Abstract
The stability of antipseudomonal β-lactams in concentrated solutions was examined in view of their potential administration by continuous infusion with external pumps (for intensive care patients) or with portable pumps carried under clothing (for cystic fibrosis patients). Aztreonam (100 g/liter), piperacillin (128 g/liter, with tazobactam), and azlocillin (128 g/liter) remained 90% stable for up to more than 24 h at 37°C (mezlocillin [128 g/liter] was stable at 25°C but not at 37°C). Ceftazidime (120 g/liter), cefpirome (32 g/liter), and cefepime (50 g/liter) remained 90% stable for up to 24, 23.7, and 20.5 h at 25°C but only for 8, 7.25, and 13 h at 37°C, respectively. The control of temperature therefore appears to be critical for all three cephalosporins that cannot be recommended for use in portable pumps carried under clothes for prolonged periods for reasons of stability. Cefpirome and cefepime solutions developed an important color change (from light yellow to dark red) upon exposure when stored at 30°C or higher. Degradation of ceftazidime was accompanied by the liberation of pyridine which, at 37°C, was in excess of what is allowed by the U.S. Pharmacopeia, i.e., 1.1 mg/liter, after 8 and 12 h for drug concentrations of 12 and 8.3%, respectively. Imipenem and meropenem are too unstable (10% degradation at 25°C after 3.5 and 5.15 h, respectively) to be recommended for use by continuous infusion. Faropenem, examined in comparison with imipenem and meropenem, proved as stable as aztreonam or piperacillin.
β-Lactams are one of the most typical antibiotic classes for which the pharmacodynamic data, collected in vitro, as well as in animal studies, clearly indicate a potential advantage of a continuous infusion over the more conventional, discrete mode of administration (see references 6, 22, 23, 38, and 41 for reviews). Accordingly, several clinical trials have been initiated over the last half-decade, most notably in difficult-to-treat situations such as suspected gram-negative infections in critically ill patients (3), severe infections in intensive care patients (8, 16, 21, 24, 37), septicemia (1), severe sepsis (20), and cystic fibrosis (29, 40). Yet, as clearly pointed out by one of the first promoters of the continuous infusion of β-lactams (6), this mode of administration, through programmable pumps or other similar devices, requires the drug to remain sufficiently stable in solution over the projected duration of the infusion. In a previous study (30), we examined the stability of ceftazidime in solution and found it to be critically dependent on the temperature within the 20 to 37°C range (68 to 99°F). This sets clear limitations on the potential use of ceftazidime by continuous infusion if administrations every 24 h are contemplated without periodic changes of the solutions. Chemical considerations suggest that the stability of the main β-lactams currently registered for human medicine use may vary to a meaningful extent. Unfortunately, these variations, and their consequences as far as continuous infusion is concerned in the clinics, cannot always be deduced in a straightforward fashion from the manufacturer's stability data. The latter, indeed, mainly pertains to shelf storage conditions. In the present study, we examined and compared the stability of nine parenteral β-lactams (one monobactam, three ureidopenicillins, three broad-spectrum cephalosporins, and two carbapenems) with potential use for continuous infusion in severe infections in intensive care patients and in cystic fibrosis. All of these drugs show activity against gram-negative organisms, especially Pseudomonas aeruginosa, and have registered indications in this context. For the sake of comparison, we also included faropenem based on pilot studies with meropenem and imipenem and chemical considerations.
MATERIALS AND METHODS
Origin of products.
