Abstract
The efficacy of intravenously administered liposomal amphotericin B (AmBisome [AmBi]) for the treatment of experimental coccidioidal meningitis was compared with those of oral fluconazole (FLC) and intravenously administered conventional amphotericin B (AMB). Male New Zealand White rabbits were infected by intracisternal inoculation of arthroconidia of Coccidioides immitis. Starting 5 days postinfection, animals received one of the following: 5% dextrose water diluent; AMB given at 1 mg/kg of body weight; AmBi given at 7.5, 15, or 22.5 mg/kg intravenously three times per week for 3 weeks; or oral FLC given at 80 mg/kg for 19 days. One week after the cessation of therapy, all survivors were euthanatized, the numbers of CFU remaining in the spinal cord and brain were determined, and histological analyses were performed. All AmBi-, FLC-, or AMB-treated animals survived and had prolonged lengths of survival compared with those for the controls (P < 0.0001). Treated groups had significantly lower numbers of white blood cells and significantly lower protein concentrations in the cerebrospinal fluid compared with those for the controls (P < 0.01 to 0.0005) and had fewer clinical signs of infection (e.g., weight loss, elevated temperature, and neurological abnormalities including motor abnormalities). The mean histological scores for AmBi-treated rabbits were lower than those for FLC-treated and control rabbits (P < 0.016 and 0.0005, respectively); the scores for AMB-treated animals were lower than those for the controls (P < 0.0005) but were similar to those for FLC-treated rabbits. All regimens reduced the numbers of CFU in the brain and spinal cord compared with those for the controls (P ≤0.0005). AmBi-treated animals had 3- to 11-fold lower numbers of CFU than FLC-treated rabbits and 6- to 35-fold lower numbers of CFU than AmB-treated rabbits. Three of eight animals given 15 mg of AmBi per kg had no detectable infection in either tissue, whereas other doses of AmBi or FLC cleared either the brain or the spinal cord of infection in fewer rabbits. In addition, clearance of the infection from both tissues was achieved in none of the rabbits, and neither tissue was cleared of infection in AMB-treated animals. Overall, these data indicate that intravenously administered AmBi is superior to oral FLC or intravenous AMB and that FLC is better than AMB against experimental coccidioidal meningitis. These data indicate that AmBi may offer an improvement in the treatment of coccidioidal meningitis. Additional studies are warranted.
Meningitis due to Coccidioides immitis infection is a life-threatening, devastating disease (11, 12, 25). The therapeutic options available for the treatment of this form of coccidioidomycosis are less than optimal, with many patients failing therapy while they are receiving treatment or recrudescing after treatment has been stopped. This is particularly true for azole therapy (8). In addition, azole therapy is considered noncurative, requiring lifelong treatment (8). Although amphotericin B (AMB) remains the standard of therapy for the treatment of nonmeningeal disseminated coccidioidomycosis, its utility for the treatment of coccidioidal meningitis is limited by the need for intrathecal administration, since it is ineffective when given intravenously (9-11, 13, 25). In addition, toxicities may severely limit the use of conventional AMB (13). Thus, there is a need for improved therapeutic options for the treatment of coccidioidal meningitis.
Reductions of the toxicities associated with AMB have been accomplished to various extents by the use of one of several different lipid carrier systems (17). However, the efficacies of these formulations are often less than those of conventional AMB when the efficacies are compared on the basis of milligrams per kilogram of body weight, and thus, higher dosages are needed. Liposomal AMB (AmBisome [AmBi]) has been demonstrated to have potential utility for the treatment of meningeal infections due to Cryptococcus neoformans (2, 6, 7, 17, 27) and Candida albicans (14). Although AmBi has been reported to have efficacy against experimental murine pulmonary coccidioidomycosis (3), no information on its efficacy against coccidioidal meningitis is available. AmBi, which is a unilamellar liposomal form of AMB, has fewer side effects and appears to be better able to penetrate the cerebrospinal fluid (CSF) (1, 17, 19). In addition, AmBi has been shown to accumulate in the brain tissue of uninfected rabbits to a greater degree than other lipid formulations of AMB or conventional AMB (A. Groll, N. Giri, C. Gonzales, T. Sein, J. Bacher, S. Piscitelli, and T. J. Walsh, Abstr. 37th Intersci. Conf. Antimicrob. Agents Chemother., abstr. A-90, p. 19, 1997). Thus, in light of the efficacy of AmBi against cryptococcal and candidal meningitis that has been described, as well as the penetration of AmBi into brain tissue and CSF that has been described, we sought in the present study to examine the potential efficacy of AmBi given intravenously in a rabbit model of coccidioidal meningitis.
MATERIALS AND METHODS
Infection model.
The rabbit model of coccidioidal meningitis used in these studies was similar to that described previously (22, 23, 28). In brief, 2.5- to 3.0-kg male New Zealand White rabbits (Myrtle's Rabbitry, Thompson Station, Tenn.) received an intramuscular injection of 2 mg of hydrocortisone sodium succinate (Solu-Cortef; Pharmacia & Upjohn) per kg of body weight on day −1 through day 3 after infection (five doses) to aid in the establishment of meningeal disease. On the day of infection, rabbits were anesthetized with a mixture of ketamine (35 mg/kg), xylazine (3.5 mg/kg), and acepromazine (0.5 mg/kg) given intramuscularly (22, 23, 28). After a surgical plane of anesthesia was achieved, the rabbits were infected by direct inoculation of 5 × 104 arthroconidia of C. immitis strain Silveira into the cisterna magna (22, 23, 28). The inoculum was administered in 0.2 ml of saline suspension, followed by a flush with 0.6 ml of sterile saline. Following infection, yohimbine (0.2 mg/kg) was administered intravenously to aid in recovery from anesthesia. On day 14 or 15 of infection and at the conclusion of the study 30 days after infection and prior to euthanasia, blood and CSF samples were obtained from the rabbits while they were under a surgical plane of anesthesia (by use of up to 4.5% isoflurane with 1.5 liter of oxygen/min).
