Short abstract
Deciding whether to fund treatments that do good one by one tends to lead to a positive decision. However, this can cause wider harmful effects, as West Midlands' experience in the funding of enzyme replacement therapy for lysosomal storage diseases shows
Orphan drugs tend to be expensive for two reasons. Firstly, development and production costs need to be offset in low volume sales, and, secondly, the monopoly position of manufacturers (entrenched within legislation to provide an incentive to develop treatments for rare conditions) permits large profit margins. Historically, the NHS has paid for expensive orphan drugs. It could do so because treatments for these diseases were so rare that the effect on health services was negligible. This policy is increasingly being questioned. As more and more expensive orphan drugs come on to the market, the impact on other health services is becoming substantial. In addition, since the establishment of the National Institute for Clinical Excellence (NICE) in 1999, the idea that technologies should reach minimum standards of cost effectiveness has become widely accepted.
However, efficiency is not the only principle in resource allocation: we also value equity and caring. Indeed, the abrogation of the principle of efficiency (by more generous reimbursement of treatments for rare diseases) is usually defended on such grounds. However, as we move towards more explicitness in decision making, which requires us to show that principles are being applied consistently, incoherence and tensions within the equity argument have begun to surface. McCabe and colleagues cite various reasons given in support of a more generous reimbursement policy for orphan drugs and refute each on theoretical grounds.1 We approach the issue from a different perspective, that of the frontline commissioner. We tell the story of what happened during 2002-5, when commissioners responsible for health services in the West Midlands tried to approach such decisions in an explicit, justifiable manner.
The context
In England, primary care trusts are responsible for securing health services for their local populations. For high cost, low volume activities, trusts are expected to collaborate with neighbouring trusts to commission specialised services.2 Services covering populations of 3-6 million are commissioned regionally, whereas services with a national caseload under 400 tend to be commissioned nationally through the National Specialist Commissioning Advisory Group.3
The question
The decision facing West Midlands concerned enzyme replacement therapy for lysosomal storage diseases. These are a group of rare inherited deficiencies in enzymes that degrade cellular material. Treatment aims to replace the deficient enzyme, thereby preventing accumulation of material and consequent ill health.
In 2001, the West Midlands was funding enzyme replacement therapy for Gaucher's disease, the only lysosomal storage disease that had a specific treatment at the time. In 2002, a new enzyme was licensed for Fabry's disease, and primary care trusts needed to decide whether to fund it. No comprehensive framework for making such decisions was in place.
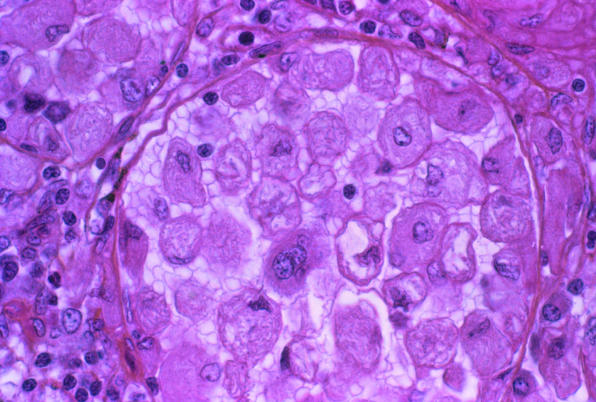
Figure 1.
Spleen of patient with Gaucher's disease: enzyme replacement therapy can prevent accumulation of glucocerebroside
Credit: CNRI/SPL
Although the evidence supporting enzyme replacement therapy is thin, this was not the main issue. Even if the drugs were 100% effective, the question remained whether they produced enough benefit to justify their cost, given other claims on resources. Over 5000 diseases are classified as rare in the European Union, and, in just four years, orphan drug designations and approvals have increased more than 10-fold. The potential effect of increasing numbers of high cost treatments is enormous. At the time, it was known that an enzyme replacement therapy for mucopolysaccharidosis 1 would shortly become available, and similar treatments for other lysosomal storage diseases were on the horizon. West Midlands primary care trusts decided, therefore, to develop a coherent commissioning approach to these orphan drugs that was compatible with their primary aim of providing comprehensive health care and their legal duty to stay within budget.
Process and facts
The West Midlands Specialist Services Agency formally investigated the ethical, legal, and other policy considerations of funding orphan drugs; commissioned reports on the public perspective,4 ethical issues,5 and clinical and cost effectiveness (Burls et al, unpublished data)6,7 and sought legal advice. The public were reluctant to engage in prioritisation, believing that: “NHS treatment should be provided regardless of cost if it could improve a patient's condition,” with more money simply being made available through taxes.4 They thought that: “If this kind of decision, about whether or not to fund particular treatments, had to be made at the local level, it should be by a group of people that included people with different areas of expertise such as finance managers and doctors who had expert knowledge...”4
After lengthy deliberations, it was concluded that rarity and being identifiable were not in themselves overriding factors to be considered in the decision. No principled argument could be identified that distinguished patients with rare disease from those with common conditions, and all patients are potentially identifiable if they have a treatment need that is not being met. The cost effectiveness for all enzyme replacement therapies was over £200 000 ($350 000, €290 000) for each quality adjusted life year (QALY), well above the oft cited £30-40 000 UK threshold (table).
