001). Voriconazole was well tolerated; the most commonly reported adverse events in voriconazole-treated subjects were mild to moderate headache, rash, and abnormal vision. Visual function tests detected no further abnormalities during voriconazole treatment.
Voriconazole is a novel antifungal agent that is a derivative of fluconazole, having one triazole moiety replaced by a fluoropyrimidine grouping and a methyl group added to the propanol backbone (K. Richardson, A. S. Bell, R. P. Dickinson, S. Narayanaswami, and S. J. Ray, Abstr. 35th Intersci. Conf. Antimicrob. Agents Chemother., abstr. F69, p. 125, 1995). This change in structure results in a highly selective action against the ergosterol biosynthetic pathway of numerous fungi (1, 2, 3, 6, 9, 11; F. Barchiesi, M. Restrepo, D. A. McGough, and M. G. Rinaldi, Abstr. 35th Intersci. Conf. Antimicrob. Agents Chemother., abstr. F71, p. 125, 1995). In vitro voriconazole exhibits 1.6- and 160-fold greater inhibition of ergosterol P-450-dependent 14α-demethylase than fluconazole in Candida albicans and Aspergillus fumigatus lysates, respectively (C. A. Hitchcock, G. P. Oliver, G. W. Pye, and P. F. Troke, Abstr. 35th Intersci. Conf. Antimicrob. Agents Chemother., abstr. F72, p. 125, 1995).
In vitro investigations have demonstrated that voriconazole exhibits potent, broad-spectrum activity against clinically important fungi, such as Candida spp. (1, 10), Aspergillus spp. (7; C. J. Clancy, C. Y. Yu, and M. H. Nguyen, Abstr. 37th Intersci. Conf. Antimicrob. Agents Chemother., abstr. E-88, 1997), and Cryptococcus neoformans (8; C. J. Clancy and M. H. Nguyen, Abstr. 13th Congr. Int. Soc. Hum. Anim. Mycol., abstr. P477, 1997), and dimorphic fungi, such as Blastomyces dermatitidis, Coccidioides immitis, and Histoplasma capsulatum (6). This activity also extends to emerging and less common mold pathogens, including several species of Fusarium and Penicillium marneffei (9).
The clinical pharmacokinetics of voriconazole have been studied in over 2,000 subjects following single- and multiple-dose oral and intravenous (i.v.) dosing regimens (5; B. E. Patterson and P. E. Coates, Abstr. 35th Intersci. Conf. Antimicrob. Agents Chemother., abstr. F78, p. 126, 1995; B. E. Patterson, S. Roffey, S. G. Jezequel, and B. Jones, Abstr. 35th Intersci. Conf. Antimicrob. Agents Chemother., abstr. F79, p. 126, 1995). Voriconazole is rapidly and almost completely absorbed following oral administration, with a Tmax (time to the first occurrence of the maximum observed concentration in plasma [Cmax]) of less than 2 h. The bioavailability of voriconazole is estimated to be greater than 90%. Voriconazole exhibits nonlinear pharmacokinetics, possibly due to saturable first-pass metabolism and systemic clearance. Dose-dependent accumulation (up to eightfold) and decreased systemic clearance are observed with administration of multiple doses of voriconazole. The elimination half-life of voriconazole is dose dependent and is approximately 6 to 9 h at 3 mg/kg i.v. or 200 mg orally. Voriconazole has a plasma protein binding level of 58%, which is independent of the concentration ranges observed therapeutically.
Although the clinical pharmacokinetics of voriconazole following both oral and i.v. dosing have been well characterized, it is expected that many hospitalized patients will require treatment with an i.v. regimen initially and will then be switched to an oral regimen when they are able, to maintain plasma voriconazole concentrations comparable to those achieved following i.v. administration. Therefore, in this study we evaluated the safety, tolerability, and pharmacokinetic profiles of three i.v.- to oral-dose regimens of voriconazole, each consisting of a loading dose, followed by 6 days of i.v. maintenance, followed by 7 days of oral maintenance, in healthy male volunteers.
MATERIALS AND METHODS
Study subjects.
A total of 42 healthy male subjects were screened and enrolled following the provision of written informed consent. All subjects were age 18 to 43 years old, weighed 60 to 93 kg, and were within the permitted weight range for their height and frame according to Quetelet's index (18 to 28 kg/m2).
Volunteers were excluded if evidence of clinically significant disease, laboratory test abnormality, or allergy (especially drug sensitivity) was observed at screening. In addition, volunteers were excluded if they had received any prescribed or over-the-counter drug (excluding paracetamol at ≤3 g/day) in the 3 weeks before the start of the study or any experimental drug in the 4 months before the study (determined by questioning the subject); showed evidence of drug abuse (determined by a urine test for drugs of abuse) or alcohol abuse; smoked >5 cigarettes or the equivalent per day; intended to donate blood or blood products immediately before, during, or for 1 month after the study; were human immunodeficiency virus or HBsAg positive; or had a history of clinically significant visual impairment (e.g., visual field abnormality, glaucoma, cataract, retinitis pigmentosa, or color blindness).