Treatment groups.
Groups of 8 to 10 animals were assigned to each treatment group. The treatments consisted of sterile 5% dextrose water (D5W); AmBi (Gilead Sciences, Inc., Foster City, Calif.) at 7.5, 15, or 22.5 mg/kg; fluconazole (FLC; Pfizer, Groton, Conn.) at 80 mg/kg; or deoxycholate-formulated AMB (Pharma-Tek, Inc., Huntington, N.Y.) at 1 mg/kg. AmBi was reconstituted according to the instructions of the manufacturer to contain a final concentration of 2 mg of AMB per ml as AmBi in D5W. Rabbit weight was used to determine each dosage. The control diluent for AmBi was prepared by combining 50 ml of sterile H2O with 50 ml of D5W, and each animal was given a volume equivalent to a dose of 15 mg of AmBi per kg (by weight). AMB was reconstituted according to the instructions of the manufacturer and added to sterile D5W to obtain a final concentration of 0.15 mg of AMB per ml. Control D5W was prepared by adding an equivalent volume of sterile H2O to the D5W; volumes for dosing were also based on body weight. Treatment began 5 days postinfection and continued through day 23 of infection. D5W, AmBi, and AMB treatments were given intravenously in a lateral ear vein three times a week (Monday, Wednesday, and Friday) for 3 weeks (nine doses) at flow rates of 2 ml/min. FLC was given orally in polyethylene glycol 200 once a day for 19 consecutive days.
Because of the number of animals involved, these studies were divided into blocks of the same overall experiment. Treatment groups consisted of four or five animals per run, with two runs required to complete a block for a treatment arm. Block 1 consisted of treatment with D5W, FLC, or AmBi at 15 mg/kg; block 2 consisted of treatment with D5W or AMB; and block 3 consisted of treatment with D5W, 7.5 of AmBi per kg, or 22.5 mg of AmBi per kg. Each block was completed before a new block was begun.
Clinical parameters.
All animals were clinically assessed twice daily throughout the 30-day duration of the infection. Clinical assessments included documentation of body weight, temperature, hydration, posturing, mobility, and presence of paresis (23, 28). Those animals showing signs of dehydration and weight loss were given 100 to 150 ml of lactated Ringer's solution by subcutaneous clysis; nutritional supplements were also given to those animals that were found to be consuming reduced amounts of food. Animals exhibiting signs of chronic discomfort or pain were given 0.03 mg of buprenorphine per kg subcutaneously twice daily.
Animals meeting the criteria for euthanasia to alleviate pain and suffering (e.g., paresis, >20% weight loss, and coma), as mandated by the Institutional Animal Care and Use Committee of the California Institute for Medical Research, were euthanatized by injection of 1 ml (390 mg/ml; an overdose) of pentobarbital (Euthasol; Delmarva Laboratories) while under a surgical plane of anesthesia induced with isoflurane as described above. Animals euthanatized due to the severity of infection were tallied as though they had succumbed to infection. CSF and blood samples were obtained prior to euthanasia. All animals were necropsied after euthanasia, with the brain and spinal cord removed for microbiological and histological analyses. Quantitative plating of organ homogenates was done to determine the numbers of CFU in the tissues. Organs were mechanically homogenized in sterile saline, and dilutions were plated on Mycosel agar plates. The numbers of colonies arising were enumerated after 3 to 5 days of growth at 37°C. The number of CFU was expressed as the log10 number of CFU per gram of tissue.
Histology.
Tissues for histological analyses were placed in 10% buffered formalin and embedded in paraffin, and sections were stained with hematoxylin-eosin to assess the associated tissue response and pathology. The histological observations were scored on the basis of the severity of meningitis on a scale from 0 to 6, with 0 being normal, 2 being mild (i.e., rare foci of small meningeal inflammatory infiltrates), 4 being moderate (i.e., more numerous and larger meningeal inflammatory foci), and 6 being severe (i.e., large numbers of confluent inflammatory infiltrates with numerous granulomas). The scores were assigned by a neuropathologist blinded to the clinical status of the animals and their respective treatments.
Other parameters.
Samples of CSF and blood were taken from each animal 14 or 15 days after infection and just prior to euthanasia. The numbers of white blood cells (WBCs) in the CSF were determined by counting with a hemacytometer, and the numbers of CFU of C. immitis free in the CSF were determined by quantitative plating immediately after sampling, as described previously (23, 28). The remaining sample of CSF was divided and stored at −80°C for protein and lactic acid analyses as described elsewhere (23, 28). At various times postdosing, serum was collected from the whole blood and was stored at −80°C for determination of drug concentrations. In some instances, serological analysis was done to determine the titers of antibodies against coccidioidal antigens, as described previously (18).
Determination of drug levels.
The concentrations of AMB in the serum and CSF were determined by bioassay with Paecilomyces variotii as the indicator organism, as described previously (15, 16, 23, 26). The lower limit of detection by this assay was 0.031 μg/ml. The AMB in samples from animals that had received AmBi was extracted with cold 70% methanol, the extracts were dried, and the residue was resuspended in normal rabbit serum prior to bioassay. FLC concentrations in the serum and CSF were determined by bioassay with Candida kefyr as the indicator organism, as described previously (20). The lower limit of detection by this assay was 2.0 μg/ml for both serum and CSF.
