Abstract
Chlamydia pneumoniae may play a role in atherogenesis and vascular diseases, and antibiotics may prove useful in these conditions. Three groups of New Zealand White rabbits (24 per group) were infected via the nasopharynx with C. pneumoniae on three separate occasions (2 weeks apart). Group I was untreated and sacrificed at 12 weeks; group II received clarithromycin at 20 mg/kg/day for 8 days, beginning 5 days after each inoculation (early treatment); and group III received a similar dose of clarithromycin starting 2 weeks after the third inoculation and continued for 6 weeks thereafter (delayed treatment). To test for a possible anti-inflammatory effect of clarithromycin, two other groups of uninfected rabbits (12 animals in each) were fed 0.5% cholesterol-enriched chow, and one of these groups was treated with clarithromycin at 30 mg/kg/day for 6 weeks. Of 23 untreated infected rabbits, 8 developed early lesions of atherosclerosis, whereas 2 of the 24 early-treated group II had similar changes (P = 0.036 [75% efficacy]). However, in the delayed-treatment group, group III, 3 of 24 rabbits developed early lesions of atherosclerosis, thus demonstrating 62.5% reduction compared to the untreated controls (P = 0.07 [trend to statistical significance]). C. pneumoniae antigen was detected in 8 of 23 group I (untreated) rabbits versus 1 of 24 of the early-treated (group II) rabbits and 4 of 24 animals in the delayed group III (P = 0.009 and 0.138, respectively). All of the untreated, cholesterol-fed rabbits had moderate to advanced atherosclerosis (grade III or IV); clarithromycin had no effect on reducing the prevalence of but did reduce the extent of atherosclerosis in the cholesterol-fed rabbits by 17% compared to untreated controls. Thus, clarithromycin administration modified C. pneumoniae-induced atherosclerotic lesions and reduced the ability to detect organism in tissue. Early treatment was more effective than delayed treatment.
Recent preliminary clinical trials have suggested that newer macrolides may reduce secondary events after heart attack (15, 16). These studies were done on the premise that atherosclerosis and vascular complications may be partially caused by an infectious agent, i.e., Chlamydia pneumoniae.
C. pneumoniae is a common respiratory pathogen responsible for community-acquired pneumonia, sinusitis, and bronchitis (11-13). It is also strongly implicated in the pathogenesis of atherosclerosis based on seroepidemiological studies (6, 14, 32, 33), histopathological findings of the organisms in atheromatous plaques (1, 2, 4, 20, 36), the recovery of viable organisms from carotid and coronary diseased arteries (19, 22), and various animal models. We and others were able to induce vascular damage of the aorta resembling early lesions of atherosclerosis in the rabbit by C. pneumoniae infection by itself (7, 21) or by C. pneumoniae infection in combination with a low-cholesterol-enriched chow (27) that can enhance atherosclerosis.
Clarithromycin, like all macrolides, shows potent in vitro activity against C. pneumoniae and is a recommended treatment for community-acquired pneumonia and lower-respiratory-tract infection with this organism. However, there is no report of clarithromycin on C. pneumoniae-related atherosclerosis in the animal model. Furthermore, the newer macrolides have anti-inflammatory activity, and studies are needed to determine whether any possible modifying effect on the development of atherosclerosis could be related to this nonspecific activity rather than the antimicrobial effect.
The objectives of the present study were twofold, First, we wanted to determine whether a macrolide (clarithromycin) active against C. pneumoniae could prevent atherosclerosis after infection in the rabbit by early treatment or reverse the changes with delayed treatment. Second, we sought to determine whether clarithromycin could prevent or reduce atherosclerotic changes in rabbits fed cholesterol-enriched chow but that were not infected with C. pneumoniae, since macrolides have anti-inflammatory properties (35, 38).
MATERIALS AND METHODS
This study was approved by our institution's (St. Michael's Hospital) Animal Care Committee, and the guidelines were strictly adhered to.
Animals.
One-month-old, male, New Zealand White, specific-pathogen-free rabbits were used in all of the experiments. Three groups I to III (24 animals per group) were fed standard chow without cholesterol supplementation and were infected with C. pneumoniae. Two additional groups (12 animals per group) were fed 0.5% cholesterol enriched chow but were not infected with C. pneumoniae. Euthanasia was achieved at sacrifice by injection of concentrated pentobarbitol (340 mg/ml) at 0.3 ml/kg in the marginal ear lobe vein.