Study design.
This randomized, placebo-controlled, parallel-group, double-blind i.v. escalation and i.v.-to-oral switchover study received ethics committee approval and was conducted according to the revised declaration of Helsinki (Hong Kong, 1989).
Subjects who had given written informed consent were enrolled in the study and were allocated to one of two cohorts: 28 subjects were in cohort 1 and a further 14 subjects were in cohort 2 (Table 1).
TABLE 1.
Dosing regimens
| Cohorta (n) | Periodb | Dose on day:
|
||
|---|---|---|---|---|
| 1 (i.v. loading) | 2-7 (i.v. maintenance) | 8-14 (oral maintenance) | ||
| 1 (28) | 1 | 6 mg/kg b.i.d. | 3 mg/kg b.i.d. | 200 mg b.i.d. (days 8-13), 200 mg o.d.c (day 14) |
| 2 | 6 mg/kg b.i.d. | 5 mg/kg b.i.d. | 400 mg b.i.d. (days 8-13), 400 mg o.d. (day 14) | |
| 2 (14) | 6 mg/kg b.i.d. | 4 mg/kg b.i.d. | 300 mg b.i.d. (days 8-13), 300 mg o.d. (day 14) | |
Subjects received either active drug or matched placebo in the regimens shown (active/placebo ratio, 1:1) throughout the study.
For cohort 1, there was a washout period of at least 7 days between periods 1 and 2.
o.d., once a day.
Subjects in cohort 1 took part in two 14-day study periods, involving 15 consecutive overnight stays, separated by a minimum 7-day washout period. Fourteen subjects were randomized to active treatment, which consisted of an i.v. loading dose of voriconazole (6 mg/kg twice a day [b.i.d.]) on day 1 followed by i.v. maintenance (3 mg/kg b.i.d.) on days 2 to 7. Subjects were then switched to an oral maintenance regimen (200 mg b.i.d.) on days 8 to 14. After a minimum 7-day washout, the dosing regimen was repeated on days 21 to 34 with higher maintenance doses of voriconazole (5 mg/kg b.i.d. i.v. and then 400 mg b.i.d. orally). The remaining 14 subjects received a placebo during both 14-day study periods.
Fourteen subjects were enrolled in cohort 2 and took part in one 14-day study period. Seven subjects received a placebo and seven received intermediate maintenance doses of voriconazole (4 mg/kg b.i.d. i.v. and then 300 mg b.i.d. orally).
All i.v. doses were infused from a minibag over a 1-h period with an infusion pump.
Pharmacokinetic sampling.
Venous blood samples (sufficient to provide 2 ml of plasma) were collected into heparinized tubes. On days 1 to 6 these samples were collected immediately before (predose) and immediately prior to the end of the morning infusion. On day 7, plasma samples were taken predose and at frequent intervals for up to 12 h following the start of infusion. On days 8 to 13, samples were taken prior to the morning dose only. On day 14, plasma samples were taken predose and at frequent intervals for up to 12 h following oral administration.
Blood samples were centrifuged at 1,500 × g for 10 min at 4°C within 60 min of collection. Prior to analysis, the plasma samples were stored upright in screw-cap polypropylene tubes at −20°C.
On days 7 and 14 of each study period, a 2-ml sample of saliva was collected at the same time points as the day 7 and 14 plasma pharmacokinetic samples. Saliva flow was stimulated using polytetrafluoroethylene tape. Saliva samples were stored in 30-ml polystyrene universal tubes at −20°C.
Voriconazole assay.
Plasma samples were assayed using a previously validated high-performance liquid chromatography assay (Huntingdon Life Sciences, Cambridge, United Kingdom). The interbatch imprecision and inaccuracy of the plasma assay, as assessed from the coefficients of variation of the quality control samples analyzed during the test sample analysis, were in the ranges of 3.3 to 13.1% and −2.3 to 7.9%, respectively, over the concentration range of 25 to 2,500 ng/ml. The lower limit of quantification was 10 ng/ml, with imprecision and inaccuracy at this level, determined from the back-calculated concentrations of calibration standards, of 1.0 and 0.2%, respectively.
Saliva samples underwent an assay procedure employing liquid extraction and separation by high-performance liquid chromatography and UV detection (Scotlab Analytical, Lanarkshire, United Kingdom). During the study, the imprecisions for the overall method were 3.54, 4.09, and 4.86% for voriconazole at concentrations of 1,500, 500, and 75 ng/ml, respectively. The inaccuracies at these concentrations ranged from −1.60 to 1.80%. The lower limit of quantification was 10 ng/ml, and the upper limit of the calibration curve was 2,000 ng/ml.