Statistical analyses.
Survival comparisons were done by χ2 tests for homogeneity and log rank tests with GraphPad Prism software (version 3.02) for Windows (GraphPad Software, San Diego Calif.). The comparative numbers of CFU recovered from the tissues were analyzed by a Mann-Whitney U test (21). Statistical comparisons of histological scores were done by a Student's t test.
RESULTS
Survival.
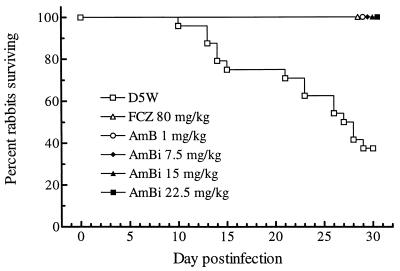
The survival of all rabbits was monitored through 30 days of infection. All animals given AmBi, FLC, or AMB survived, whereas only 9 of 24 control animals survived; no significant variation in the overall length of survival of the D5W-treated animals was found among the blocks (Fig. 1 and Table 1). Statistical comparison of survival by time was done by the log rank test, in which the the results obtained with the treatment regimens run in a block were compared with those obtained with the D5W control treatment in that block. These results showed that AMB, FLC, and AmBi at 15 mg/kg significantly prolonged survival (P < 0.0005, 0.0007, and 0.0023, respectively) (Table 1). In block 3 of the experiment, in which animals received 7.5 or 22.5 mg of AmBi per kg, fewer control animals succumbed to infection (three of eight versus six of eight animals receiving D5W treatment in the other arms of the study), and treatment with each AmBi dose showed a trend toward prolongation of survival (P = 0.0628) (Table 1).
FIG. 1.
Cumulative mortality of rabbits infected intracisternally with C. immitis and given one of the indicated treatments. A χ2 test for homogeneity was done on the survival in the D5W treatment arms (Table 1), in which the total number of animals surviving in blocks 1, 2, and 3 were compared (e.g., two of eight, two of eight, and five of eight animals, respectively) and showed that P was equal to 0.2019 (χ2 = 3.2; two degrees of freedom). Thus, the survival results for the D5W treatments were pooled across the blocks. The statistical analyses of survival times by a log rank test showed that treatment with FLC (FCZ), AMB, and all doses of AmBi significantly prolonged survival compared with that with D5W treatment (P = 0.0001).
TABLE 1.
Survival of rabbits in each experimental block infected intracisternally with C. immitis and treated with AmBi, AMB, FLC, or D5W
| Block | Experiment run | No. of surviving rabbits/total no. in group
|
|||||
|---|---|---|---|---|---|---|---|
| D5Wa | FLC at 80 mg/kg | AMB at 1 mg/kg | AmBi at:
|
||||
| 7.5 mg/kg | 15 mg/kg | 22.5 mg/kg | |||||
| 1b | A1 | 2/4 | 5/5 | 4/4 | |||
| A2 | 0/4 | 5/5 | 4/4 | ||||
| 2c | B1 | 1/4 | 4/4 | ||||
| B2 | 1/4 | 4/4 | |||||
| 3d | C1 | 4/4 | 4/4 | 4/4 | |||
| C2 | 1/4 | 4/4 | 4/4 | ||||
| Total | 9/24 | 10/10 | 8/8 | 8/8 | 8/8 | 8/8 | |
A χ2 test for homogeneity was done on the replications within each block and showed the following results: block 1, two of four versus zero of four animals (P = 0.4286; two-sided); block 2, one of four versus one of four animals (P = 1.5714; two-sided); block 3, four of four versus one of four animals (P = 0.1429, 2-sided). Comparison of the survival times of D5W-treated groups across blocks by a log rank test showed that P was equal to 0.36 when all three blocks were compared. Individual comparisons showed the following: for block 1 versus block 2, P = 0.787; for block 1 versus block 3, P = 0.1837; and for block 2 versus block 3, P = 0.2213.
Comparison of the survival times in block 1 by a log rank test showed that treatment with FLC and AmBi at 15 mg/kg each prolonged survival over treatment with D5W (P = 0.0007 and 0.0023, respectively).
Comparison of the survival times in block 2 by a log rank test showed that treatment with AmB prolonged survival over treatment with D5W (P = 0.0005).
Comparison of the survival times of all three groups in block 3 by a log rank test showed that P was equal to 0.032 overall. Individual comparisons of each AmBi treatment group versus D5W showed that P was equal to 0.0628.
The Kaplan-Meier survival curves generated by pooling of the D5W data from all blocks of the experiment are shown in Fig. 1; for the D5W regimen, n was equal to 24; for the FLC regimen, n was equal to 10, and for the AMB or the AmBi regimens, n was equal to 8. Neither the χ2 test for homogeneity (Fig. 1) nor log rank analysis of the comparative survival times for the three D5W-treated groups showed significant differences in survival times (P > 0.05) (Table 1). Thus, the data for these groups were pooled, and statistical analyses of the Kaplan-Meier survival curves by the log rank test showed that all treatment regimens significantly prolonged survival (P < 0.0001).
Clinical parameters.