C. pneumoniae strains and inoculum.
TWAR strain AR-39 (Seattle, Wash.) and ATCC strain VR1310 (originally respiratory isolates) were used in the studies. Strain AR-39 was used in the first inoculation, and strain VR1310 was used in the subsequent two inoculations for groups I to III. The organisms were grown in HEP-2 cells (31). Infected cells were harvested with sterile glass beads and ultrasonic disruption after 72 h. Cell culture grown organisms were partially purified by one cycle each of low- and high-speed centrifugation, resuspended in sucrose-phosphate-glutamic acid buffer, and frozen in 1.0-ml aliquots at −70°C. Inoculum preparations were adjusted to contain 1.5 × 107 to 2.6 × 107 inclusion-forming units of C. pneumoniae in 1.0 ml. Contamination by Chlamydia trachomatis, Chlamydia psittacci, or Mycoplasma species was excluded by analysis with PCR genus- and species-specific primers (23) and monoclonal antibody stain.
Design. (i) Group I.
Group I (untreated controls) were inoculated with C. pneumoniae via the posterior nasopharynx on three separate occasions, 2 weeks apart, by using a small catheter without any sedation, and were sacrificed at 12 weeks after the first inoculation.
(ii) Group II.
Group II animals were inoculated as described above, but 5 days after each inoculation the rabbits were treated with clarithromycin at 20 mg/kg/day by oral gavage for 8 days each time. Animals were sacrificed at 12 weeks after the first inoculation.
(iii) Group III.
Group III animals were inoculated similarly, but treatment was delayed until 2 weeks after the final inoculation, with the same dose of clarithromycin but given daily for 6 weeks until sacrifice, which occurred 12 weeks after the first inoculation.
(iv) Group IV.
Rabbits in group IV were fed cholesterol-enriched chow, were not infected, and were sacrificed at 12 weeks (untreated cholesterol control).
(v) Group V.
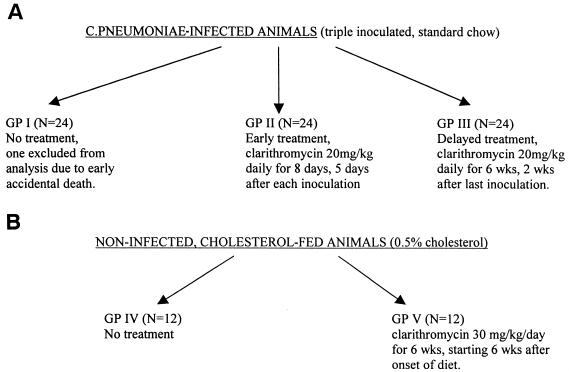
After 6 weeks of being fed cholesterol-enriched chow, the rabbits in group V were treated with clarithromycin at 30 mg/kg/daily for 6 weeks until sacrifice 12 weeks after starting. (See Fig. 1 for a flow diagram of the study design.)
FIG. 1.
Flow diagram of the study design.
The experiments on the separate groups were performed in batches at separate times with decontamination of the room after each experiment. The treatment was not blinded, but the investigator assessing the histopathology and immunostain was blinded to the specific group or treatment.
The initial dosage of clarithromycin chosen was based on preliminary pharmacokinetic data noted in a pilot study of four rabbits receiving a dose of 20 mg of clarithromycin/kg that resulted in levels in blood similar to those of seen in humans; unfortunately, the initial assay used was subsequently found to be inaccurate. The dosage of 30 mg of clarithromycin/kg was subsequently used in the cholesterol-fed rabbits when we realized that the 20-mg/kg dosage resulted in blood levels that were lower than predicted. A higher daily dose was not chosen since rabbits were poorly tolerant of clarithromycin at a higher dose (Abbott Laboratories [unpublished data]).
Antibiotic assay.
Blood was taken from a random sample of rabbits in groups II, III, and V for the assay of the concentrations of clarithromycin and 14-hydroxyclarithromycinserum in serum. Four rabbits received 20 mg of clarithromycin/kg/day, and four received 30 mg/kg/day. The blood was taken after the second day of dosing at 4, 12, and 24 h of dosing. The serum assay was performed by BAS Analytics (West Lafayette, Ind.) by high-pressure liquid chromatography and mass spectrophometry. BAS Analytics, a division of Bioanalytical Systems, Inc., had validated a high-performance liquid chromatographic method for the determination of clarithromycin and 14-hydroxyclarithromycin in rabbit serum. This assay utilizes liquid-liquid extraction, followed by liquid chromatography coupled to triple quadrupole mass spectrometry (LC/MS/MS).