All drugs that were commercially available in Belgium were obtained through a hospital pharmacy as brand name products distributed for clinical use by their original marketing authorization holder as follows: aztreonam (Azactam; Bristol-Myers/Squibb), piperacillin (Pipcil; Wyeth-Lederlee/American Home Products), piperacillin-tazobactam (Tazocin; Wyeth-Lederlee/American Home Products), ceftazidime (Glazidim; Glaxo-SmithKline), cefepime (Maxipime; Bristol-Myers/Squibb), cefpirome (Cefrom; Aventis-Pharma), imipenem-cilastatin (Tienam; Merck, Sharp & Dohme), and meropenem (Meronem; AstraZeneca). Samples of azlocillin and mezlocillin (which are not commercially available in Belgium) were kindly provided by Bayer AG, Wuppertal, Germany; samples of faropenem were obtained from Suntory, Ltd., Gunma, Japan. Water for dissolving the drugs was obtained from a a MilliQ Academic ultrapure water system (Millipore Corp., Bedford, Mass.). All other reagents were of analytical grade (or for high-pressure liquid chromatography [HPLC] analysis) and were provided by E. Merck AG (Darmstadt, Germany) or Sigma-Aldrich (Steinheim, Germany).
Choice of concentrations and conditions of stability.
The highest, clinically acceptable daily doses of each drug were determined based on officially approved recommendations for severe infections in intensive care patients or cystic fibrosis or on widely accepted clinical practice. Faropenem (which has no useful activity against P. aeruginosa) was only used for comparison purposes with imipenem and meropenem and was used at the same concentrations as were used for meropenem. All final figures are shown in Table 1. For intensive care patients, we considered that the daily amount of drug would be placed in a volume of 48 ml (i.e., the standard volume of motor-operated syringe pumps) and infused at 25°C (room temperature). For cystic fibrosis patients, for whom home therapy was contemplated, we considered that the daily drug amount would be placed in a 125-ml motorless elastomeric pump carried under clothing (and, therefore, at temperatures close to 37°C). These considerations gave us a maximal drug concentration, as well as a range of temperatures, for practical stability testing. For most drugs, however, this maximal concentration proved to be impractical, either because of the development of an important color change at 37°C (cefpirome and cefepime at >32 g/liter and >50 g/liter, respectively, assessed against a white background by a trained inspector with solutions left at 37°C for 2 h after the solutions were prepared), unacceptable viscosity for unhindered flow from elastomeric pumps (e.g., meropenem at >64 g/liter, aztreonam at >100 g/liter, and ureidopenicillins at >128 g/liter, with a flow rate falling to <75% of the nominal flow), or limited solubility (imipenem plus cilastatin at >8 g/liter or the presence of nondissolved material when solutions were examined with the naked eye on a white and a black background 1 h after preparation). These drugs were therefore tested only at the highest practical concentration. Table 1 shows the maximal values actually used in our study.
TABLE 1.
Maximum daily drug dosages projected, maximum concentrations tested, and pH values of the the corresponding solutions
| Drug(s) | Projected daily dosage (g)a | Maximum concn tested (g/liter)b | pHc |
|---|---|---|---|
| Aztreonam | 8 | 100* | 4.8 |
| Piperacillin | 16 | 128* | 5.7 |
| Piperacillin + tazobactam | 16 | 128* | 6.2 |
| Azlocillin | 20 | 128* | 7.3 |
| Mezlocillin | 20 | 128* | 5.7 |
| Ceftazidime | 6 | 120 | 7.4 |
| Cefepime | 6 | 50 | 4.5 |
| Cefpirome | 4 | 32 | 7.3 |
| Imipenem + cilastatin | 4 | 8 | 7.8 |
| Meropenem | 6 | 64* | 8.2 |
| Faropenem | 64 | 6.8 |
That is, the highest recommended dosage for severe, life-threatening infection and/or cystic fibrosis, based on the official registration information (Belgian package inserts for all, except for imipenem [U.S. package insert data]); for faropenem, for which no pertinent registration data are available, the values of meropenem were used.
Corresponding to the maximum daily dose in a volume of 48 ml (used for intensive care patients) for ceftazidime. For all other drugs, lower concentrations had to be used for the reasons indicated. Symbols: *, higher concentrations caused unacceptable viscosity; , higher concentrations caused a marked change of color after a few hours at 37°C; , limit of solubility (precipitation observed at >10 g/liter).