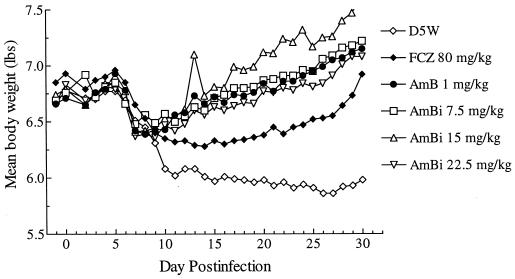
The major clinical signs and symptoms of coccidioidal meningitis that have been found to be useful for monitoring of the course of infection are body weight, body temperature, and mobility (28). Figure 2 illustrates the changes in body weights of treated and control rabbits during the 30 days of infection. These data reflect initial decreases in the weights of all groups by days 7 to 9 through about day 14. Thereafter, the animals in the control groups showed continued weight loss, whereas the animals in the AmBi-treated groups gained weight for the remainder of the study; the results in terms of body weight changes in animals in the AMB-treated group were similar to those for the animals in the AmBi-treated group. The weights of the animals in the FLC-treated group stabilized after the initial decline but did not increase until about day 25.
FIG. 2.
Mean body weights of rabbits in each treatment arm (n = 8 to 24, depending on the group) monitored during the course of infection. FCZ represents FLC.
Posture changes reflecting the animals' resting positions ranged from mild (resting on their hind legs) to severe, indicated by an opisthotonic posture, which reflects meningeal discomfort and progression of disease. The animals in the D5W-treated groups had sharp increases in abnormal postures beginning on about day 8; these abnormal postures progressively increased in severity throughout the study. The FLC-treated animals had mild increases in abnormal postures beginning on about day 9, with a gradual worsening until day 18, followed by steady improvement to near normal postures by day 28. AmBi- and AMB-treated rabbits showed no signs of aberrant posture.
All groups had increases in daily body temperature, indicative of fever, during the first week of infection. In general, for the D5W-treated animals, the body temperatures remained higher than those of any of the treated animals, and they remained higher for a longer period. After the initiation of therapy, the body temperatures of AmBi- and AMB-treated animals returned to normal by day 10, whereas the body temperatures of the FLC-treated animals were slower to return to normal (day 19).
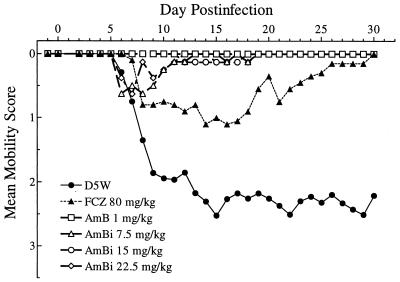
The assessment of mobility reflecting neurological impairment, shown in Fig. 3, was graded from normal to severe, i.e., ataxia and/or paresis. In general, as early as day 5 of infection the D5W-treated animals began to show signs of difficulty in movement, which progressively worsened. FLC-treated animals had a less severe loss of mobility that eventually returned to normal, and AmBi- or AMB-treated animals showed few to no signs of difficulty in movement. Overall, 16 of 24 D5W-treated animals showed paresis of one or both hind limbs prior to euthanasia. None of the animals given AmBi, AMB, or FLC showed any signs of paresis.
FIG. 3.
Mean mobility scores for rabbits in each treatment arm (n = 8 to 24, depending on the group) during the course of infection. Mobility decreases as the scores increase. FCZ represents FLC
Histology.
Histopathological assessments were performed with samples of the brain and spinal cord of all animals. All D5W-treated animals had severe coccidioidal meningitis. The characteristics of the meningitis often included granulomas, arteritis, encephalitis, cord ischemia, infarcts, and parenchymal invasion of inflammation. The histopathologies of the tissues from treated animals varied from severe meningitis in two of the FLC-treated animals and one animal given AmBi at 7.5 mg/kg to mild meningitis with cerebritis and focal invasion or arteritis to a very mild meningitis or normal tissues in other treated animals. Most animals receiving one of the doses of AmBi showed only minimal evidence of meningitis, whereas those given AMB showed mild to moderate meningitis.
A semiquantitative scoring system was used to allow comparisons of the severities of the histological responses of the animals among the treatment arms. The mean score for D5W-treated animals (all three blocks) was 6.0; the mean scores for animals treated with AmBi at 7.5, 15, and 22.5 mg/kg were 3.3 ± 0.7, 0.9 ± 0.4, and 1.9 ± 0.4, respectively (P < 0.01 versus D5W-treated animals in blocks 3, 1, and 3, respectively); the mean score for animals treated with FLC was 2.7 ± 2.0 (P < 0.0035 versus D5W-treated animals in block 1); and the mean score for animals treated with AMB was 2.4 ± 0.9 (P < 0.0005 versus D5W-treated animals in block 2). Among the treated animals, the lowest mean scores and the fewest associated histopathologies were for animals given AmBi at 15 or 22.5 mg/kg. In a direct comparison, animals that received AmBi at 15 mg/kg had significantly fewer associated pathologies than FLC-treated animals (P < 0.05). In block 3, which comprised the 7.5- and 22.5-mg/kg doses of AmBi, animals given AmBi at 22.5 mg/kg had significantly fewer associated pathologies than those given AmBi at 7.5 mg/kg (P = 0.01). Comparisons across experimental blocks showed that AmBi at 15 mg/kg was better than all other regimens (P < 0.05 to 0.01, depending on the comparison). Except for AMB at 1 mg/kg being better than AmBi at 7.5 mg/kg (P < 0.05), there were no differences among the other comparisons.
CSF parameters.
Progression of infection was also monitored during the course of these experiments by assessing the numbers of WBCs in the CSF, the protein concentration in the CSF, and in some instances, the lactic acid concentration in the CSF. The number of WBCs in the CSF and the protein concentrations are presented in Table 2 . WBC counts were highest for the control animals at 14 days postinfection, as well as at the ends of the experiments. Animals in the treatment groups had fewer WBCs in their CSF than the animals in the respective D5W-treated control group. Animals given AmBi at 15 or 22.5 mg/kg had the fewest WBCs, indicating a minimal inflammatory response.