Clarithromycin (A-56268) and 14-hydroxyclarithromycin (A-62671) were extracted from rabbit serum (0.200 ml) by liquid-liquid extraction. Before the extraction, erythromycin B (A-24091.0; internal standard for A-56268 and A-62671, 50 μl of a 5-μg/ml working solution), water (1.0 ml), carbonate solution (1.0 ml), and methyl-t-butyl ether (2 ml) were added to the sample. The samples were vortexed and centrifuged. The ether layer was transferred to a clean tube and evaporated under nitrogen to dryness. The residue was reconstituted in 100 μl of 23% acetonitrile-77% (50.0 mM) ammonium acetate (pH 4.90 ± 0.05). The extract was injected into an atmospheric pressure chemical ionization LC/MS/MS system utilizing two short cyano columns coupled in series, with a 23% acetonitrile-77% (50.0 mM) ammonium acetate mobile phase.
Calibration standard samples of A-56268 were created from blank rabbit serum spiked in the concentration range from 1,000 to 10.0 ng/ml. Calibration standard samples of A-62671 were created from blank rabbit serum spiked in the concentration range from 800 to 10.0 ng/ml. The quality control sample concentrations for both compounds were 750, 150, and 30.0 ng/ml.
The precision for six replicates for the upper limit of quantitation (set at 1,000 ng/ml) was ±3.6%, and the accuracy of the mean was 101.6%. The precision for the lower limit of detection (set at 10 ng/ml) for six replicates was ±4.2%, and the accuracy of the mean was 96.2 to 98.2%.
Pathology.
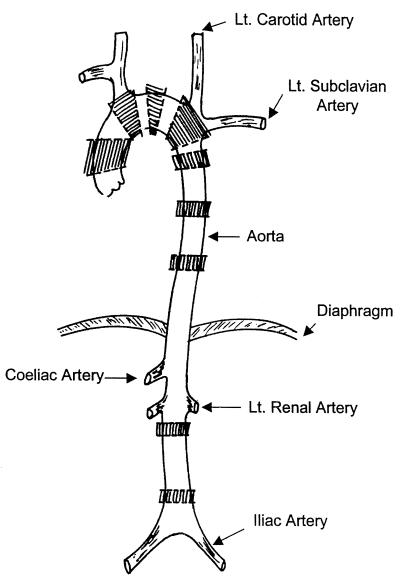
At sacrifice, the entire aorta from the ascending aorta to the iliac bifurcation was removed and cleansed of any fat. The aorta was split longitudinally, and sections were taken from the ascending, arch, descending, and abdominal portions of the aorta. Multiple sections were taken from the ascending, arch, descending, and abdominal portions of the aorta by a standardized method from rabbits in all of the infected groups (Fig. 2).
FIG. 2.
Schematic diagram of the aorta of the rabbit. The areas of sectioning for histopathology are indicated by shading.
All specimens obtained were fixed in 10% buffered formalin, processed, and paraffin embedded. Hematoxylin and eosin (H&E) staining and elastic (Movat's pentachrome) staining were performed on sections for histological examination. Selected sections were processed for examination under electron microscopy. Aortic lesions were graded histologically, modified from Daley et al. (5), as follows. For grade I, an early fatty streak is defined as consisting of foamy cells (identified as macrophages by immunohistochemical stain) in the intima. These lesions are similar to Stary's type I and II lesions (37). For grade II, advanced fatty streaks are defined as consisting of approximately equal numbers of foam cells and spindle-shaped cells (fibrofatty lesions similar to Stary's type III lesion). For grade III, a spindle cell lesion is defined as consisting of spindle-shaped (smooth muscle) cells and other products (this lesion is unlike any lesion in Stary's classification). For grade IV, an advanced atheromatous lesion is defined as the presence of a core containing pools of extracellular lipid and/or necrotic debris and fibrous cap (similar to Stary's type IV lesions, seen in the cholesterol fed but not the infection model). Advanced spindle cell lesion with calcification in the infection model was also considered grade IV (similar histologically to Stary's type VII lesion but does not grossly impinge on or narrow the lumen, as is typically seen with Stary's type VII lesion. Foam cells were recognized by their microscopy appearance upon H&E staining and electron microscopy. The cholesterol concentration of the infection-induced lesions could not be measured because of their small, discrete, patchy nature.
Other vascular changes in the infection model that were noted but not classified as atherosclerotic lesions included myxoid changes and “periaortitis.” Myxoid lesions were defined as isolated accumulation of ground substance in the aortic intima and intima-medium junction, demonstrated by light blue-green staining on Movat's pentachrome stain. In experimental atherosclerosis with either injury or hypercholesterolaemic models, there is increased ground substance or glycosaminoglycans during early atherogenesis (34). Periaortitis represents the focal accumulation of inflammatory mononuclear cells between the outer wall of the aorta and the adventitia. These cells consist of macrophages and T and B lymphocytes, as shown by immunohistochemical stains with specific monoclonal antibodies.