Study drugs.
Voriconazole was supplied as a 10-mg/ml infusate in sulfobutyl ether-β-cyclodextrin sodium salt for a 1-h i.v. infusion and as 50- and 200-mg capsules for oral administration. Sulfobutyl ether-β-cyclodextrin sodium salt infusion and matching capsules (containing no active excipient) were used as placebos for the i.v. and oral arms of the study, respectively.
Safety assessments.
All adverse events that occurred during treatment or up to 7 days after treatment were coded according to the Coding Symbols for Thesaurus of Adverse Reaction Terms dictionary. Where possible, information on the severity, time of onset, and duration of adverse events was recorded together with the investigator's assessment of their relationship to treatment. Investigators were also requested to report any serious adverse events occurring up to 30 days after the end of the study.
The following tests were used to assess visual disturbance: City Universal Color Test (2nd ed.) (color vision), Snellen chart (visual acuity), funduscopy (exudates, hemorrhages, or microaneurysms), and a visual disturbance questionnaire. In addition, standard clinical testing methods were used to assess visual fields and eye reflexes. Visual disturbance tests were carried out at 1 day before dosing, 2 h after the start of the morning infusion on day 1, 1 h after the start of the morning infusion on day 7, 1 h after the morning dose on days 8 and 14, predischarge on day 15, and at a follow-up visit which was 7 to 10 days after the final dose of treatment. The predose screening tests for the oral dosing period were performed on a separate day from the follow-up visit for the i.v. period.
Routine clinical laboratory, hematology, and clinical chemistry tests were performed on samples taken at screening, on days 1 and 7 of each study period before the morning dose, and at the follow-up visit. An additional blood sample was taken for liver function tests prior to the morning dose on days 6 and 11 of each study period.
Samples for urinalysis were collected during the 24-h periods between screening and the first dosing day; prior to the morning dose on days 1, 7, and 14 of each study period; and 24 h before the follow-up visit.
Measurements of vital signs (pulse rate and supine and standing systolic and diastolic blood pressure) and a 12-lead electrocardiogram were performed once in the 3 weeks prior to the start of the study and once again during the 7 to 10 days following the final dose of treatment.
Pharmacokinetic parameters.
The Cmax, the predose plasma concentration (trough), and the Tmax were obtained directly from the plasma concentration- time curves on days 7 and 14 of each treatment period. The area under the concentration-time curve within a dosage interval (AUCτ) was calculated by the linear trapezoidal rule. The clearance of voriconazole for the fraction of the dose absorbed (CL/F) was calculated as the dose/AUCτ ratio. The terminal-phase elimination rate constant (kel) was estimated from the terminal phase of the plasma concentration-time curve by using log-linear regression. The volume of distribution based on the terminal phase for the fraction of the dose absorbed (V/F) was estimated as dose/(kel · AUC0-∞). The volume of distribution at steady state (Vss/F) was calculated as Vss/F = dose · (AUCτ + τAUC0-∞)/AUC0-τ2, where AUCτ-∞ is the area under the curve extrapolated from the end of dosing interval to infinity.
Individual concentrations of voriconazole in plasma and saliva were listed for each cohort and dosing regimen, and summary statistics are presented. Individual profiles of voriconazole concentrations in plasma and saliva against time postdose were plotted for i.v. and oral dosing in each cohort. Similarly, mean concentrations in plasma and saliva against time postdose were plotted for i.v. and oral dosing separately. For each cohort and each period, trough concentrations were plotted for the i.v. maintenance dosing followed by the oral maintenance dosing as a continuation of the same plot.
Statistical analysis.
To compare the pharmacokinetics of voriconazole following i.v. and oral dosing regimens within each cohort, natural log-transformed Cmax and AUCτ values and untransformed Tmax, Vss/F, V/F, CL, and kel values were subjected to an analysis of variance procedure, which allowed for variation due to subject and treatment. For these comparisons the differences between the mean values for each dose were estimated together with the associated standard errors and 95% confidence intervals (CIs). The relationship between concentrations in saliva and plasma was investigated using regression techniques for each dose regimen separately. The model used was y = α + βx, where y is the concentration in plasma, x is the concentration in saliva, α is the intercept of the fitted line on the axis, and β is the slope of the fitted line. Plots of the concentrations in plasma versus those in saliva indicated that the variation in the data increased with increasing concentration. To reduce this variation, the concentrations were log transformed prior to analysis.
The sample size was estimated based on pharmacokinetic data obtained from previous studies using i.v.- and oral-dose regimens. The calculations indicated that 12 subjects were needed to provide sufficient degrees of freedom to detect a ratio of 85% (compared to 100%) between the oral and i.v. doses with 80% power. Therefore, 28 subjects were entered in cohort 1 (14 on placebo and 14 on voriconazole), with the intention that 12 subjects in each group would complete the pharmacokinetic assessments. No sample size calculation was performed for cohort 2. All analyses and tabulations were performed using SAS (12).