TABLE 2.
WBC and protein concentrations in the CSF of infected animals
| No. of WBCs/mm3a
| ||||||||
|---|---|---|---|---|---|---|---|---|
| Day | Block 1
|
Block 2
|
Block 3
|
|||||
| D5W | FLC | AmBi (15 mg/kg) | D5W | AmB | D5W | AmBi (7.5 mg/kg) | AmBi (22.5 mg/kg) | |
| 0 | 0 | 12.5 (39) | 0 | 46.9 (93) | 15.6 (41.7) | 109 (205) | 31 (58) | 16 (44) |
| 15 | 1,437 (1,139) | 825 (646) | 172 (176) | 910.7 (871) | 375 (453) | 4,703 (3,706) | 1,750 (2,989) | 804 (1,050) |
| 30 | 979 (776) | 400 (269) | 31 (58) | 2,953 (1,019) | 603 (370) | 2,063 (1,012) | 328 (149) | 63 (94) |
The protein concentrations in the CSF of all animals were equivalent at day 0 (Table 2). Thereafter, the concentrations progressively increased in animals given D5W. Similarly, the concentrations increased in treated animals, but to lower ultimate concentrations. The concentrations in animals given AmBi at 15 mg/kg showed the least change from the day 0 baseline concentrations, with mean concentrations slightly lower at day 30 than at day 14. Animals given AmBi at 7.5 or 22.5 mg/kg showed a delayed rise in protein concentrations, with the concentrations at day 30 higher than those at day 14; similar results were found for AMB-treated animals, although the concentrations were higher than those in animals given the low dose of AmBi. The protein concentrations were unchanged from 14 to 30 days postinfection for FLC-treated animals. The changes in the lactic acid concentrations in CSF were similar to the changes in protein concentrations that were detected (data not shown).
Analysis of the titers of specific antibodies against coccidioidal antigen in serum was done with samples from animals given D5W, FLC, or AmBi at 15 mg/kg. Seven of eight D5W-treated control rabbits were positive for coccidioidal-specific immunoglobulin G (IgG), and the other animal was positive for IgM. Thus, all eight animals were serologically positive. In comparison, only 4 of 10 animals given FLC were positive for IgG (1 of the 4 was also positive for IgM) at either day 15 or day 30. Of the eight rabbits treated with 15 mg of AmBi per kg, only a single animal was serologically positive for coccidioides-specific IgG at either day 15 or day 30. These results are in accord with the CFU data.
CFU in tissues.
The numbers of CFU of C. immitis remaining in the tissues were determined after euthanasia. These results are presented in Table 3. D5W-treated animals had the highest mean burden of organisms in the spinal cord and the brain. All treatment regimens significantly reduced the numbers of CFU in both tissues in comparison with the numbers in the D5W-treated controls in that block (P ≤0.0005). AmBi at 15 mg/kg was superior to FLC at reducing the numbers of CFU in the spinal cord (P < 0.01), but it was not significantly superior to FLC at reducing the numbers of CFU in the brain. In block 3, AmBi at 7.5 and AmBi at 22.5 mg/kg were equivalent at reducing the numbers of CFU in both tissues. Examination of the fold reductions in the numbers of CFU caused by the treatments compared with the counts for the controls in the respective blocks of the experiment for each tissue showed that AmBi at 15 mg/kg caused the greatest reduction in the numbers of CFU in both tissues, whereas AMB was the least effective (Table 3).
TABLE 3.
Recovery of C. immitis from the brains and spinal cords of rabbits
| Block and treatment (dose [mg/kg]) | No. of animals cured/total no. in group | Log10 geometric mean no. of CFU/g of spinal cord | Fold reduction in no. of CFU in spinal cord that for versus controla | Log10 geometric mean no. of CFU/g of brain tissue. | Fold reduction no. of CFU in brain versus that for control |
|---|---|---|---|---|---|
| Block 1 | |||||
| D5W | 0/8 | 3.67 (2.22-5.13, 0)b | 2.78 (1.33-4.24, 0) | ||
| FLC (80) | 0/10 | 1.45 (0.15-2.75, 1) | 166 | 0.92 (0-2.23, 2) | 72 |
| AmBi (15) | 3/8 | 0.42 (0-1.87, 5) | 1,778 | 0.46 (0-1.95, 4) | 209 |
| Block 2 | |||||
| D5W | 0/8 | 4.14 (3.92-4.36, 0) | 3.61 (3.38-3.83, 0) | ||
| AmB (1) | 0/8 | 2.43 (2.13-2.69, 0) | 51 | 2.06 (1.83-2.28, 0) | 35 |
| Block 3 | |||||
| D5W | 0/8 | 3.47 (3.07-3.87, 0) | 2.93 (2.34-3.52, 0) | ||
| AmBi (7.5) | 0/8 | 1.56 (0.94-2.17, 1) | 81 | 1.18 (0.66-1.70, 1) | 56 |
| AmBi (22.5) | 0/8 | 1.44 (0.65-2.22, 2) | 107 | 1.11 (0.80-1.41, 0) | 66 |
The fold reductions in CFU were determined by subtracting the log10 geometric number of CFU for the treatment group from the log10 geometric number of CFU for the D5W control group in the same block. The antilog of the resulting number was taken to give the arithmetic fold reduction.
The values in parentheses are 95% confidence intervals, numbers of animals free of infection.