Table 1.
Enzyme replacement therapies licensed for use in the UK
| Treatment | Disease | Annual drug cost for average adult (£) | Cost/QALY (£) | Approximate No of patients in England and Wales |
|---|---|---|---|---|
| Imiglucerase | Gaucher's disease | 90 0008 | 400 0008 | 2508 |
| Agalsidase beta | Fabry's disease | 119 0009 | 252 0009 | 1509 |
| Agalsidase alpha | ||||
| Laronidase | Mucopolysaccharidosis 1* | 450 000† | Not available but >450 000 | 969 |
Hurler, Hurler-Scheie, and Scheie's syndromes.
Charge to West Midlands region for annual cost of this drug for one patient in 2005 (National Specialist Commissioning Advisory Group, personal communication.)
Primary care trusts wanted a consistent, justifiable policy. Legal advice was that it is inconsistent to fund treatment for one lysosomal storage disease and not another unless there was a principled argument to distinguish them. None was apparent. This meant that as trusts were funding enzyme replacement therapy for Gaucher's disease, they should also fund these treatments for the other lysosomal storage diseases. However, the trusts were also advised that consistency did not require the continued funding of a service because it had been funded in the past.
Although manufacturers argued that European orphan medical products regulations obliged primary care trusts to fund orphan drugs, this is untrue. The legislation is directed at encouraging the development of drugs for orphan diseases by reducing development costs and providing market incentives; it is not about reimbursement decisions.
The decision
The commissioning group's recommendation, endorsed by the boards of all 30 primary care trusts, was not to support funding of enzyme replacement therapy for Fabry's disease, mucopolysaccharidosis 1, and new patients with Gaucher's disease. The drugs were considered poorly cost effective. The potential long term costs, possibly reaching £20m/patient, could not be justified on the grounds of equity given that many more patients, with equal capacity to benefit from treatment, would be deprived of treatments.
Consequences
The decision caused consternation. Patients and carers were understandably worried, and patient groups lobbied hard on their behalf. Lawyers from Genzyme raised concerns about their “legitimate commercial expectations.” Politicians were concerned: discrepant decisions about funding across the country raised the spectre of postcode prescribing (treatment being determined by where you live rather than medical need).
The Department of Health decided to move commissioning for lysosomal storage diseases to the national level; from April 2005 and for the next two years, treatments for these diseases are being commissioned by the National Specialist Commissioning Advisory Group.10 It has placed no restrictions on the use of enzyme replacement therapy in accordance with licensed indications. So, a happy end to the West Midlands' funding dilemma? Unfortunately, not. The new commissioning arrangement did not bring with it funding; the costs are directly levied from primary care trusts.11 Moreover, the cost to trusts doubled, from around £3.2m to £6.7m and “To be of such an order of magnitude... as to significantly distort and limit budgets available to PCTs to commission and develop other services in 2005/06” (West Midlands Specialised Commissioning Group, letter to Department of Health, 8 June 2005).
The National Specialist Commissioning Advisory Group's 2005-6 service and drug budget for lysosomal storage diseases alone is £63m. Although their criteria say a service needs “To be able to justify its costs when set against alternative uses of NHS funds,”3 none of its published documents examine the cost effectiveness or opportunity costs of enzyme replacement therapy. These costs are very real. The increase levied on West Midlands primary care trusts for this single service was greater than the entire increase in all acute regionally commissioned services, including several high cost and growing specialty areas such as blood and marrow transplantation, blood products for haemophiliacs, neonatal intensive care and special care services for babies, paediatric intensive care, adult and paediatric burns services, and laboratory and clinical genetics services.
Discussion
Although most people would like every effective treatment to be available, this is not possible within current, and probable future, budgets, and commissioners have to make difficult decisions about resource allocation. Although efficiency is important, so is equity. Funding enzyme replacement therapy for lysosomal storage diseases is not efficient in that it does not maximise health gain, but is it equitable? Obviously it would be unfair not to treat patients only because their disease is rare, but is it unfair not to treat them because it is very expensive? If the answer is “yes,” does it not follow that it must be unfair not to treat patients with common conditions because they are very expensive? Indeed in Canada enzyme replacement therapy for mucopolysaccharidosis 1 was turned down for precisely this reason: “Reimbursement of laronidase would raise questions about equity, since drugs that have not been shown to be cost-effective for other diseases are not generally reimbursed.”12
Organisations representing patients with rare disorders tend (legitimately) to advocate on an individual patient basis, citing precedents to support their case. This evokes our natural sympathy by highlighting the patient's health problems but obscures the opportunity cost and the wider policy implications. Although it is important that individuals have an opportunity to make appeals against policy decisions, these appeals should be approved only on principled arguments that are generalisable. Otherwise, the process would be unfair and inconsistent.