RESULTS
Patient demographics.
A total of 28 subjects were enrolled in cohort 1 (14 on voriconazole and 14 on placebo), and 14 subjects were enrolled in cohort 2 (7 on voriconazole and 7 on placebo). All subjects completed the study except for one individual who withdrew from cohort 1 in period 2 as a result of a laboratory abnormality (elevated liver function test). All subjects were included in the analysis of pharmacokinetics and safety. All available pharmacokinetic data from the withdrawn subject were included in the analysis.
The demographic characteristics of subjects are summarized in Table 2. All subjects were male, with ages and body weights ranging from 18 to 43 years and 60 to 93 kg, respectively. There was no significant difference in age and weight among the four dosing groups. With the exception of one subject who had a visual field abnormality identified at screening (but who was not excluded from the study), none of the subjects had a history of visual disturbances.
TABLE 2.
Demographic characteristics
| Cohort | Treatment | No. of subjects | Mean (SE):
|
||
|---|---|---|---|---|---|
| Age (yr) | Wt (kg) | Ht (cm) | |||
| 1 | Voriconazole | 14 | 26.5 (1.48) | 78.7 (1.93) | 179.5 (1.88) |
| Placebo | 14 | 24.8 (1.08) | 74.2 (2.29) | 180.5 (1.95) | |
| 2 | Voriconazole | 7 | 24.7 (2.37) | 73.2 (2.12) | 180.9 (1.78) |
| Placebo | 7 | 23.3 (1.54) | 72.4 (4.37) | 176.9 (2.32) | |
Pharmacokinetics.
Mean pharmacokinetic parameters for voriconazole in plasma and saliva are summarized in Table 3.
TABLE 3.
Pharmacokinetic parameters in plasma and saliva
| Sample and parameter (unit) | Mean value (95% CI) for:
|
|||||
|---|---|---|---|---|---|---|
| Low dose
|
Medium dose
|
High dose
|
||||
| 3 mg/kg (n = 14) | 200 mg (n = 14) | 4 mg/kg (n = 7) | 300 mg (n = 7) | 5 mg/kg (n = 14) | 400 mg (n = 14) | |
| Plasma | ||||||
| Cmax (ng/ml) | 3,006 (2,656-3,400) | 1,885 (1,515-2,347) | 5,402 (3,968-7,361) | 4,839 (3,499-6,698) | 7,184 (6,199-8,331) | 5,272 (4,321-6,431) |
| AUCτ (ng · h/ml) | 13,919 (10,471-18,501) | 9,765 (6,990-13,655) | 29,467 (15,595-55,677) | 30,940 (17,816-53,753) | 43,374 (33,638-55,971) | 37,549 (28,822-48,879) |
| Tmax (h) | 1.07 (1.00-1.14) | 1.50 (1.25-1.75) | 1.04 (0.88-1.22) | 1.43 (1.25-1.61) | 1.02 (0.98-1.06) | 1.81 (1.50-2.12) |
| Vss/F (ml) | 1,478 (1,284-1,671) | NAa | 1,176 (969-1,384) | NA | 817 (750-884) | NA |
| V/F (ml) | 1,874 (1,395-2,354) | 160,241 (124,230-196,252) | 1,391 (1,032-1,750) | 106,917 (86,065-127,768) | 904 (816-992) | 123,868 (110,919-136,816) |
| CL/F (ml/h) | 200 (138-263) | 19,985 (14,130-25,840) | 120 (45-196) | 8,358 (3,881-12,835) | 86 (57-116) | 8,100 (5,699-10,501) |
| kel (h−1) | 0.131 (0.151-0.111) | 0.147 (0.122-0.171) | 0.088 (0.054-0.123) | 0.105 (0.143-0.067) | 0.085 (0.104-0.066) | 0.096 (0.116-0.076) |
| Saliva | ||||||
| Cmax (ng/ml) | 2,081 (1,877-2,308) | 1,309 (1,050-1,632) | 3,353 (2,556-4,402) | 2,796 (2,055-3,805) | 4,308 (3,970-4,672) | 3,287 (2,810-3,847) |
| AUCτ (ng · h/ml) | 8,591 (6,715-10,981) | 5,970 (4,530-7,876) | 19,249 (11,497-32,214) | 18,634 (11,043-31,460) | 24,981 (20,378-30,631) | 22,047 (17,588-27,640) |
| Tmax (h) | 1.00 | 1.21 (0.92-1.5) | 1.04 (0.96-1.12) | 1.29 (1.04-1.54) | 1.09 (0.92-1.26) | 1.54 (1.16-1.92) |
NA, not applicable.