In addition to the superiority of AmBi at 15 mg/kg in reducing the numbers of CFU, this regimen also cleared both tissues of detectable infection in three of eight animals. No other treatment cleared both tissues of detectable infection. However, the number of animals in the group receiving AmBi at 15 mg/kg cleared of detectable infection was not significantly different from the numbers of animals in groups receiving regimens in which no animals were cleared of detectable infection.
AMB and FLC concentrations.
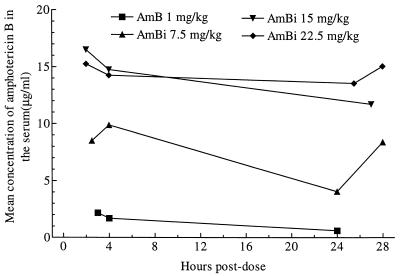
Although the study was not intended to be an extensive pharmacokinetic study, the concentrations of AMB in serum and CSF were determined by bioassay at various times after administration of the fifth dose of AMB or AmBi. FLC concentrations were determined after the administration of 10 doses of FLC. Drug concentrations were also determined at the termination of the experiment, on day 30 of infection (1 week after the cessation of treatment). The AMB concentrations at each time point were determined for two to four rabbits. The mean AMB concentrations in serum after the administration of five doses of drug for each of the groups are shown in Fig. 4. When the drug was given as AMB, the AMB concentrations in serum were the lowest among the groups, but when the treatment consisted of AmBi at 7.5 mg/kg, the AMB concentrations were about four- to eightfold higher, depending on the time postdosing. After the administration of repeated doses of AmBi at 15 or 22.5 mg/kg, the concentrations of AMB were higher than those after dosing with AmBi at 7.5 mg/kg. Approximately twofold increases in the concentrations in serum were noted from the 7.5-mg/kg dose to the 15-mg/kg dose, but administration of a 22.5-mg/kg dose of AmBi resulted in no further increase in serum AMB concentrations over those obtained from the 15-mg/kg regimen. In contrast to the results for serum, detectable concentrations of AMB in CSF were found in only three animals, and each had received AmBi at 22.5 mg/kg (i.e., at 2 h postdosing one animal had a concentration of 0.35 μg/ml, and at 25 h postdosing two animals had concentrations of 0.73 and 0.63 μg/ml, respectively).
FIG. 4.
Mean concentrations of AMB in serum at various times after the administration of dose 5 of AmBi or AMB.
On day 30, the concentration of AMB in serum had decreased in all treatment groups. Only four of eight AmB-treated animals had detectable AMB concentrations (mean concentration, 0.12 μg/ml). Five of eight animals given AmBi at 7.5 mg/kg had detectable levels of AMB (mean concentration, 0.39 μg/ml), eight of eight animals given AmBi at 15 mg/kg had detectable levels (mean concentration, 1.15 μg/ml), and seven of eight animals given AmBi at 22.5 mg/kg had detectable levels (mean concentration, 1.32 μg/ml). Similar to the results on day 15 for CSF, AMB was detectable in the CSF of only two rabbits (in both rabbits, at a concentration of 0.4 μg/ml), both of which were given AmBi at 22.5 mg/kg; both had detectable concentrations in their CSF at day 15 as well.
The concentrations of FLC in serum and CSF were also determined. At 2, 4, 5, 6, and 8 h postdosing on day 15, the mean concentrations (for one to three rabbits, but for two rabbits at most time points) of FLC in serum were 91, 63, 70, 76, and 104 μg/ml, respectively. Similarly, the mean concentrations of FLC in CSF at the same time points were 56, 53, 56.5, >64, and 60 μg/ml, respectively. At day 30 postinfection, only 4 of 10 animals had detectable concentrations of FLC (1.05 to 2.0 μg/ml) in serum, and 1.5 μg/ml was also detectable in the CSF of a single animal.
DISCUSSION
Previous studies have demonstrated the efficacies of lipid formulations of AMB in murine models of pulmonary and systemic coccidioidomycosis (3, 5). However, no previous studies have been reported on the efficacies of these types of formulations against coccidioidal meningitis. Thus, we studied the efficacy of one lipid-based AMB formulation, AmBi, against this manifestation of coccidioidomycosis.
Our results demonstrate that AmBi administered intravenously has utility in the treatment of experimental acute coccidioidal meningitis. Animals treated with AmBi showed improved clinical status, with minimal manifestations of neurological disease (reduced mobility, paresis, etc.), improved overall health (i.e., weight gain, reduced fever, etc.), reduced histopathological severity, prolongation of survival, and significant reductions in the burdens of C. immitis remaining in the brain and spinal cord 1 week after the cessation of therapy. FLC treatment was also efficacious, further corroborating our previous results (23); conventional AMB treatment also proved efficacious, but to a lesser degree than FLC treatment, and both of those treatments were less effective than AmBi given at 15 mg/kg.
Interestingly, the efficacy of AmBi was only partially dose responsive; i.e., there was improved efficacy with an increase in the dose from 7.5 to 15 mg/kg, but an increase in the dose of AmBi to 22.5 mg/kg did not result in a further reduction in the numbers of CFU in the tissues. Thus, there appears to be a point at which larger doses are not beneficial. One might speculate that the 22.5-mg/kg dose of AmBi resulted in some deleterious toxicities. However, assays for blood urea nitrogen and serum creatinine concentrations in three to four animals in each treatment arm showed no significant differences in the concentrations over the baseline concentrations for the animals before they became infected (data not shown). More telling may be the serum AMB concentrations after the administration of AmBi. Comparison of these data shows that the increase in the serum AMB concentration was not proportional when the dosage was increased from 15 to 22.5 mg/kg. These pharmacokinetic results are similar to the nonlinear pharmacokinetics of AmBi demonstrated previously in beagle dogs (4). Thus, the 22.5-mg/kg dosage might effectively saturate the reticuloendothelial system and possibly reduce the effectiveness of the macrophages and/or granulocytes in killing the organism. This possibility remains to be investigated but would provide a possible explanation of why the 22.5-mg/kg dose of AmBi appeared to be less effective in reducing the infectious burden and the overall clearance of the organism from the tissues.