Value observed after dissolution of the product (supplied as for hospital-based usage, except for azlocillin, mezlocillin, and faropenem) at the concentration indicated without adjustment.
Conditions of experiments.
Antibiotics were dissolved in sterile water; diluted to the desired concentrations; immediately brought to 4, 25, or 37°C; and maintained at that temperature for up to 24 h under normal artificial laboratory light (aimed at mimicking the light environment of an intensive care unit). No attempt was made to use any diluent other than water since all solutions were highly hypertonic and infusion to patients would always take place at very low rates (typically between 33 and 86 μl/min). No control of pH was attempted after we observed that the value reached for each drug upon dissolution in water from its clinically available form or from the sample received from the corresponding manufacturer (see Table 1) was actually offering the best stability. Samples were taken at appropriate times, and the drug residual concentration was determined immediately by HPLC after suitable dilution and then compared to freshly prepared standards. If the assays had to be delayed, samples were frozen at −80°C after we verified that no degradation would occur for several days under these conditions.
Analyses.
Separations by HPLC for assessment of the stability of the β-lactams were made on LiChrospher 100RP-18 column (25 by 0.4 cm; 5 μm; Merck KgaA, Darmstadt, Germany), except for cefepime for which a XTerrra RP-18 column (0.46 by 15 cm; 5-μm-size beads; Waters, Milford, Mass.) was used. Analyses were made on a Spectra Physics P2000 system (Thermoquest, San Jose, Calif.) or on a Waters 2690 System (Waters Corp.), both of which were equipped with a diode array UV detector, and by using a injection volume of 25 μl. Elution was isocratic for all drugs except for imipenem. (See Table 2 for details of the methodology and references to the original publications and the ranges of linearity and intraday coefficients of variation for all drugs [except in special circumstances, all assays for each experiment were made over a single 24-h period].) All conditions were selected to provide a retention time (RT) of ca. 5 min and satisfactory separation from known degradation products (obtained by keeping samples for several days at 37°C; for example, the conditions used for ceftazidime allowed us to unambiguously detect the appearance of its Δ2 isomer). To ensure that no degradation product would comigrate with the nondegraded drug, the full spectrum of the major peak (associated with the putatively nondegraded drug) in all samples showing signs of degradation was systematically recorded and compared to that of the intact drug. In preliminary experiments with aztreonam, we also verified that the intactness of the drug, as assessed by HPLC, corresponded to the maintenance of an unaltered microbiological activity (based on a disk-plate assay with Escherichia coli as the test organism). Pyridine released by ceftazidime was assayed as recently described (11) (XTerrra RP-18 column; mobile phase, 10 mM ammonium acetate buffer [pH 5]-acetonitrile [94:6, vol/vol]; detection, 257 nm; retention time, 3.38 min [versus 4.44 min for ceftazidime]; lowest limit of detection, 5 ng; limit of quantification, 20 ng; linearity, up to 1,000 ng [R2 = 0.999]).
TABLE 2.
