Abstract
Reduction in electron transport is associated with decreased production in alpha-toxin despite the fact that Staphylococcus aureus is able to grow from 1 CFU to >107 CFU. Similarly, under anaerobic conditions, S. aureus does not produce alpha-toxin. Although the pathways that connect oxidative metabolism and toxin production are unknown, agents are available that exhibit greater inhibition of plant versus mammalian electron transport. Herbicides block electron transport in plants by inhibiting the formation of phosphoquinol (QH2) in plants. Commercial use in farming is possible because these compounds are much less active against the quinones found mammalian mitochondria. Because bacterial electron transport systems are closer to plant than mammalian systems, we hypothesized that inhibitors of respiration might be able to reduce S. aureus electron transport and block the production of alpha-toxin. We studied two compounds and found that the effective dose for the inhibition of bacterial respiration was 50 to >3,500 times lower than the concentration required to cause similar inhibition of rat mitochondrial respiration. Compounds I and II also reduced toxin production in S. aureus without causing overt toxicity to cultured endothelial cells. Finally, the compounds reduced the damage caused by S. aureus when cocultured with the endothelial cells. This raises the possibility that compounds that inhibit bacterial respiration might be prove valuable for the prevention of toxin production in S. aureus.
While studying the ability of Staphylococcus aureus to lyse cultured endothelial cells, we found that a site-directed hla (alpha-toxin) mutant was able to persist within bovine endothelial cells (19). This finding suggested a mechanism for the persistence of S. aureus within host tissues and led to a search for naturally occurring alpha-toxin mutants. We discovered that most clinical alpha-toxin-negative organisms were auxotrophs for either hemin or menadione (16), which leads to slow growth and small colonies on unsupplemented media (1, 8, 12-17). Hemin and menadione participate in electron transport in cytochromes and menaquinone, respectively (1, 8, 12-17, 21). Hence, these small-colony variants (SCVs) grow slowly due to reduced availability of ATP that is needed for cell wall biosynthesis and other growth-related synthetic activities. Also, when S. aureus organisms are grown anaerobically, they produce small, nonpigmented, nonhemolytic colonies that are identical to S. aureus SCVs (1). This suggests a link between alpha-toxin production and electron transport. Our initial thoughts were that this was simply due to decreased amino acid import since this is an energy-dependent process, but further investigation made this less likely because the levels of ATP were only decreased to ∼50% of wild-type values (14). In addition, SCVs are able to grow from an initial low inoculum to >107 CFU, but they only produce small amounts of alpha-toxin (1, 21). Finally, SCVs produce normal or increased amounts of fibronectin binding protein, proteases, and β-lactamase (P. E. Vaudaux et al. and I. M. Jonsson et al., unpublished data), but they make decreased amounts of coagulase (1, 13, 15, 16, 21). Hence, SCVs show a selective decrease in the production of exoproteins.
To begin to define the interactions between electron transport and the production of several exoproteins in S. aureus (1, 13-17), we made a site-directed hemB mutant, which showed reduced mRNA for alpha-toxin (21). This mutant grows multiple orders of magnitude, but agr and alpha-toxin are not expressed even when the mutant goes into stationary phase. Supplementation of the mutant with hemin or complementing it with hemB on a plasmid allows for the production of alpha-toxin. Hence, hla, the alpha-toxin gene, is clearly undamaged, but it is downregulated in the hemB mutant. Although we are currently studying the interactions between electron transport and factors that control alpha-toxin production, the ability to interrupt electron transport with drugs might also be of value clinically. We hypothesized that a drug-induced interruption of electron transport might lead to decreased alpha-toxin production and decreased damage to host cells.