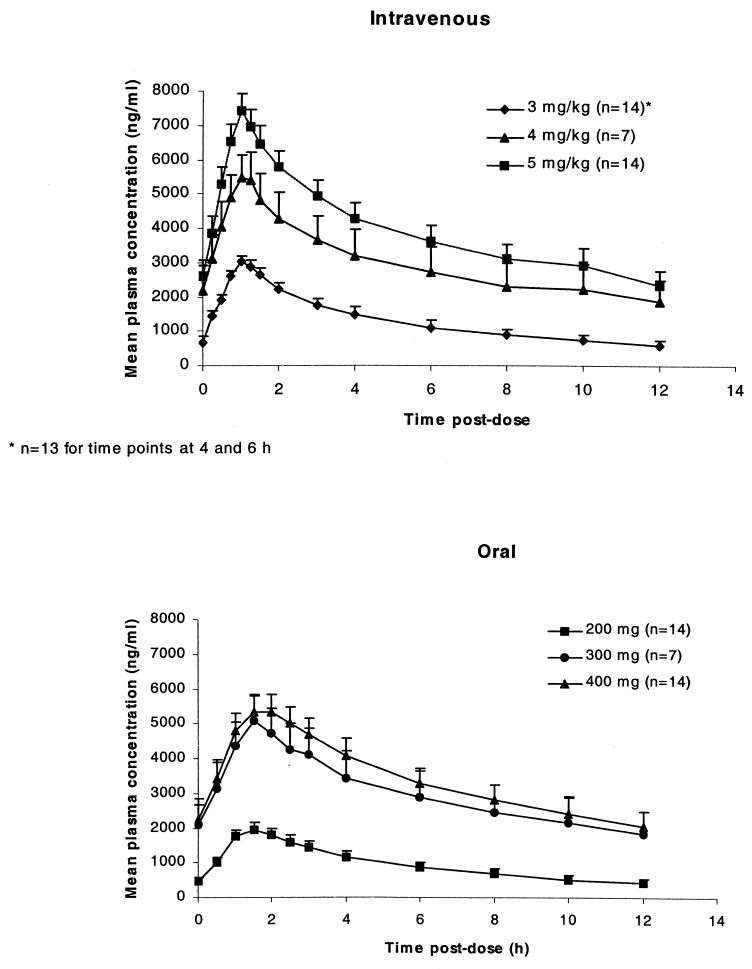
Mean plasma voriconazole concentration-time profiles after i.v. (day 7) and oral (day 14) administration are illustrated in Fig. 1. Cmax occurred at the end of the 1-h i.v. infusion and between 1.4 and 1.8 h after oral administration. For cohort 1, both Cmax and AUCτ increased disproportionately with dose for both i.v. and oral dosing. For i.v. dosing a 1.7-fold increase in dose (i.e., an increase from 3 to 5 mg/kg) resulted in 2.4-fold (95% CI, 2.09 to 2.73) and 3.1-fold (95% CI, 2.72 to 3.58) increases in Cmax and AUCτ, respectively. Similarly, a 2-fold increase in oral dosing (i.e., an increase from 200 to 400 mg b.i.d.) resulted in 2.8-fold (95% CI, 2.45 to 3.23) and 3.9-fold (95% CI, 3.40 to 4.51) increases in Cmax and AUCτ, indicating nonlinearity of voriconazole pharmacokinetics. Data from cohort 2 (given 4 mg/kg i.v. followed by 300 mg b.i.d. orally) could not be used to interpret nonlinearity, as they were obtained from subjects different from those in cohort 1.
FIG. 1.
Mean (+standard error) plasma voriconazole concentration-time profiles following i.v. dosing (day 7) and following oral dosing (day 14) of voriconazole.
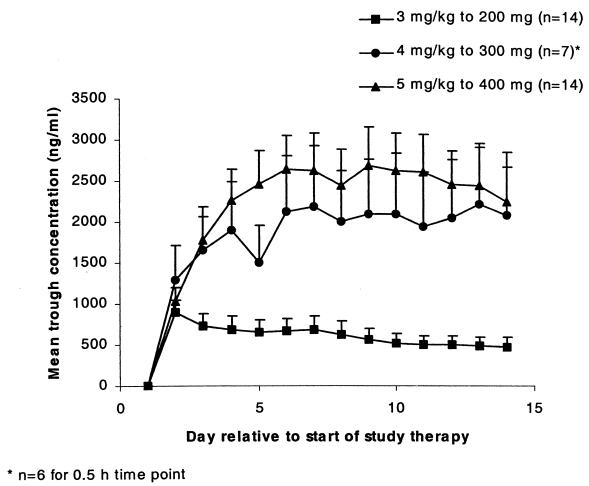
Mean values for Cmax observed following oral dosing were lower than those obtained after i.v. administration, ranging from 62.7 to 89.6% of the i.v. value for the three dose levels (Table 3). Although Cmax values fell after the switch from i.v. to oral dosing, most subjects achieved steady state on day 4, and mean trough concentrations remained above the MICs for Candida spp. (Fig. 2). For each i.v.-to-oral switchover, there was no significant difference in Vss/F, V/F, CL, or kel between the i.v. and oral dosings.