Conditions of assay of β-lactams by HPLC
| Drug | Elution buffer (vol/vol)a | Reference | λ detector (nm)b | Range of linearity in μg (r2) | Intraday Covc (%) |
|---|---|---|---|---|---|
| Aztreonam | TBAHSO4, 5 mM, pH 4.2; acetonitrile (80:20) | 27 | 254 | 1-400 (0.999) | 0.5 |
| Piperacillin | NH4 acetate, 70 mM, pH 5; acetonitrile (78:22) | 2 | 220 | 1-100 (0.999d) | 3d |
| Azlocillin | Na acetate, 20 mM, pH 5; acetonitrile (80:20) | 19 | 220 | 1-10 (0.999) | 1.4 |
| Mezlocillin | Na acetate, 20 mM, pH 5; acetonitrile (76:24) | 19 | 220 | 1-10 (0.999) | 3.2 |
| Ceftazidime | Na acetate, 10 mM, pH 4; acetonitrile (89:11) | 13 | 258 | 0-400 (0.999) | 0.3 |
| Cefepime | Na acetate, 20 mM, pH 5; acetonitrile (95:5) | -e | 258 | 50-200 (0.999) | 0.6 |
| Cefpirome | Na acetate, 20 mM, pH 5; acetonitrile (86:14) | -e | 258 | 1-50 (0.998) | 1.7 |
| Imipenem | Gradient (12 min): Na borate, 200 mM, pH 7.2; acetonitrile (95:5 to 85:15) | 15 | 220 | 1-10 (0.998) | 1.9 |
| Meropenem | NH4 acetate, 10.53 mM, pH 4; acetonitrile (90:10) | 9 | 297 | 10-100 (0.993) | 2.7 |
| Faropenem | NH4 acetate, 10.53 mM, pH 4; acetonitrile (86:14) | -f | 308 | 1-25 (0.999) | 0.4 |
TBAHSO4, tetrabutylammonium hydrogen sulfate.
λ of maximum absorbance, as shown by the instrument readings (diode-array photometer).
COV, coefficient of variation.
Similar values for piperacillin plus tazobactam (tazobactam was not eluted within the time frame of the piperacillin chromatographic run).
Conditions were adapted from those of ceftazidime.
Conditions were adapted from those of meropenem.
RESULTS
Stability studies.
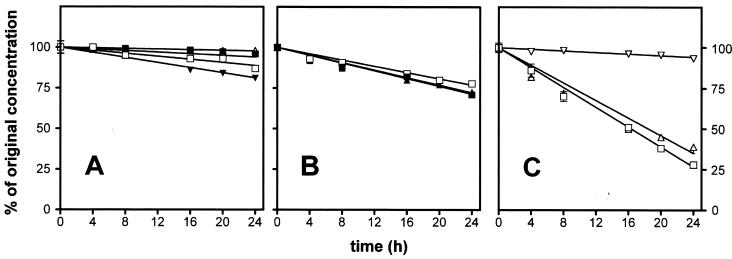
Figure 1 and Table 3 show the rate of degradation of all β-lactams investigated upon incubation for up to 24 h at 37°C and at the highest concentration tested. Table 3 gives the time during which each drug remained >90% stable, which is the limit set up by the U.S. Pharmacopeia (39) for most β-lactams, including those studied here. If we consider aztreonam and the ureidopenicillins first, it is evident that aztreonam and the piperacillin-tazobactam mixture had a satisfactory stability for at least 24 h at 37°C. Piperacillin without tazobactam was less stable, since 10% of the drug was already degraded at 22 h. Mezlocillin was the least stable molecule in this group. Moving to the cephalosporins, it is clear that three drugs tested showed essentially similar behaviors, being less stable than aztreonam or piperacillin since they had already undergone 10% degradation within 7 to 13 h. The greatest instability, however, was observed for the two parenteral penems, imipenem and meropenem (imipenem was tested only in the presence of cilastatin since pharmaceutical preparations of imipenem alone for clinical use were unavailable). These drugs, indeed, remained 90% stable for less than 3 h at 37°C and were degraded by up to 60 and 70% within 24 h at that temperature. In sharp contrast, however, faropenem displayed a remarkable stability with no more degradation than that shown by piperacillin-tazobactam within 24 h.
FIG. 1.
Stability of the β-lactams in water at 37°C over time at the maximum concentration tested. (A) Symbols: ▵, 10% aztreonam; □, 12.8% piperacillin; ▪, 12.8% piperacillin plus tazobactam (since the slope for 12.8% azocillin was almost identical to that for piperacillin-tazobactam, it was omitted for the sake of clarity); ▾ 12.8% mezlocillin. (B) Symbols: ▪, 12% ceftazidime; □, 5% cefepime; ▴, 3.2% cefpirome. (C) Symbols: □, 0.8% imipenem plus cilastatin; ▵, 6.4% meropenem; ▿, 6.4% faropenem. All values are the means of three independent determinations ± the standard deviation (SD; symbols without bars indicate values for which the SD is smaller than the symbol size).