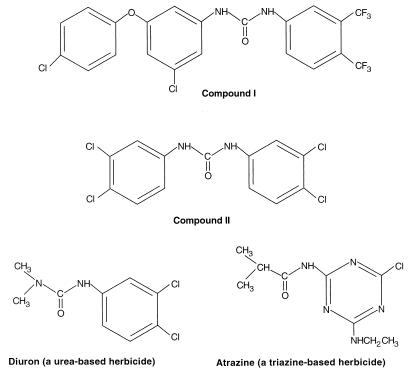
Many electron transport inhibitors are too toxic to consider using in humans; however, some relatively nontoxic compounds are available that interrupt electron transport in plants are much less active against mammalian electron transport systems. These compounds are used as herbicides. Some herbicides kill weeds by interfering with the action of electron transport involved in photosynthesis, specifically photosystem II (18). Herbicides that are urea derivatives, such as diuron, and triazine derivatives, such as atrazine (Fig. 1), bind to the QB subunit and block the formation of phosphoquinol (QH2), thereby blocking electron transport in plants (18). Because bacterial quinones and electron transport have similarities to plant photosynthetic systems, urea compounds similar to diuron might be more active on bacterial electron transport compared to mitochondrial electron transport (18). In screening a large number of compounds at Zeneca Pharmaceuticals for antibacterial activity, several urea-based compounds were identified that decreased growth via reduced respiration compounds I and II (Fig. 1). This formed the basis of the collaboration for the studies presented here, wherein we found that these two diuron-like urea derivatives decreased the release of cytotoxic bacterial products into supernatants of S. aureus cultures. This was tested by quantifying the amount of damage caused by bacterial supernatants to cultured endothelial cells, by determining the direct toxicity caused by compounds I and II to the monolayers, and by measuring the inhibition of mammalian mitochondria oxidative activity in the presence of compounds I and II.
FIG. 1.
Urea- and tiazine-based electron transport inhibitors. Some herbicides kill weeds by interfering with the action of photosystem II. Urea derivatives, such as diuron, and triazine derivatives, such as atrazine, bind to the QB subunit and block the formation of phosphoquinol (QH2). This is a plant quinone. Bacterial quinones and electron transport have similarities to plant photosynthetic systems (especially purple bacteria); hence, urea compounds similar to diuron might affect bacterial electron transport.
MATERIALS AND METHODS
Compounds I and II.
Compounds I and II were provided by W. W. Nichols (Zeneca Pharmaceuticals, MacClesfield, United Kingdom) and dissolved at 10 mg/ml of dimethyl sulfoxide. Dimethyl sulfoxide was added as a control in the oxygen and protein synthesis experiments at the highest concentrations tested, and it had no effect.
Preparation of bacterial strains.
S. aureus 6850 is a clinical isolate that caused bacteremia and metastatic abscesses and produces β-lactamase (1). For oxygen uptake studies, a 1-ml overnight culture was added to 500 ml of IST broth (IsoSensitest; Oxoid) and grown with shaking at 37°C until an optical density at 550 nm (OD550) of 0.2 to 0.3 was reached, and then the bacteria were harvested (5,000 rpm, 10 min, 4°C). Bacteria were resuspended in 2 ml of ice-cold IST broth and stored on ice. For production of supernatants for testing for endothelial cell lytic activity, S. aureus 6850 was cultured in Ryan Red tissue culture medium for 12 to 14 h (1), and the supernatants were harvested (5,000 rpm, 10 min, 22°C). Because of the different rates of growth in the presence or absence of compounds I or II, supernatants were harvested from the growth medium when the organisms reached early stationary phase (10 to 12 h). Supernatants were passed through 0.45-μm (pore-size) filters and tested for sterility, and the pH was adjusted to 7.4. To test the effects of compounds I and II on toxin production, bacteria were grown in the presence of the compounds at one-quarter and one-half of the MICs in Ryan Red tissue culture medium. Inocula were adjusted to an OD540 of 0.6, diluted 20-fold, and counted in a Petroff-Hauser chamber to give ca. 108/ml of tissue culture fluid, and the numbers of CFU were determined.
Microbiology tests.
Minimal inhibitory testing of compounds I and II were performed by standard broth dilution methods in a 96-well microtiter plate with Mueller-Hinton broth and according to NCCLS guidelines (10). Plates were read after overnight incubation at 35°C. Colonial morphology was determined for size, pigmentation, and hemolysis on rabbit and sheep blood plates, as well as on Mueller-Hinton agar. Colonies were considered to have the SCV phenotype if they were nonhemolytic, nonpigmented, and >10-fold smaller than colonies formed by untreated bacteria. Growth curves were performed as previously described (21).
Hemolytic activity.
Hemolytic activity was determined by examining zone sizes on Mueller-Hinton agar plates containing rabbit erythrocytes. Compounds I and II were either not added to agar or added at one-quarter or at one-half of their MICs.
Oxygen electrode measurements.