FIG. 2.
Mean (+standard error) trough concentrations of voriconazole after i.v. dosing followed by oral dosing.
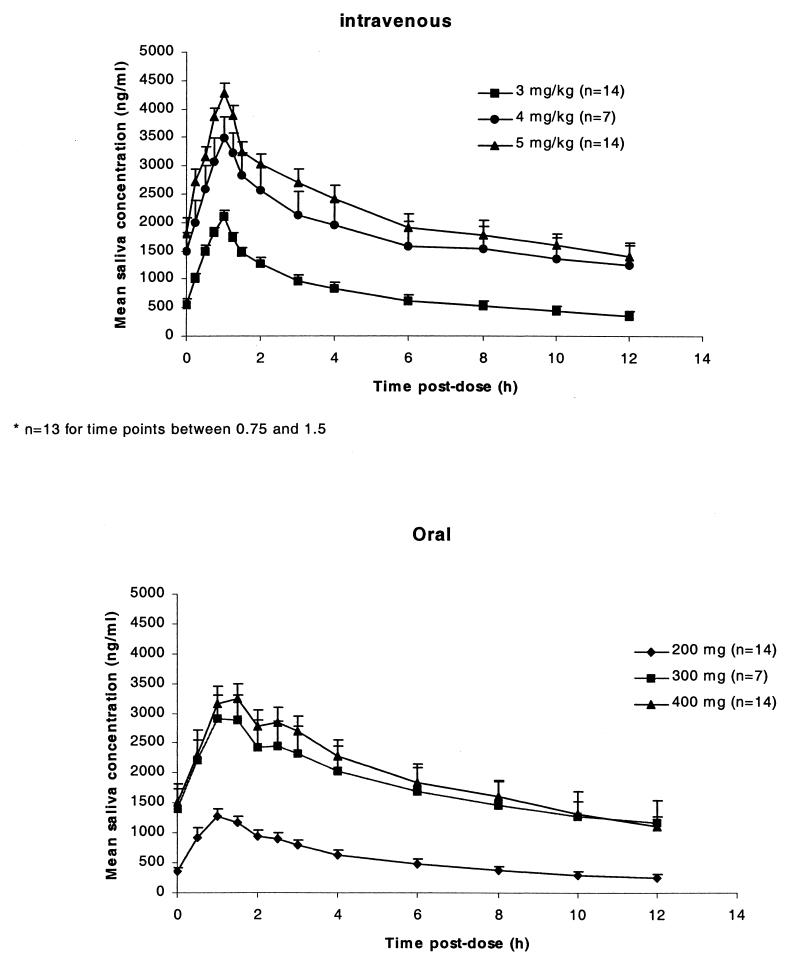
Mean saliva voriconazole concentration-time profiles after i.v. (day 7) and oral (day 14) administration are illustrated in Fig. 3. The pharmacokinetic parameters for saliva followed a pattern similar to those observed for plasma. The average saliva/plasma voriconazole concentration ratio across the different i.v. doses was 66.0% (coefficient of variation, 30%) and that across the oral doses was 64.2% (coefficient of variation, 29%). The relationship between the plasma and saliva voriconazole concentrations was described by log−Cp = intercept + coefficient · log Cs, where Cp and Cs are the concentrations in plasma and saliva, respectively. The intercepts and regression coefficients for each dosing regimen are shown in Table 4.
FIG. 3.
Mean (+standard error) saliva voriconazole concentration-time profiles following i.v. dosing (day 7) and following oral dosing (day 14) of voriconazole.
TABLE 4.
Regression analysis for relationship between log voriconazole concentrations in plasma and saliva at steady state
| Cohort and dosing | Mean (95% CI):
|
|
|---|---|---|
| Intercept | Coefficient | |
| 1 | ||
| i.v. (3 mg/kg b.i.d.) | 0.12 (−0.18, 0.42) | 1.04 (1.00, 1.09) |
| Oral (200 mg b.i.d.) | −0.03 (−0.35, 0.28) | 1.08 (1.03, 1.13) |
| i.v. (5 mg/kg b.i.d.) | 0.07 (−0.39, 0.52) | 1.06 (1.00, 1.12) |
| Oral (400 mg b.i.d.) | 0.27 (−0.19, 0.73) | 1.03 (0.97, 1.09) |
| 2 | ||
| i.v. (4 mg/kg b.i.d.) | −1.02 (−1.57, −0.47) | 1.19 (1.12, 1.26) |
| Oral (300 mg b.i.d.) | −0.17 (−0.66, 0.33) | 1.09 (1.02, 1.15) |
There was no significant difference between plasma and saliva voriconazole levels for all dose regimens. A regression analysis showed a highly statistically significant relationship (P < 0.0001) between log-transformed plasma and log-transformed saliva voriconazole concentration data. The imprecision of the predicted plasma voriconazole level for a given saliva voriconazole value is within a 95% CI of 0.61 to 1.65 times the predicted value.