TABLE 3.
Time during which β-lactams remains >90% stable at the highest concentration tested (see )
| Drug(s) | Time (h, min)a at:
|
|
|---|---|---|
| 37°C | 25°C | |
| Aztreonam | >24 | ND |
| Piperacillin | 21, 40 | ∼30 |
| Piperacillin + tazobactam | >24 | ≫72b |
| Azlocillin | >24 | ≫72b |
| Mezlocillin | 14 | 46, 30 |
| Ceftazidime | 8 | 24 |
| Cefepime | 13 | 20, 30 |
| Cefpirome | 7, 15 | 23, 40 |
| Imipenem + cilastatin | 2, 45 | 3, 30 |
| Meropenem | 1, 50 | 5, 15 |
| Faropenem | >24 | ∼80 |
Decays were monitored for 24 h; the slope was calculated by linear regression and used to determine the 90% stability time point. All data were rounded to the closest 15-min value. ND, not determined.
90% stability for at least 72 h, but the slope was too weak to calculate the 90% intercept value with accuracy from the 24-h decay data.
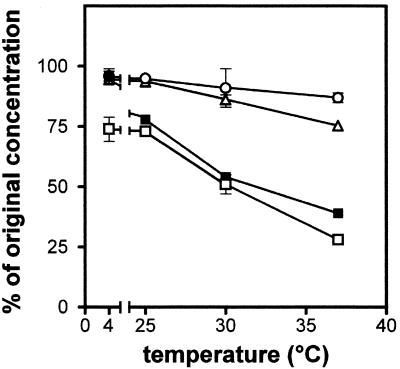
These experiments were repeated at 4, 25, and 30°C to more fully delineate the limits of stability of each molecule. The results are presented summarized in Fig. 2 (where only one representative drug in each group is shown for the sake of clarity) and in Table 3 (where only data obtained at 25°C are presented since this temperature is of direct interest for practical clinical usage). It is clear that the degradation of all drugs was temperature dependent. Degradation could be almost completely abolished if the drugs were maintained at 4°C, with the notable exception of imipenem, which suffered almost as much degradation at 4°C as at 25°C (ca. 30% in 24 h). At 25°C, aztreonam and the two ureidopenicillins suffered only minor degradation after 24 h, whereas the three cephalosporins showed ca. 10% degradation within 20 to 24 h. The two parenteral penems showed a fast degradation (10% in 3 h 30 min and 5 h 15 min, respectively). Yet, faropenem demonstrated an almost complete stability. Finally, in view of the slight differences in stability between piperacillin and piperacillin plus tazobactam, all data concerning these two preparations were subjected to statistical evaluation by paired t test on all matched points (Table 4), but this comparison showed only a trend toward a greater instability of the piperacillin alone versus the piperacillin plus tazobactam (lowest P value = 0.0736).
FIG. 2.
Influence of temperature on the degradation of selected β-lactams (24 h of incubation) at the maximum concentration tested as follows: 12.8% piperacillin (○), 5% cefepime (▵), 6.4% meropenem (▪), and 0.8% imipenem plus cilastatin (□). All values are the means of three independent determinations ± the SD (symbols without bars indicate values for which the SD is smaller than the symbol size).
TABLE 4.