A Clark-type oxygen electrode was used for polarographic measurements of oxygen uptake. S. aureus 6850 (0.02 ml) was added to 2.98 ml of prewarmed (37°C) IST broth in a water-jacketed chamber, and the mixture was continually stirred. Oxygen utilization was calculated from the slopes of the graphs as nanograms of atoms of oxygen/minute/milligram (dry weight) of S. aureus. Rat liver mitochondria were prepared from an overnight starved rat (to deplete glycogen and fatty acids) as follows: the animal was killed by decapitation and allowed to exsanguinate as much as possible, the liver was sliced into small fragments and homogenized (Potter-Elvehjem) in 2 ml of ice-cold buffer/g of liver, the cellular debris was removed (by centrifugation at 1,000 × g), and the mitochondrial fraction was harvested (at 10,000 × g, 10 min) (4). All preparative steps were performed at 4°C. Homogenizing buffer contained 0.3 M sucrose, 10 mM EDTA, 5 mM morpholinepropanesulfonic acid (pH 7.4), 5 mM KH2PO4, and 0.1% bovine serum albumin (fatty acid-free; Sigma Chemical Co., St. Louis, Mo.). Each day, a fresh 2-ml batch of whole mitochondria was removed from the −80°C freezer and sonicated on ice (output 3, continuous pulse) for 10 2-s bursts, with a cooling pause between each burst, or until the suspension appeared translucent (4). The mitochondrial reaction mixture contained 20 μl of mitochondria, 2 μl of NADH stock solution (750 mM), 2 μl of test compound, and buffer to make to 3 ml. The NADH was added to the prewarmed (37°C) mix to start the reaction. The oxygen electrode measurements for mitochondria were made with NADH (0.5 mM) as the substrate by using submitochondrial particles prepared by sonication of rat liver mitochondria. Oxygen utilization was calculated from the slopes of the graphs as nanograms of atoms of oxygen/minute/milligram of mitochondrial protein.
Endothelial cell lysis assay.
One milliliter of bacterial supernatant was added to one milliter of tissue culture fluid with confluent cultured bovine endothelial cell monolayers in 16-well tissue culture plates. The damage to the monolayer was observed over a 24-h period, and the damage was scored as follows: 0, no damage; 1, minor disruption of the monolayer; 2, some loss of cells and alteration in shape; 3, most of the cells were lysed, and the remaining cells showed gross distortion of normal morphology; and 4, total disruption of the monolayer. Scores were performed by two observers, and the mean scores for three wells were recorded. To obtain reproducible results, the monolayers needed to have just reached confluence since older monolayers gave inconsistent results.
Bacterial persistence assay.
The persistence assay was performed as described previously (1) and is briefly summarized here. S. aureus 6850 were grown overnight at a one-half MIC of compound I or compound II, washed, counted (OD determination followed by direct counts), adjusted to ∼108 with tissue culture medium, and added to the bovine endothelial monolayer. After 3.5 h, the monolayer was washed and lysostaphin was added. At the times indicated, the monolayers were washed, endothelial cells were released by treatment with trypsin-EDTA, and the bacteria persisting within endothelial cells were released by sonication. The sonicate was diluted, and numbers of CFU were determined by plating on Mueller-Hinton agar.
Protein synthesis inhibition assay.
A human fibroblast cell line, KB2, was used to determine inhibition of protein synthesis. Radiolabeled amino acid incorporation into total cellular proteins was measured over a 48-h period. The data are presented as the concentration of the compound that produced a 50% inhibition of incorporation.
RESULTS
The MICs of compound I and compound II for S. aureus 6850 were determined so that these compounds could be tested at concentrations, which were below the bactericidal level. The MICs for compound II and compound I were 1.0 and 2.0 μg/ml, respectively (Table 1). These agents inhibited oxygen uptake in S. aureus at concentrations 100- and 5-fold less than the MICs for compound I and compound II (Table 1). In contrast, they did not reach a 50% inhibition of NADH-stimulated mitochondrial oxygen uptake until they reached 50 to >3,500 times higher than that needed to block bacterial respiration. Similarly, high concentrations of compound I and compound II were required to effect a 50% inhibition of protein synthesis in cultured KB2 cells (Table 1). These results show that differential inhibition of electron transport can be obtained between bacterial and mammalian cells. The compounds were also added to cultured endothelial cells at the highest concentrations tested, and they did not alter endothelial cell viability, as assessed by direct visualization of the monolayer.
TABLE 1.