Most encouraging from these studies are the results demonstrating that intravenously administered AmBi appears to have excellent efficacy against coccidioidal meningitis. Somewhat surprisingly, conventional AMB given intravenously also showed some efficacy in this model, in contrast to its lack of efficacy in humans when it was given intravenously (24). However, AMB was substantially less effective than FLC in this model, which is in accord with the comparative activities of these drugs in patients (8, 24, 25). The clearance of detectable infection from tissues showed that AmBi at 15 mg/kg was very effective and resulted in the cure of three of the eight treated rabbits. Whether additional or more frequent dosing would result in complete cure in all animals remains to be determined. In contrast, FLC at a dosage of 80 mg/kg, a dosage equivalent to 5.6 g/day in a 70-kg human, did not clear the infection from either the brain or the spinal cord of any animals, nor did a nearly toxic dose of 1 mg of AMB per kg clear the organism from the tissues of any of the animals. Thus, the possibility that a cure may result from intravenous treatment with AmBi in patients with this acutely severe manifestation of coccidioidomycosis is extremely promising and might provide the impetus for improved therapy of patients with coccidioidal meningitis that would avoid the potential neurotoxicity of intrathecal AMB (24).
TABLE 2.
Continued
| Protein concn (mg/dl)a
| ||||||||
|---|---|---|---|---|---|---|---|---|
| Day | Block 1
|
Block 2
|
Block 3
|
|||||
| D5W | FLC | AmBi (15 mg/kg) | D5W | AmB | D5W | AmBi (7.5 mg/kg) | AmBi (22.5 mg/kg) | |
| 0 | 25.6 (5.4) | 20.7 (3.4) | 23.5 (4.1) | 26.5 (7.7) | 28.7 (4.2) | 23.6 (2.4) | 22.1 (3.1) | 24 (4.1) |
| 15 | 229.6 (55.6) | 103.2 (50.9) | 37.5 (7.7) | 155.9 (83.8) | 67.6 (30.4) | 293.8 (232.8) | 63.1 (30.5) | 38.6 (20.2) |
| 30 | 257 (89.9) | 101.2 (55.4) | 33.6 (7.1) | 337.8 (283.5) | 142.8 (91.3) | 652.7 (431.8) | 92.5 (44.1) | 59.3 (13.2) |
The values are means (standard deviations).
Acknowledgments
These studies were funded in part by Gilead Pharmaceuticals, Inc., and the Bank of Stockton, Stockton, Calif.
We thank Byron Brown, Department of Health and Policy, Stanford University, for advice and assistance with the statistical analyses. We thank David Hewitt at Kaweah Delta District Hospital for performing the CSF assays. We thank K. Howell, L. Calderon, R. Ramirez, M. Martinez. and P. Kamberi for assistance during these studies.
REFERENCES
- 1.Adler-Moore, J. 1994. AmBisome targeting to fungal infections. Bone Marrow Transplant. 14:S3-S7. [PubMed] [Google Scholar]
- 2.Adler-Moore, J. P., S. Chiang, A. Satorius, D. Guerra, B. McAndrews, E. J. McManus, and R. T. Proffitt. 1991. Treatment of murine candidosis and cryptococcosis with a unilamellar liposomal amphotericin B formulation (AmBisome). J. Antimicrob. Chemother. 28:63-71. [DOI] [PubMed] [Google Scholar]
- 3.Albert, M. M., K. Adams, M. J. Luther, S. H. Sun, and J. R. Graybill. 1994. Efficacy of AmBisome in murine coccidioidomycosis. J. Med. Vet. Mycol. 32:467-471. [DOI] [PubMed] [Google Scholar]
- 4.Bekersky, I., G. W. Boswell, R. Hiles, R. M. Fielding, D. Buell, and T. J. Walsh. 1999. Safety and toxicokinetics of intravenous liposomal amphotericin B (AmBisome) in beagle dogs. Pharm. Res. 16:1694-1701. [DOI] [PubMed] [Google Scholar]
- 5.Clemons, K. V., and D. A. Stevens. 1991. Comparative efficacy of amphotericin B colloidal dispersion and amphotericin B deoxycholate suspension in treatment of murine coccidioidomycosis. Antimicrob. Agents Chemother. 35:1829-1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clemons, K. V., and D. A. Stevens. 1998. Comparison of Fungizone, Amphotec, AmBisome, and Abelcet for treatment of systemic murine cryptococcosis. Antimicrob. Agents Chemother. 42:899-902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coker, R. J., M. Viviani, B. G. Gazzard, B. Du Pont, H. D. Pohle, S. M. Murphy, J. Atouguia, J. L. Champalimaud, and J. R. Harris. 1993. Treatment of cryptococcosis with liposomal amphotericin B (AmBisome) in 23 patients with AIDS. AIDS 7:829-835. [DOI] [PubMed] [Google Scholar]
- 8.Dewsnup, D. H., J. N. Galgiani, J. R. Graybill, M. Diaz, A. Rendon, G. A. Cloud, and D. A. Stevens. 1996. Is it ever safe to stop azole therapy for Coccidioides immitis meningitis? Ann. Intern. Med. 124:305-310. [DOI] [PubMed] [Google Scholar]