Influence of tazobactam on the degradation of piperacillin at 37°C
| Drug(s) | Mean % recovery ± SDa after 24 h of incubation at:
|
||
|---|---|---|---|
| 4°C | 25°C | 37°C | |
| Piperacillin alone | 99 ± 2.97 | 93 ± 0.99 | 87 ± 2.36 |
| Piperacillin + tazobactam | 97 ± 3.43 | 96 ± 1.50 | 94 ± 1.31 |
Means of the data were subjected to unpaired t test which revealed a “not-quite-significant” effect of the temperature for the degradation of piperacillin alone (P = 0.079) and no significant effect (P > 0.1) for piperacillin-tazobactam. The same t test was used to assess a possible effect of the presence of tazobactam at each temperature point but again revealed a not-quite-significant effect (P = 0.0736) at 37°C only. The significance of the differences between piperacillin alone and piperacillin-tazobactam was as follows: not significant (P > 0.1) at 4 and 25°C and P = 0.0736 at 37°C.
Release of pyridine from ceftazidime.
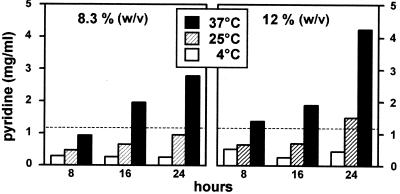
Ceftazidime contains a pyridinium moiety in its C3 side chain and is known to release pyridine upon chemical degradation (42). The U.S. Pharmacopeia (39) states that the pyridine content of ceftazidime solutions for intravenous administration cannot exceed 1.1 mg/ml. We therefore tested for release of pyridine by quantitative methods. As shown in Fig. 3, pyridine was found to be released in a time-, temperature-, and drug concentration-dependent fashion. While only minimal amounts of pyridine (<0.1 mg/ml) could be detected in freshly prepared samples, the storage of ceftazidime (12% [wt/vol]) at 37°C for 24 h caused a pyridine release of >4 mg/ml. The limit set forth by the U.S. Pharmacopeia (1.1 mg/ml) was actually already attained after less than 8 h at 37°C and between 16 and 24 h at 25°C at the highest concentration tested (i.e., 12%). This limit was not reached at 25°C within the 24-h time span if a lower drug concentration (8.3%) was used.
FIG. 3.
Release of pyridine from ceftazidime upon incubation at 4°C (□), 25°C (▨), and 37°C (▪) at two different initial concentrations (left panel, 8.3% [wt/vol]; right panel, 12% [wt/vol]). Freshly prepared samples contained <0.1 mg of pyridine/ml. The dotted line indicates the upper limit allowed for pyridine content in ceftazidime solutions according to the U.S. Pharmacopeia (39).
DISCUSSION
In vitro and animal pharmacodynamic studies have clearly demonstrated that β-lactams are time-dependent antibiotics that are more effective when administered by continuous infusion (7). However, these drugs tend to be intrinsically unstable in aqueous media because of the high reactivity of their four-member β-lactam ring (which is critical for their antibacterial action [17, 33]). This ring is indeed very susceptible to hydrolysis through base catalysis combined with nucleophilic attack on the nitrogen atom (25). It is therefore no surprise to observe significant degradation of β-lactams after dissolution in water-based media, especially if maintained at temperatures that are too high. Three key findings, with regard to the practical use of β-lactams, made here are that (i) at 37°C a significant degradation may indeed occur for some β-lactams, a finding that has immediate implications in daily practice; (ii) degradation rates vary widely among β-lactams in present clinical use, making determinations of the stability of each molecule essential in the context of its potential use by continuous infusion; and (iii) clear warnings must issued concerning the unsuitability of some β-lactams and certain clinical conditions for this type of administration. We know of no easily accessible and validated information in this context.