Sudan IV staining.
Sudan IV staining was carried out according to a previously described method (30) on aortic surfaces from the aortic arch to the iliac bifurcation of the cholesterol-fed groups (groups IV and V) to detect intimal fat for quantitation of gross atherosclerosis (24). The extent of staining was evaluated by measuring the total area and the stained area by using Sigma Scan Pro(Jandel Scientific, San Rafael, Calif.) software and an IBM computer with a scanner (30).
Immunohistochemical study.
Immunohistochemical staining (20) for C. pneumoniae antigen was performed on paraffin-embedded sections by the labeled (strept)avidin-biotin-peroxidase method (3) by using the Histo-Stain kit (Zymed). The antisera used included C. pneumoniae-specific monoclonal antibody RR-402 and Chlamydia genus-specific antibody CF-2 (Washington Research Foundation, Seattle), as well as a second C. pneumoniae-specific monoclonal antibody Chlamydia Cel Pn (Cell Lab). In selected cases, factor VIII and smooth muscle actin (Dako), T cells, B cells, and macrophage markers specific for rabbits (Serotec) were added to adjacent sections to identify endothelium cells, smooth muscle cells, T cells, B cells, and macrophages, respectively.
A negative control experiment with phosphate-buffered saline instead of primary antibodies was performed in each run. Positive controls for C. pneumoniae included positive lung and spleen samples from our previous study (7), and cell pellets of the C. pneumoniae inoculum prepared in Hep-2 cells were fixed in formalin and embedded in paraffin.
Blinding.
The pathologist reading the histopathology and the immunohistochemical slides was blinded to the groups from which the sections of aorta were obtained.
Data analysis.
The prevalence of atherosclerotic lesions in the treated groups was compared to the untreated group by Wilcoxon rank sum test by using SAS software (version 7). A quantitative assessment could not be performed since the lesions were small, discrete, patchy foci in groups I to III. The difference in the surface area of the aorta staining red with the Sudan IV between groups IV and V was analyzed by analysis of covariance with adjustment for the total area, and the prevalence of C. pneumoniae antigen in the various groups was compared by using χ2 analysis or Fisher's exact test.
RESULTS
One of the rabbits in the untreated group died accidentally before completion of the experiment and was excluded from the analysis. Of the 23 untreated rabbits (group I), 8 developed early patchy lesions of atherosclerosis in the arch and descending aorta (predominantly grade III lesions, with mainly spindle cell proliferation). These lesions were grossly visible as discrete foci of slightly raised, pale patches. Another five rabbits had myxoid-like changes accumulating between the intima and media, without other changes of atherosclerosis; and eight animals showed focal accumulation of mononuclear cells on the outer wall of the abdominal aorta, adjacent to the adventitia, a condition we classified as “periaortitis.” The results of early and delayed treatment with clarithromycin are shown in Table 1. In the early treated group II, 2 of 24 animals developed grade II and III lesions of the aorta; in the delayed-treatment group III, 3 of 24 rabbits had atherosclerotic lesions (one with a grade I lesion and two with grade IV lesions) (P = 0.036 and 0.07, respectively, for the early versus delayed-treatment groups).
TABLE 1.
Effect of early and delayed treatment with clarithromycin on atherosclerotic lesions of the aorta
| Group | n | No. of animals with lesion(s)
|
Total no. of animals with lesions (%) | % Efficacy | Pa | |||
|---|---|---|---|---|---|---|---|---|
| Grade I | Grade II | Grade III | Grade IV | |||||
| Controls (group I) | 23 | 7 | 1 | 8 (34.8) | ||||
| Early treatment (group II) | 24 | 1 | 1 | 2 (8.3) | 75 | 0.036 | ||
| Delayed treatment (group III) | 24 | 1 | 2 | 3 (12.5) | 62.5 | 0.07 | ||
As determined by Wilcoxon rank sum one-tail test.
Early treatment after infection was moderately effective (75%) in preventing changes of early atherosclerosis of the aorta. Delayed treatment was less effective (62.5%), with a trend toward a significant difference compared to the untreated controls. Treatment was not effective in preventing the myxoid changes seen in the untreated group, but the periaortitis lesions were absent in the two treated groups. In the cholesterol-fed groups (IV and V), all rabbits developed advanced atherosclerotic changes (grade III to IV), with grossly visible diffuse raised lesions. The morphometric analysis of the Sudan IV-stained aortas showed ca. 17% difference in the mean areas of involvement between the untreated and the clarithromycin-treated groups (68.1% ± 10.8% [standard deviation] versus 51.2% ± 21.1%, respectively [P = 0.022]). The mean total concentrations of cholesterol in serum in these two groups (groups IV and V) were similar: 57.7 versus 56.8 mmol/liter.