- 9.Drutz, D. J., and A. Catanzaro. 1978. Coccidioidomycosis. Part I. Am. Rev. Respir. Dis. 117:559-585. [DOI] [PubMed] [Google Scholar]
- 10.Drutz, D. J., and A. Catanzaro. 1978. Coccidioidomycosis. Part II. Am. Rev. Respir. Dis. 117:727-771. [DOI] [PubMed] [Google Scholar]
- 11.Galgiani, J. 1997. Coccidioidomycosis Curr. Top. Clin. Infect. Dis. 17:188-204. [PubMed] [Google Scholar]
- 12.Galgiani, J. 1993. Coccidioidomycosis. West. J. Med. 159:153-171. [PMC free article] [PubMed] [Google Scholar]
- 13.Gallis, H. A., R. H. Drew, and W. W. Pickard. 1990. Amphotericin B: 30 years of clinical experience. Rev. Infect. Dis. 12:308-329. [DOI] [PubMed] [Google Scholar]
- 14.Groll, A. H., N. Giri, V. Petraitis, R. Petraitiene, M. Candelario, J. S. Bacher, S. C. Piscitelli, and T. J. Walsh. 2000. Comparative efficacy and distribution of lipid formulations of amphotericin B in experimental Candida albicans infection of the central nervous system. J. Infect. Dis. 182:274-282. [DOI] [PubMed] [Google Scholar]
- 15.Hanson, L. H., A. M. Perlman, K. V. Clemons, and D. A. Stevens. 1991. Synergy between cilofungin and amphotericin B in a murine model of candidiasis. Antimicrob. Agents Chemother. 35:1334-1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hanson, L. H., and D. A. Stevens. 1992. Comparison of antifungal activity of amphotericin B deoxycholate suspension with that of amphotericin B cholesteryl sulfate colloidal dispersion. Antimicrob. Agents Chemother. 36:486-488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hiemenz, J. W., and T. J. Walsh. 1996. Lipid formulations of amphotericin B: recent progress and future directions. Clin. Infect. Dis. 22:S133-S144. [DOI] [PubMed] [Google Scholar]
- 18.Pappagianis, D., and B. Zimmer. 1990. Serology of coccidioidomycosis. Clin. Microbiol. Rev. 3:247-268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Proffitt, R. T., A. Satorius, S. M. Chiang, L. Sullivan, and J. P. Adler-Moore. 1991. Pharmacology and toxicology of a liposomal formulation of amphotericin B (AmBisome) in rodents. J. Antimicrob. Chemother. 28(Suppl. B):49-61. [DOI] [PubMed] [Google Scholar]
- 20.Rex, J. H., L. H. Hanson, M. A. Amantea, D. A. Stevens, and J. E. Bennett. 1991. Standardization of a fluconazole bioassay and correlation of results with those obtained by high-pressure liquid chromatography. Antimicrob. Agents Chemother. 35:846-850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sokal, R. R., and F. J. Rohlf. 1981. Biometry, p. 432-435. Biometry, 2nd ed. W. H. Freeman & Co., San Francisco, Calif.
- 22.Sorensen, K. N., R. A. Sobel, K. V. Clemons, L. Calderon, K. J. Howell, P. R. Irani, D. Pappagianis, P. L. Williams, and D. A. Stevens. 2000. Comparative efficacies of terbinafine and fluconazole in treatment of experimental coccidioidal meningitis in a rabbit model. Antimicrob. Agents Chemother. 44:3087-3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sorensen, K. N., R. A. Sobel, K. V. Clemons, D. Pappagianis, D. A. Stevens, and P. L. Williams. 2000. Comparison of fluconazole and itraconazole in a rabbit model of coccidioidal meningitis. Antimicrob. Agents Chemother. 44:1512-1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stevens, D. A. 1986. Coccidioidal menigitits, p. 1074-1076. In A. Braude (ed.), Infectious diseases and medical microbiology, vol. II, 2nd ed. The W. B. Saunders Co., Philadelphia, Pa.
- 25.Stevens, D. A. 1995. Coccidioidomycosis. N. Engl. J. Med. 332:1077-1082. [DOI] [PubMed] [Google Scholar]
- 26.Tucker, R. M., J. N. Galgiani, D. W. Denning, L. H. Hanson, J. R. Graybill, K. Sharkey, M. R. Eckman, C. Salemi, R. Libke, R. A. Klein, and D. A. Stevens. 1990. Treatment of coccidioidal meningitis with fluconazole. Rev. Infect. Dis. 12(Suppl. 3):S380-S389. [DOI] [PubMed] [Google Scholar]
- 27.Viviani, M. A., G. Rizzardini, A. M. Tortorano, M. Fasan, A. Capetti, A. M. Roverselli, A. Gringeri, and F. Suter. 1994. Lipid-based amphotericin B in the treatment of cryptococcosis. Infection 22:137-142. [DOI] [PubMed] [Google Scholar]
- 28.Williams, P. L., R. A. Sobel, K. N. Sorensen, K. V. Clemons, L. M. Shuer, S. S. Royaltey, Y. Yao, D. Pappagianis, J. E. Lutz, C. Reed, M. E. River, B. C. Lee, S. O. Bhatti, and D. A. Stevens. 1998. A model of coccidioidal meningoencephalitis and cerebrospinal vasculitis in the rabbit. J. Infect. Dis. 178:1217-1221. [DOI] [PubMed] [Google Scholar]