Effects of compounds I and II on bacterial respiration, mitochondrial oxygen uptake, cultured human cell protein biosynthesis, and bacterial colonial morphologya
| Compound | MIC (μg/ml) | IC50 (μg/ml) for O2 uptake by:
|
IC50 (μg/ml) for protein synthesis by KB2 cells | One-half MIC (μg/ml) | Pigment formation on rabbit blood agar | Colony size | Hemolytic on rabbit blood agar | |
|---|---|---|---|---|---|---|---|---|
| S. aureus | Mitochondria | |||||||
| No drug | NAa | Bright yellow | Normal | 4-mm zone | ||||
| Compound I | 2.0 | 0.02 | 71 | >48 | 1.0 | None | Small | No zone |
| Compound II | 1.0 | 0.2 | 10 | 15 | 0.5 | None | Small | No zone |
IC50, 50% inhibitory concentration.
NA, not applicable.
Growing S. aureus 6850 in the presence of one-half the MIC of compounds I and II blocks pigment formation and lysis of rabbit erythrocytes and also reduces colony size (Table 1). A fourfold increase in the gentamicin MIC was also noted. These changes are the same as those seen when electron transport is deficient in S. aureus SCVs (1, 8, 9, 12-15, 17, 21). Interruption of electron transport decreases pigmentation since carotenoid biosynthesis is linked to electron transport (12, 13). Also, S. aureus strains that are defective in hemin or menadione biosynthesis are unable to construct an intact electron transport chain, and these strains are unable to produce hemolysins or grow rapidly (1, 8, 9, 12-17, 21). Hence, compounds I and II reproduce the SCV phenotype; these are variants that are much less lytic to rabbit erythrocytes and cultured endothelial cells (1, 8, 16, 21). When 50 treated colonies are subcultured on rabbit blood agar that does not contain compounds I or II, the SCV phenotype was not seen. Also, when the organism was subcultured into medium that did not contain compounds I or II at the MIC, growth began at 2 h, as indicated by an increase in the OD540. Consequently, the phenotypic change does not appear to be a stable change. Finally, growth in the presence of one-quarter or one-half the MIC of compounds I or II increased the lag phase from 60 to 120 min, increased the length of the log phase from 6 h to 8 h, shifted the time to peak growth from 10 to 12 h, and reduced the maximum number of CFU/ml from 6 × 1010 to 8 × 108.
When S. aureus organisms were grown in sub-MICs of compound I or compound II, the supernatant culture medium was less toxic for cultured endothelial cells than it was when the bacteria were grown in the absence of compound. When no compound was present during growth of the S. aureus, supernatants caused moderate loss of cells and altered the endothelial cell shape within 5 h (score = 2). The damage progressed over time: the score was 2.75 by 8 h, and total disruption of the monolayer occurred by 30 h (score = 4). In contrast, compound I prevented damage to the monolayers for the first 8 h, with only minor damage to the monolayers by 23 and 30 h (score = 1). Compound II prevented all damage to the monolayers for 23 h, with only slight damage to the monolayers by 30 h (score = 0.5). The cytolytic activity of S. aureus 6850 supernatants was also removed by boiling for 30 min, a finding which is consistent with the thermal lability of S. aureus alpha-toxin. Of note, the addition of compound I or compound II to the cultured endothelial cells did not result in disruption of the monolayers. Indeed, endothelial cell growth in the monolayer seems to have been somewhat enhanced. Other time points were assessed (growth of the bacteria from 8 to 16 h), and similar results were obtained, i.e., no toxin was produced.
We found that S. aureus treated with compounds I and II was able to persist within cultured endothelial cells. The numbers of persistent bacteria were 3- and 10-fold higher at 24 h (for compounds II and I, respectively), but this is less dramatic persistence than that found with S. aureus strains that have biosynthetic defects in electron transport chain proteins (1, 8, 21). Persistence has been correlated in the past with reduced alpha-toxin production (1, 19, 21).
DISCUSSION
The development of drugs that are able to reduce the virulence of bacteria, even if they fail to kill pathogens, may allow for the discovery of agents that are unrelated to currently used antibiotics. As a novel group of compounds, there is less likelihood that the bacteria would show cross-resistance to drugs that are currently available. Also, the compounds decreased the virulence of bacteria at concentrations much lower than that required to kill the bacteria; hence, this allows for lower doses and less toxicity.
When compounds I and II were added to the monolayers, no visual damage to the endothelial monolayers was apparent. Oxygen uptake by rat mitochondria remained NADH stimulatable at levels of compound 50 to >3,500 times higher than that needed to block bacterial respiration. Furthermore, protein synthesis in KB2 cells was inhibited only at concentrations 200 to 300 times higher than that needed to inhibit bacterial toxin production. This inhibitory activity against the bacteria compared to the toxicity to mammalian cells indicates that selective action can be achieved with these compounds, raising the hope that safe agents related to compounds I and II may be developed for human use.