The concentrations of clarithromycin and its 14-hydroxy metabolite in the sera of rabbits treated with two different doses of clarithromycin are summarized in Table 2. The results of the immunostain for C. pneumoniae antigen are summarized in Table 3. Positive immunostain for C. pneumoniae antigen was localized to atherosclerotic and periaortitis lesions and rarely to areas of myxoid changes. In the untreated animals, 8 of 23 (group I) had detectable C. pneumoniae antigen in the aorta, whereas 1 of 24 of the early treatment group II and four of the 24 delayed-treatment rabbits (group III) had detectable antigen (P = 0.009 and 0.138, respectively).
TABLE 2.
Mean concentration of clarithromycin and 14-hydroxyclarithromycin in serum at two different dosages after the second day
| Time (h) postdose | Mean concn (μg/ml)a ± SD in:
|
|||
|---|---|---|---|---|
| Uninfected rabbits fed cholesterol-enriched dietb (n = 4)
|
Infected rabbits on normal chowc (n = 4)
|
|||
| CLA | 14-HCLA | CLA | 14-HCLA | |
| 4 | 0.534 ± 0.269 | 0.271 ± 0.326 | 0.168 ± 0.084 | 0.126 ± 0.078 |
| 12 | 0.172 ± 0.138 | 0.03 ± 0.17 | 0.010 ± 0.007 | 0.011 ± 0.011 |
| 24 | <0.01 | <0.01 | <0.01 | <0.01 |
CLA, clarithromycin; 14-HCLA, 14-hydroxyclarithromycin.
At dose of 30 mg/kg/day.
At dose of 20 mg/kg/day.
TABLE 3.
Prevalence of C. pneumoniae antigen in the aortas of rabbits left untreated or treated with clarithromycin
| Group | n |
C. pneumoniae antigen prevalence
|
|||
|---|---|---|---|---|---|
| No. of segments positive/total no. examined (%) | Pa | No. of animals positive (%) | Pa | ||
| Controls (group I) | 23 | 11/69 (15.9) | 8 (34.8) | ||
| Early treatment (group II) | 24 | 1/72 (1.4) | 0.002 | 1 (4.2) | 0.009 |
| Delayed treatment (group III) | 24 | 7/72 (9.7) | 0.196 | 4 (16.7) | 0.138 |
As determined by Fisher's exact test.
DISCUSSION
C. pneumoniae can induce changes in the aortas of rabbits not receiving a cholesterol-supplemented diet that resemble the early lesions of atherosclerosis. Macroscopically, the lesions in the aorta are small, slightly raised, barely visible, discrete, and few in number, whereas the cholesterol-induced lesions were more diffuse, prominent, and widespread. Microscopically, the lesions in the infection model contain less foam cells than the cholesterol model and were more fibromuscular (with predominantly spindle cells). However, we have previously shown that the lesions produced in the rabbit aorta by C. pneumoniae infection closely resemble microscopically the lesions produced by a 0.15% cholesterol diet that result in cholesterol levels in serum similar to those recommended for humans (8). We have demonstrated that these changes do not occur in sham-infected or Mycoplasma pneumoniae-infected rabbits (8) and thus do not appear to be the nonspecific effects of any chronic infection. C. pneumoniae infection has also been demonstrated to enhance the aortic changes seen with a cholesterol-enriched diet in the rabbit (27) and to accelerate atherosclerosis in hypercholesterolemic murine models (18, 25). The enhancement of intimal thickening or atherosclerotic changes seen in cholesterol-fed animals infected with C. pneumoniae may not be specific for this microorganism, as is also seen in rabbits infected with Pasteurella multocida and fed a cholesterol-enriched diet (30). However, unlike an infection with C. pneumoniae, a P. multocida infection alone without cholesterol supplementation does not induce aortic damage (Mary Richardson, unpublished data).
Our study has shown that acute treatment with clarithromycin soon after inoculation is moderately effective (75%) in preventing atherosclerotic changes. Similar results were seen with early treatment with azithromycin administered at 30 mg/kg/day initially for 3 days and then every 6 days until sacrifice (87% efficacy) (9). However, delayed treatment with clarithromycin was partially effective (62.5% efficacy), and a larger sample size might have shown greater statistical significance. The delayed treatment with clarithromycin appears promising and more effective than that seen with intermittent dosing of azithromycin for 4 weeks (which showed no reduction of lesions) (9). Early treatment represents a preventative strategy following respiratory infection and before the development of aortic lesions, whereas delayed treatment can be used to assess the ability of the antibiotic to reverse preformed lesions, since intimal lesions can develop within 4 weeks (7). Clearly, the preventative strategy is more effective. The reasons for the apparent greater efficacy of clarithromycin compared to azithromycin after delayed treatment may be due to daily dosing and longer duration of therapy rather than intrinsic activity. Although the in vitro activity of clarithromycin against C. pneumoniae is greater than that of azithromycin (90% MIC of 0.03 μg/ml versus 0.125 to 0.25 μg/ml [17]), the intracellular concentration of azithromycin is much higher (200- to 500-fold the extracellular level versus 10- to 20-fold [29]) and the terminal half-life is much longer (10).