We support the principle of commissioning services and treatments for rare diseases at a national level. However, the current situation, where a national body commissions services but costs fall, without consultation, on a budget serving other patients for whom the national body is not responsible, is of concern. It is particularly concerning as England has no national policy or ethical framework for dealing with treatments whose costs per QALY well exceed accepted levels. We believe this “fudge” is neither efficient nor fair and is unlikely to be sustainable in the longer term. It has meant that important services in the West Midlands cannot be commissioned.
It is time to educate ourselves, policy makers, and the public. We need to learn how to make trade-offs between equity and efficiency that are explicit, principled, and generalisable and how to admit openly when there are treatments and services that are not being funded. In the words of one lay member of NICE's Citizen's Panel:
Difficult decisions do have to be made within the NHS. Too often these decisions are made in secret. But they should know that we do support them and we know they have to keep within a budget. The NHS shouldn't be frightened of the public finding out about all this—they should discuss it more with the public. They'll only keep our confidence if they level with us about the difficult choices that have to be made.13
Summary points
In a system with finite resources that do not meet all needs, money spent on one service means that some other service cannot be provided (opportunity cost)
Commissioning decisions should not be posed as isolated questions but need to take into account other priorities
A national decision to fund expensive enzyme replacement therapies for lysosomal storage disorders prevented primary care trusts funding other equally vital services
Cost effectiveness thresholds are a shorthand way of taking opportunity cost into account; therefore, if decisions breach these norms they should be justified by principled argument
The current system, which obscures the opportunity cost, is inefficient, unfair, and unsustainable given the growth in orphan drugs
Contributors and sources: The opinions expressed in this paper are those of the authors and not the views of the organisations for which they work. DA chaired the regional group that supported the development of the West Midlands' commissioning policy on enzyme replacement therapy and commissioned the reports on the effectiveness and cost-effectiveness of enzyme replacement therapies in lysosomal storage disorders. DM and AB were authors on full health technology assessments commissioned by the National Coordinating Centre for Health Technology Assessment. DA subsequently represented commissioners during the NICE feasibility study on ultra-orphan drugs. Information used in this paper comes from the working documents of the commissioning review and the systematic reviews and technology assessments produced.
Competing interests: None declared.
References
- 1.McCabe C, Claxton K, Tsuchiya A. Orphan drugs and the NHS: should we value rarity? BMJ 2005;331: 1016-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Department of Health. Guidance on commissioning arrangements for specialised services. London: DoH, 2003.
- 3.Department of Health. National Specialist Commissioning Advisory Group (NSCAG). London: DoH, 2005.
- 4.McIver S. The views of the public about funding enzyme replacement therapy. Birmingham: West Midlands Specialised Services Agency, 2004.
- 5.Sheehan MP. Enzyme replacement therapy, resource allocation and distributive justice. Birmingham: West Midlands Specialised Services Agency, 2004.
- 6.Aggressive Research Intelligence Facility. Recombinant human alpha-L-iduronidase for the treatment of patients with mucopolysaccharidosis type 1. Birmingham: West Midlands Specialised Services Agency, 2004.
- 7.Aggressive Research Intelligence Facility. Recombinant human alphagalactosidase for the treatment of patients with Fabry's disease. Birmingham: West Midlands Specialised Services Agency, 2004.
- 8.Connock MJ, Burls A, Frew E, Fry-Smith A, Juarez-Garcia A, McCabe C, et al. The clinical effectiveness and cost-effectiveness of enzyme replacement therapy for Gaucher's disease. Health Technol Assess (in press). [DOI] [PubMed]
- 9.Connock MJ, Frew E, Juarez-Garcia A, Mans A, Dretzke J, Fry-Smith A, Moore D. The clinical effectiveness and cost-effectiveness of enzyme replacement therapies for Fabry's disease and mucopolysaccharidosis type 1. Health Technol Assess (in press). [DOI] [PubMed]
- 10.National Specialised Commissioning Advisory Group. Lysosomal storage disorders: NSCAG's policy on the funding of enzyme replacement therapy and substrate reduction therapy. London: DoH, 2005.
- 11.Department of Health. Mechanisms and funding of NSCAG. www.advisorybodies.doh.gov.uk/nscag/how.htm (accessed 2 Oct 2005).
- 12.Canadian Coordinating Office for Health Technology Assessment. Laronidase CEDAC Final recommendations and reasons for recommendation. Ottowa: CCOHTA, 2005.
- 13.National Institute for Clinical Excellence. NICE citizen's council report on ultra orphan drugs. London: NICE, 2004.