Thus, our study shows that aztreonam and the ureidopenicillins (with the exception of mezlocillin) entirely fulfill the requirements of stability set forth by the U.S. Pharmacopeia (39), even when kept for 24 h at temperatures up to 37°C. These molecules can therefore be used in almost all clinical conditions, including administration from portable pumps carried under clothes, as occurs in the at-home therapy of cystic fibrosis patients. The situation is less favorable for the cephalosporins since the three drugs we tested here (and which probably represent the most useful ones in cases of severe infections) showed significantly less stability than penicillins. Chemical considerations suggest that cephalosporins are more reactive than penicillins, and thus less stable in water based-media, because of their C3 substituent, which acts as a leaving group (4, 5) (this hypothesis has, however, been challenged [26]). Since the C3 substituents of ceftazidime, cefepime, and cefpirome (a pyridinium, a pyrrolidinium, and a 5H-cyclopentapyridinium, respectively) are all cationic, a common behavior of these molecules with respect to stability is not surprising. Globally, and of direct interest for the clinician, is the fact that neither of these three cephalosporins can be used for 24-h infusion if they are maintained at a temperature of >25°C. This conclusion is somewhat at variance with other studies, wherein it was concluded that ceftazidime and cefepime solutions could be kept at 24°C, or even at 30°C, and at pH 4.6 to 6.5 for ca. 2 days and yet maintain 90% of their initial concentration (14, 28, 43). These studies, however, (i) used more diluted solutions, (ii) had the drug prepared in buffered media (which are not available to clinicians in a pharmaceutically validated form and may also not be sufficient in view of the high drug concentrations needed for the projected clinical use), and (iii) were not specifically designed to test the drug stability under pertinent conditions of clinical use.
Apart from the loss of active product, the release of potentially harmful degradation products upon storage of β-lactams must also be considered. As shown in our previous study (30), the degradation of ceftazidime is accompanied by the release of at least three products: its Δ2 isomer, an ethanal derivative, and pyridine. The latter two compounds arise from degradation of the β-lactam ring and the ensuing release of the C3 substituent of ceftazidime. While little is known about the safety of the ethanal derivative, pyridine is a well-known toxic, and the level of its release needs therefore to be accurately determined. This has already been studied by several authors (10, 11, 32, 42), but we considered it useful to reexamine this issue in order to unambiguously establish the link between drug instability and pyridine release in the projected conditions of clinical use. Our results not only confirm clearly this relationship but also demonstrate that pyridine release exceeds the limit considered acceptable by the U.S. Pharmacopeia (39) if the drug is kept at 37°C for more than a few hours. This reinforces our previous conclusion (30) that ceftazidime should not be recommended for use by continuous infusion at temperatures greater than 25°C (see also the comment by Plasse et al. [J. C. Plasse, J. C., C. Chabloz, A. Terrier, and G. Bellon, Letter, Pediatr. Pulmonol. 33:232-233, 2002]). We are aware of a recent study (29) in which ceftazidime was infused to cystic fibrosis patients with the aid of portable pumps changed only every 24 h. Presumably, these pumps were carried under clothes, and the drug was therefore exposed to temperatures exceeding 25°C. While no adverse effect was reported, these authors did not address specifically the issue of pyridine toxicity nor did they check for the intactness of ceftazidime and release of pyridine under these conditions of use. We did not check in the present study for the release of degradation products from cefepime and cefpirome. However, the degradation of both cephalosporins has been shown to be accompanied by the release of compounds originating from their C3 substituents (namely, N-methylpyrrolidine for cefepime [12] and 6,7-dihydro-5H-1-pyrindine for cefpirome [34]). We could not find public data concerning the toxicity of these compounds. It is also of interest that degradation studies in water have demonstrated the destruction of the cephem ring for cefepime (28) and the release of isomers for cefpirome (epi-cefpirome, Δ2-cefpirome, and anti-cefpirome [34]). Again, no public information could be found concerning the toxicity potential of these compounds. Based on all of these considerations, it can be concluded that it is not safe to use ceftazidime, cefepime, or cefpirome for any clinical use that involves the storage of these drugs in aqueous solution at temperatures higher than 25°C for more than a few hours. More specifically, portable pumps carried under clothes should not be used in this way unless their content is renewed at least every 8 h. Conversely, ceftazidime, cefepime, and cefpirome appear to be suitable for infusion from external pumps in countries and, during periods of the year, in which the air temperature remains or can be made to remain lower than 25°C. The degradation of cefepime has been claimed to be slowed down by avoiding its exposure to light (28), but we consider this approach to be unpractical and unreliable under clinical conditions.