The concentrations measured in the serum of rabbits demonstrate that the mean peak levels (at 4 h after dosing) were less than the concentrations reported in humans after 500-mg or 250-mg multiple dosings, which usually average 2 to 3 μg/ml and 1.0 μg/ml, respectively (28). The concentrations of clarithromycin in serum achieved in the rabbits, despite being very low, had an antimicrobial effect, as indicated by the number and severity of the atherosclerotic lesions and a reduction in the ability to detect C. pneumoniae antigen in the treated animals. However, the concentration of clarithromycin achieved in rabbit serum is still severalfold higher than the MIC (0.03 μg/ml), and one can expect the intracellular levels to be 10- to 20-fold higher (29).
In addition to the protective effect of clarithromycin in reducing the prevalence of early atherosclerotic lesions, the rate of C. pneumoniae antigen detection was also substantially decreased by early treatment. It is unclear whether the positive antigen in the delayed treatment group represents viable or dead organisms, since C. pneumoniae is extremely difficult to recover from rabbit tissues (26).
Although the newer macrolides have been reported to have anti-inflammatory properties, in our study clarithromycin had only a mild effect in reducing the extent of atherosclerosis induced by a cholesterol-enriched diet. Hence, it would appear that clarithromycin can prevent early atherosclerotic lesions in the rabbit model mainly by its antimicrobial activity. This is also consistent with the observation that clarithromycin and azithromycin appear to have only slight anti-inflammatory effects in an animal model (35). Based on our results, clarithromycin given at 250 mg twice daily for several months would be a reasonable dose to use for future clinical trials in humans to assess the value of clinical benefit in atherosclerotic heart disease.
In conclusion, our study has demonstrated that clarithromycin administered early after C. pneumoniae infection can attenuate the de novo development of early atherosclerotic lesions of the aorta in the rabbit. However, treatment initiation after lesions developed was not as effective with the treatment regimen used. Additional studies with higher doses or more frequent dosing and longer periods of treatment are needed.
Acknowledgments
This study was funded by a grant from Abbott Laboratories, Ltd.
We are grateful to D. Bajhan for assistance in preparing the manuscript and to J. Li for assistance with the statistical analysis.
REFERENCES
- 1.Blasi, F., F. Denti, M. Erba, R. Cosentini, R. Raccanelli, A. Rinaldi, L. Fagetti, G. Esposito, U. Ruberti, and L. Allegra. 1996. Detection of Chlamydia pneumoniae but not Helicobacter pylori in atherosclerotic plaques of aortic aneurysms. J. Clin. Microbiol. 34:2766-2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Campbell, L. A., E. R. O'Brien, A. L. Cappuccio, C. C. Kuo, S. P. Wang, D. Stewart, and J. T. Grayston. 1995. Detection of Chlamydia pneumoniae TWAR in human coronary arthrectomy tissues. J. Infect. Dis. 172:585-588. [DOI] [PubMed] [Google Scholar]
- 3.Cartun, R. W., and C. A. Pedersen. 1989. An immunocytochemical technique offering increased sensitivity and lowered costs with a streptavidin-horseradish peroxidase conjugate. J. Histotechnol. 12:273-277. [Google Scholar]
- 4.Chiu, B., E. Viira, W. Tucker, and I. W. Fong. 1997. Chlamydia pneumoniae, cytomegalovirus, and herpes simplex virus in atherosclerosis of the carotid artery. Circulation 96:2144-2148. [DOI] [PubMed] [Google Scholar]
- 5.Daley, S. J., K. F. Klemp, J. R. Guyton, and K. A. Rogers. 1994. Cholesterol-fed and casein-fed rabbit models of atherosclerosis. Arterioscler. Thromb. 14:105-114. [DOI] [PubMed] [Google Scholar]
- 6.Danesh, J., R. Collins, and R. Petro. 1997. Chronic infections and coronary heart disease: is there a link? Lancet 350:430-436. [DOI] [PubMed] [Google Scholar]