Safety.
There was one discontinuation because the subject had an serum glutamic-pyruvic transaminase (SGPT; alanine transaminase) elevation to greater than three times the upper limit of normal while receiving oral voriconazole at 400 mg. After withdrawal of voriconazole, SGPT values returned to the normal range within 12 days. The incidence of adverse events emerging during treatment is summarized in Table 5. The most commonly reported adverse events in voriconazole-treated subjects were headache, rash, and abnormal vision. Excluding reports of abnormal vision, treatment-related adverse events following voriconazole administration were rare in all three dosage groups; investigators considered that only one rash and none of the headaches reported were likely to be drug related. Visual disturbances, including abnormal and blurred vision, were spontaneously reported by a total of seven subjects on voriconazole and five subjects on placebo. Investigators considered these events to be related to treatment in seven subjects on voriconazole and four on placebo.
TABLE 5.
Adverse events reported by more than two subjects in any treatment group
| Adverse eventa | No. (%) of subjects with adverse events receiving:
|
|||||
|---|---|---|---|---|---|---|
| Low dose
|
Medium dose
|
High dose
|
||||
| Active (n = 14) | Placebo (n = 14) | Active (n = 7) | Placebo (n = 7) | Active (n = 14) | Placebo (n = 14) | |
| Total | 8 (57) | 8 (57) | 4 (57) | 3 (43) | 9 (64) | 5 (36) |
| Headache | 5 (36) | 2 (29) | 4 (57) | 2 (29) | 0 (0) | 1 (7) |
| Rash | 4 (29) | 1 (7) | 0 (0) | 0 (0) | 2 (14) | 0 (0) |
| Abnormal vision | 3 (21) | 3 (21) | 1 (7) | 2 (29) | 4 (29) | 1 (7) |
| Lab abnormalities | 1 (7) | 2 (14) | 1 (7) | 3 (43) | 4 (29) | 2 (14) |
All causalities.
The visual function tests conducted on all subjects detected no further abnormalities in subjects during voriconazole treatment and one abnormality (abnormal color vision test) during placebo treatment. All visual disturbances were mild to moderate in severity, and all spontaneously resolved within 2 days of onset. No subjects in any treatment group experienced a serious adverse event. The most common laboratory test abnormalities in voriconazole-treated subjects were elevated liver function tests. The highest-dose regimen was associated with an increase in liver function test abnormalities in 2 of 14 subjects. In the medium-dose group, one subject had liver function abnormalities, but there were no occurrences associated with the low-dose regimen.
DISCUSSION
This dose escalation study evaluated the pharmacokinetic and safety profiles of three different i.v.-to-oral switchover regimens of voriconazole in healthy male volunteers.
The pharmacokinetic data from this study indicate that Cmax and AUCτ increased nonlinearly with increasing voriconazole dose. Such nonlinear pharmacokinetics has been observed in earlier i.v. and oral pharmacokinetic studies of voriconazole (Patterson and Coates, 35th ICAAC). At the time that study was conducted, there was little knowledge and technology available to adequately determine the role of P450 isozymes in the metabolism of voriconazole. Later studies revealed that CYP2C19 plays a major role in the metabolism of voriconazole. This enzyme exhibits genetic polymorphism, dividing the population into poor and extensive metabolizers as a result of critical point mutations in the gene encoding the protein of CYP2C19. (4) About 5 to 7% of the Caucasian population has a deficiency in expressing the enzyme, and therefore, genotype plays a key role in the pharmacokinetics of voriconazole. The genotype confounds the determination of nonlinearity by inspection of dose-normalized AUCs if the data for different doses are collected across different individuals.
Visual inspection of the trough plasma voriconazole concentrations indicates that steady state was achieved by day 4 of i.v. dosing for most subjects. Trough levels remained well above the MICs of voriconazole for Aspergillus spp. (geometric mean MIC [GMMIC], 0.19 to 0.58 μg/ml), Candida spp. (GMMIC, 0.001 to 0.39 μg/ml), C. neoformans (GMMIC, 0.24 μg/ml), and most emerging fungal pathogens (1, 3, 6, 7, 8, 9, 10; Hitchcock et al., 35th ICAAC; Clancy et al., 37th ICAAC; Clancy and Nguyen, 13th Congr. Int. Soc. Hum. Anim. Mycol.). As expected, mean Cmax values following oral dosing were lower than those obtained following i.v. administration.