Human and animal exposure studies also show a lack of acute toxic responses to this group of compounds. In suicide attempts, even massive oral doses of diuron (38 mg/kg) have not proven to be fatal (5). Similarly, animal studies show that these compounds are well absorbed orally, and the 50% lethal doses ranged from 1,017 to 3,400 mg/kg in rats (2, 7). Thus, the development of drugs based upon compounds I and II seems rational.
One of the characteristics with agents that act against virulence factors is that they are usually highly specific. They aim at a single factor in specific strains of bacteria, e.g., antibodies aimed at a single virulence factor such as an anti-alpha-toxin antibody for S. aureus. This target specificity is a problem because of the large number of bacteria and potential virulence factors in a patient that is febrile or toxic. The lack of very rapid diagnostic techniques, i.e., a 30-min turnaround time, to direct therapy and the need to stock hundreds of virulence factor-specific agents limit enthusiasm for such highly specific drugs. Moreover, the development costs for such agents are high, and their narrow spectrum would make them much less economically feasible. However, finding compounds that reduce virulence factors in multiple species would be of great interest. Because SCVs based upon electron transport deficiencies are seen in a wide variety of species other than S. aureus (6, 12, 13, 17), similar regulatory pathways might exist in many bacterial species since these SCVs show reduced virulence and small colony size. Decreased toxin production when electron transport is interrupted is also seen in the salmonella live vaccine candidate, which carries a mutation of aroA in the Salmonella enterica serovar Typhimurium, and this alters activation of its virulence plasmid (11). The aroA mutation causes a decrease in several aromatic compounds, including menaquinone. Naturally, this vaccine strain is much less virulent than the parent strain. Similarly, P. aeruginosa SCVs are deficient in electron transport, and they are susceptible to complement-mediated killing, yet they are more prone to persist within host tissues (6). Consequently, if the interruption of bacterial electron transport causes a decrease in virulence in many genera, then such an agent would have broad appeal, both clinically and economically.
Bacterial electron transport inhibitors would be used to reduce the initial toxic response produced by the bacteria, and then the drug would be withdrawn once the patient was loaded with active antibiotics. While the production of SCVs might be problematic since they are more resistant to antibiotics (3, 9, 12), the host intracellular milieu will also produce SCVs (20). The removal of compound I or compound II allows S. aureus to return to its rapidly growing state and a baseline level of antibiotic susceptibility. In view of the increasing resistance of S. aureus to multiple antibiotics, finding drugs aimed at novel targets is of considerable importance.
Acknowledgments
R.A.P. gratefully acknowledges the support of the American Heart Association; the University of Wisconsin Graduate and Medical School Research Committees; the Alexander von Humboldt Research Foundation in Bonn, Germany; and the National Institutes of Health (RO1 AI 142072). G.P. was supported by the German Ministry for Education and Research for the Interdisciplinary Center for Clinical Research, University of Münster (project C8).
REFERENCES
- 1.Balwit, J. M., P. van Langevelde, J. M. Vann, and R. A. Proctor. 1994. Gentamicin-resistant, menadione and hemin auxotrophic Staphylococcus aureus persist within cultured endothelial cells. J. Infect. Dis. 170:1033-1037. [DOI] [PubMed] [Google Scholar]
- 2.Boyd, E. M., and V. Krupta. 1970. Protein deficiency diet and diuron toxicity. J. Agric. Food Chem. 18:1104-1107. [DOI] [PubMed] [Google Scholar]
- 3.Chuard, C., P. E. Vaudaux, R. A. Proctor, and D. P. Lew. 1997. Decreased susceptibility to antibiotic killing of small colony variants of Staphylococcus aureus in fluid phase and on fibronectin-coated surfaces. J. Antimicrob. Chemother. 39:603-608. [DOI] [PubMed] [Google Scholar]
- 4.Darley-Usmar, V. M., D. Rickwood, and M. T. Wilson. 1987. Mitochondria: a practical approach, p. 5-6. IRL Press, Oxford, England.