- 7.Fong, I. W., B. Chiu, E. Viira, M. W. Fong, D. Jang, and J. Mahony. 1997. Rabbit model for Chlamydia pneumoniae infection. J. Clin. Microbiol. 35:58-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fong, I. W., B. Chiu, E. Viira, D. Jang, and J. B. Mahony. 1999. De novo induction of atherosclerosis by Chlamydia pneumoniae in a rabbit model. Infect. Immun. 67:6048-6055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fong, I. W., B. Chiu, E. Viira, D. Jang, M. W. Fong, and J. B. Mahony. 1999. Can an antibiotic (macrolide) prevent Chlamydia pneumoniae induced in a rabbit model? Clin. Diagn. Lab. Immunol. 6:891-894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Girard, A. E., D. Girard, A. R. English, T. D. Gootz, C. R. Cimochowski, J. A. Faiella, S. L. Haskell, and J. A. Retsema. 1987. Pharmacokinetic and in vivo studies with azithromycin (CP-62,993), a new macrolide with an extended half-life and excellent tissue distribution. Antimicrob. Agents Chemother. 31:1948-1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grayston, J. T., M. B. Aldous, A. Easton, S. P. Wang, C. C. Kuo, L. A. Campbell, and J. Altrian. 1993. Evidence that Chlamydia pneumoniae causes pneumonia and bronchitis. J. Infect. Dis. 168:1231-1235. [DOI] [PubMed] [Google Scholar]
- 12.Grayston, J. T., L. A. Campbell, C. C. Kuo, C. H. Mordhorst, P. Saikku, D. H. Thom, and S. P. Wang. 1990. A respiratory tract pathogen: Chlamydia pneumoniae strain TWAR. J. Infect. Dis. 161:618-625. [DOI] [PubMed] [Google Scholar]
- 13.Grayston, J. T. 1992. Infections caused by Chlamydia pneumoniae strain TWAR. Clin. Infect. Dis. 15:757-763. [DOI] [PubMed] [Google Scholar]
- 14.Grayston, J. T. 2000. Background and current knowledge of Chlamydia pneumoniae and atherosclerosis. J. Infect. Dis. 181(Suppl. 3):S402-S410. [DOI] [PubMed] [Google Scholar]
- 15.Gupta, S., E. W. Leatham, D. Carrington, M. A. Mendall, J. C. Kaski, and A. J. Camm. 1997. Elevated Chlamydia pneumoniae antibodies, cardiovascular events and azithromycin in male survivors of myocardial infarction. Circulation 96:404-407. [DOI] [PubMed] [Google Scholar]
- 16.Gurfinkel, E., G. Bozovich, A. Daroca, E. Beck, B. Mautner, et al. 1997. Randomized trial of roxythromycin in non-Q wave coronary syndromes: ROXIS Pilot Study. Lancet 350:404-407. [DOI] [PubMed] [Google Scholar]
- 17.Hammerschlag, M. R., K. K. Qumei, and P. M. Roblin. 1992. In vitro activities of azithromycin, clarithromycin, l-ofloxacin and other antibiotics against Chlamydia pneumoniae. Antimicrob. Agents Chemother. 36:1573-1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu, H., G. N. Pierce, and G. Zhang. 1999. Atherogenic effects of chlamydia are dependent on serum cholesterol and specific to Chlamydia pneumoniae. J. Clin. Investig. 103:747-753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jackson, L. A., A. Lee, L. A. Campbell, C. C. Kuo, D. I. Rodriguez, and J. T. Grayston. 1997. Isolation of Chlamydia pneumoniae (TWAR) from the carotid atherosclerotic plaque specimen obtained by endarterectomy. J. Infect. Dis. 176:292-295. [DOI] [PubMed] [Google Scholar]
- 20.Kuo, C. C., A. M. Gown, E. P. Benditt, and J. T. Grayston. 1993. Detection of Chlamydia pneumoniae in aortic lesions of atherosclerosis by immunocytochemical stain. Arterioscler. Thromb. 13:1500-1504. [DOI] [PubMed] [Google Scholar]
- 21.Laitinen, K., A. Laurila, L. Pyhala, M. Leinonen, and P. Saikku. 1997. Chlamydia pneumoniae infection induces inflammatory changes in the aortas of rabbits. Infect. Immun. 65:4832-4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maas, M., C. Bartels, P. M. Engel, U. Momat, and H. H. Sievers. 1998. Endovascular presence of viable Chlamydia pneumoniae is a common phenomenon in coronary artery disease. J. Am. Coll. Cardiol. 31:832-837. [DOI] [PubMed] [Google Scholar]
- 23.Mahony, J. B., K. E. Luinstra, and M. A. Chernesky. 1994. Diagnosis of Chlamydia trachomatis and Chlamydia pneumoniae respiratory tract infections by multiplex PCR, p. 370-373. In J. Ofila et al. (ed.), Chlamydial infections 1994. Societa Editrice Escullapio, Rome, Italy.