Regression analyses indicated a clear and highly statistically significant relationship between plasma and saliva voriconazole levels for all dose regimens. However the 95% CIs indicated that these could be as wide as ±0.5 on the log scale, corresponding to an upper confidence limit of 1.65 times the predictive value and a lower confidence limit of 0.61 times the predictive value. Additional analyses indicated that the predictive capability is greater when data recorded earlier than 2 h postdose are excluded, suggesting that salivary voriconazole levels may have some potential use in predicting plasma levels.
The switch from i.v. to oral administration of voriconazole was well tolerated. No serious adverse events were reported, and the majority of the adverse events reported were transient and mild to moderate in severity. In addition, clinically significant laboratory safety test abnormalities were rare and resulted in treatment discontinuation in only one subject who received the high-dose regimen.
Short episodes of visual disturbances following voriconazole administration have been reported previously (B. Dupont, D. Denning, H. Lode, S. Yonren, P. F. Troke, and N. Sarantis, Abstr. 35th Intersci. Conf. Antimicrob. Agents Chemother., abstr. F81, p. 125, 1995; P. F. Troke, K. W. Brammer, C. A. Hitchcock, S. Yonren, and N. Sarantis, Abstr. 35th Intersci. Conf. Antimicrob. Agents Chemother., abstr. F73, p. 125, 1995) As a consequence, the present study was designed to assess the effect of voriconazole on vision by using a series of specifically designed visual function tests and a questionnaire. While there was a moderate incidence of adverse visual events recorded for the voriconazole group, the incidence was similar to that for the placebo group. None of the visual disturbances were rated as severe or associated with any clinical abnormality.
The results of this study with healthy male volunteers support further investigation of voriconazole following i.v.- to oral-dose administration in hospitalized patients with serious fungal infections.
REFERENCES
- 1.Barry, A. L., and S. D. Brown. 1996. In vitro studies of two triazole antifungal agents (UK-109,496 and fluconazole) against Candida species. Antimicrob. Agents Chemother. 40:1948-1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Belanger, P., C. C. Nast, R. Fratti, H. Sanati, and M. Ghannoum. 1997. Voriconazole (UK-109,496) inhibits the growth and alters the morphology of fluconazole-susceptible and -resistant Candida species. Antimicrob. Agents Chemother. 41:1840-1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Espinel-Ingroff, A. 1998. In vitro activity of the new triazole voriconazole (UK-109,496) against opportunistic filamentous and dimorphic fungi and common and emerging yeast pathogens. J. Clin. Microbiol. 36:198-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goldstein, J. A., and S. M. F. deMorais. 1994. Biochemistry and molecular biology of the human CYP2C subfamily. Pharmacogenetics 4:285-299. [DOI] [PubMed] [Google Scholar]
- 5.Koltin, Y., and C. A. Hitchcock. 1997. The search for new triazole antifungal agents. Curr. Opin. Chem. Biol. 1:176-182. [DOI] [PubMed] [Google Scholar]
- 6.McGinnis, M. R., L. Pasarell, D. A. Sutton, A. W. Fothergill, C. R. Cooper, and M. G. Rinaldi. 1997. In vitro evaluation of voriconazole against some clinically important fungi. Antimicrob. Agents Chemother. 41:1832-1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murphy, M., E. M. Bernard, T. Ishimaru, and D. Armstrong. 1997. Activity of voriconazole (UK-109,496) against clinical isolates of Aspergillus species and its effectiveness in an experimental model of invasive pulmonary aspergillosis. Antimicrob. Agents Chemother. 41:696-698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nguyen, M. H., and C. Yu. 1998. In vitro comparative efficacy of voriconazole and itraconazole against fluconazole-susceptible and -resistant Cryptococcus neoformans isolates. Antimicrob. Agents Chemother. 42:471-472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Radford, S. A., E. M. Johnson, and D. W. Warnock. 1997. In vitro studies of activity of voriconazole (UK-109,496), a new triazole antifungal agent, against emerging and less-common mold pathogens. Antimicrob. Agents Chemother 41:841-843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ruhnke, M., A. Schmidt-Westhausen, and M. Trautmann. 1997. In vitro activities of voriconazole (UK-109,496) against fluconazole-susceptible and -resistant Candida albicans isolates from oral cavities of patients with human immunodeficiency virus infection. Antimicrob. Agents Chemother. 41:575-577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sanati, H., P. Belanger, R. Fratti, and M. Ghannoum. 1997. A new triazole, voriconazole (UK-109,496), blocks sterol biosynthesis in Candida albicans and Candida krusei. Antimicrob. Agents Chemother. 41:2492-2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.SAS Institute, Inc. 1989. SAS/STAT user's guide, version 6, 4th ed. SAS Institute, Inc., Cary, N.C.