- 5.Geldmacher-von Mallinckrodt, M., and E. Schussler. 1971. Zu stoffwechsel und Toxicitaet von 1-(3,4-dichlorophenyl)-3,3-dimethyl harstoff (Diuron) beim menschen. Arch. Toxikol. 27:187-192. (In German.) [PubMed] [Google Scholar]
- 6.Goetz, M. B., R. A. Proctor, A. U. Gerber, and W. A. Craig. 1981. Complement and neutrophil mediated killing of gentamicin-resistant small colony variants of Pseudomonas aeruginosa. Clin. Res. 29:728.A. [Google Scholar]
- 7.Hodge, H. C., W. L. Downs, and B. S. Panner. 1967. Oral toxicity and metabolism of diuron (N-3,4-dicholorophenyl)-N,N-dimethylurea in rats and dogs. Food Cosmet. Toxicol. 5:513-531. [DOI] [PubMed] [Google Scholar]
- 8.Kahl, B., R. A. Proctor, A. Schulze-Everding, M. Herrmann, H. G. Koch, I. Harms, and G. Peters. 1998. Persistent infection with small colony variant strains of Staphylococcus aureus in patients with cystic fibrosis. J. Infect. Dis. 177:1023-1029. [DOI] [PubMed] [Google Scholar]
- 9.Koo, S. P., A. S. Bayer, H-G Sahl, R. A. Proctor, and M. R. Yeaman. 1996. Staphylocidal action of thrombin-induced platelet microbicidal protein (tPMP) is not solely dependent on transmembrane potential (ΔΨ). Infect. Immun. 64:1070-1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.National Committee for Clinical Laboratory Standards. 1997. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 4th ed., vol. 17, no. 2. Approved standard M7-A4. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 11.O'Callaghan, D., D. Maskell, F. Y. Lien, C. S. F. Easmon, and G. Dougan. 1988. Characterization of aromatic- and purine-dependent Salmonella typhimurium: attenuation, persistence, and ability to induce protective immunity in BALB/c mice. Infect. Immun. 56:419-423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Proctor, R. A. 1998. Bacterial energetics and antimicrobial resistance. Drug Resist. Updates 1:227-235. [DOI] [PubMed] [Google Scholar]
- 13.Proctor, R. A.2000. Microbial pathogenic factors, including small colony variants, p. 41-54. In F. Waldvogel and A. L. Bisno (ed.), Infections associated with indwelling medical devices. ASM Press, Washington, D.C.
- 14.Proctor, R. A., J. M. Balwit, and O. Vesga. 1994. Variant subpopulations of Staphylococcus aureus as cause of persistent and recurrent infections. Infect. Agents Dis. 3:302-312. [PubMed] [Google Scholar]
- 15.Proctor, R. A., and G. Peters. 1998. Small colony variants in staphylococcal infections: diagnostic and therapeutic implications. Clin. Infect. Dis. 27:419-423. [DOI] [PubMed] [Google Scholar]
- 16.Proctor, R. A., P. van Langevelde, M. Kristjansson, J. N. Maslow, and R. D. Arbeit. 1995. Persistent and relapsing infections associated with small colony variants of Staphylococcus aureus. Clin. Infect. Dis. 20:95-102. [DOI] [PubMed] [Google Scholar]
- 17.Proctor, R. A., O. Vesga, M. F. Otten, S.-P. Koo, M. R. Yeaman, H.-G. Sahl, and A. S. Bayer. 1996. Staphylococcus aureus small colony variants cause persistent and resistant infections. Chemotherapy 42(Suppl. 2):47-52.8751266 [Google Scholar]
- 18.Stryer, L. 1995. Photosynthesis, p. 665-670. In Biochemistry, 4th ed. W. H. Freeman and Co., New York, N.Y.
- 19.Vann, J. M., and R. A. Proctor. 1988. Cytotoxic effects of ingested Staphylococcus aureus on bovine endothelial cells: role of S. aureus α-hemolysin. Microb. Pathog. 4:443-453. [DOI] [PubMed] [Google Scholar]
- 20.Vesga, O., J. M. Vann, D. Brar, and R. A. Proctor. 1996. Staphylococcus aureus small colony variants are induced by the endothelial cell intracellular milieu. J. Infect. Dis. 173:739-742. [DOI] [PubMed] [Google Scholar]
- 21.von Eiff, C., C. Heilmann, R. A. Proctor, C. Woltz, B. Peters, and F. Götz. 1997. A site-directed Staphylococcus aureus hemB mutant is a small colony variant which persists intracellularly. J. Bacteriol. 179:4706-4712. [DOI] [PMC free article] [PubMed] [Google Scholar]