- 24.Mitchell, J. R. A., C. J. Schwartz, and A. Zinger. 1964. Relationship between aortic plaques and age, sex, and blood pressure. BMJ 1:205-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moazed, T. C., L. A. Campbell, M. E. Rosenfeld, J. T. Grayston, and C. C. Kuo. 1999. Chlamydia pneumoniae infection accelerates the progression of atherosclerosis in apolipoprotein (APO E)-deficient mice. J. Infect. Dis. 180:238-241. [DOI] [PubMed] [Google Scholar]
- 26.Moazed, T. C., C. C. Kuo, D. L. Patton, J. T. Grayston, and L. A. Campbell. 1996. Experimental rabbit models of Chlamydia pneumoniae infection. Am. J. Pathol. 148:667-676. [PMC free article] [PubMed] [Google Scholar]
- 27.Muhlestein, J. B., J. L. Anderson, E. H. Hammond, L. Zhao, S. Trehan, E. P. Schwobe, and J. F. Carlquist. 1998. Infection with Chlamydia pneumoniae accelerates the development of atherosclerosis and treatment with azithromycin prevents it in a rabbit model. Circulation 97:633-636. [DOI] [PubMed] [Google Scholar]
- 28.Piscitelli, S. C., and K. A. Rodvold. 1992. Clarithromycin and azithromycin: new macrolide antibiotics. Clin. Pharm. 17:137-152. [PubMed] [Google Scholar]
- 29.Rakita, R. M. 1998. Intracellular activity, potential clinical uses of antibiotics. ASM News 64:570-575. [Google Scholar]
- 30.Richardson, M., M. De Reske, K. Delaney, A. Fletch, L. H. Wilcox, and Kinlough-Rathbone, R. L. 1997. Respiratory infection in lipid-fed rabbits enhances sudanophilia and the expression of VCAM-1. Am. J. Pathol. 151:1009-1017. [PMC free article] [PubMed] [Google Scholar]
- 31.Roblin, P. M., W. Dumornay, and M. R. Hammeschlag. 1992. Use of HEP-2 cells for improved isolation and passage of Chlamydia pneumoniae. J. Clin. Microbiol. 30:1968-1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saikku, P., K. Matilla, M. S. Nieminen, P. H. Makela, J. K. Huttunen, and V. Valtonen. 1988. Serological evidence of an association of a novel chlamydia, TWAR, with chronic coronary heart disease and acute myocardial infarction. Lancet ii:983-986. [DOI] [PubMed]
- 33.Saikku, P., M. Leinonen, L. Tenkanen, E. Linnanmaki, M. R. Eckman, V. Manninen, M. Manttari, M. H. Frick, and J. K. Huttenan. 1992. Chronic Chlamydia pneumoniae infection as a risk factor for coronary heart disease in the Helsinki heart study. Ann. Intern. Med. 116:273-278. [DOI] [PubMed] [Google Scholar]
- 34.Salisbury, B. G. J., D. P. Hajjar, and C. R. Minick. 1985. Altered glycosaminoglycan metal in injured arterial wall. Exp. Mol. Pathol. 42:306-319. [DOI] [PubMed] [Google Scholar]
- 35.Scaglione, F., and G. Rossoni. 1998. Comparative anti-inflammatory effects of roxythromycin, azithromycin and clarithromycin. J. Antimicrob. Chemother. 10(Suppl. B):47-50. [DOI] [PubMed] [Google Scholar]
- 36.Shor, A., C. C. Kuo, and D. L. Patton. 1992. Detection of Chlamydia pneumoniae in coronary artery fatty streaks and atheromatous plaques. S. Afr. Med. J. 82:158-161. [PubMed] [Google Scholar]
- 37.Stary, H. C. 1996. The histological classification of atherosclerotic lesions in human coronary arteries, p. 463-474. In V. Fuster, R. Ross, and E. J. Topol (ed.), Atherosclerosis and coronary artery disease. Lippincott-Raven Publishers, Philadelphia, Pa.
- 38.Takesluta, K., I. Yamagishi, M. Harada, S. Otomo, T. Nakagawa, and Y. Mizushima. 1989. Immunological and anti-inflammatory effects of clarithromycin: inhibition of interleukin-1-production of murine peritoneal macrophages. Drug Exp. Clin. Res. 15:527-533. [PubMed] [Google Scholar]