Finally, a clear warning must be raised against the use of parenteral carbapenems (i.e., imipenem and meropenem) for continuous infusion in view of their very unsatisfactory stability. Carbapenems and penems are usually considered to be intrinsically less stable than the corresponding carbapenams and penams because of the double bond in the five-member thiazoline or dihydropyrrole rings (through a decrease in the basicity of the leaving amine brought by the introduction of a conjugation in the tetrahedral intermediate [25]). The chemical instability of imipenem is actually well known, and degradation of this drug has been shown to occur rapidly (half-life, <2 h), even in diluted solutions (35). This instability results from complex oligomerization combining an intermolecular carboxyl group attack on the β-lactam group (favored by a weakly acidic environment) and an intermolecular reaction between the β-lactam and formimidoyl groups (favored by a weakly alkaline milieu [31]). Meropenem degradation also occurs readily in water through hydrolysis and dimerization from intermolecular aminolysis, although it was claimed that improved stability, compared to other carbapenems, could result from the protective effect exerted by the 1-β-methyl group against attack of the β-lactam ring (36). Our data show that this protection is ineffective under the practical conditions of clinical use described here. It has been suggested that meropenem could be safely used for continuous infusion if kept at 4°C (18). Our data confirm that meropenem is stable for 24 h if kept at 4°C, but the safety and applicability of this approach in the clinics remain dubious. Maintaining this low temperature is an absolute requirement, since >30% of drug degradation will occur at 25°C (we did not investigate intermediate temperatures since these are not easily controlled in a clinical environment). The clinician will therefore need to carefully weigh the potential advantages of the administration of meropenem by continuous infusion against the difficulties related to this need for a strict control of the temperature and the risks associated with a significant degradation (and release of compounds of unknown toxicity) should these stringent conditions of administration not be met. The situation is even worse for imipenem, which should not be considered for continuous infusion at any rate.
It is intriguing that faropenem, which shares with imipenem and meropenem the same unsaturation in its five-member ring, shows a remarkable stability. This could result from the fact that faropenem is not a carbapenem but a penem (i.e., it possesses a S atom in its five-member ring, which may reduce the intra-ring stress). Another possibility could be the absence of protonable group in the C3 substituent of faropenem (tetrahydrocyclofuranyl), making this drug a monoanionic compound, whereas the C3 substituents of imipenem (iminomethylaminoethyl; pKa = 10.4) and of meropenem (pyrrolidinyldimethylaminocarbonyl; pKa = 8) make both drugs zwitterionic at the pH at which they spontaneously equilibrated (i.e., pH 6.8, 7.8, and 8.2) and were therefore tested (data from registry numbers 64221-86-2 [imipenem], 96036-03-02 [meropenem], and 106560-14-9 [faropenem], SciFinder Scholar version 2001 [http://www.cas.org/SCIFINDER/SCHOLAR/index.html; Chemical Abstract Services, American Chemical Society, Washington, D.C.]).
In conclusion, we show here that the poor stability of β-lactam antibiotics in solution should not be underestimated if their use by continuous infusion is contemplated. We suggest here that each clinical situation and each drug be specifically examined not only in relation to a loss of activity but also for the release of potentially toxic compounds. It is only if investigations are conducted in an orderly and practice-oriented fashion that the clinician will be provided with specific and useful guidelines that will help him or her to safely optimize patient therapy based on sound pharmacodynamic principles.
Acknowledgments
We thank E. Sonveaux (Unité de Chimie Pharmaceutique et Radiopharmacie) and J. Marchand-Brynaert (Unité de Chimie Organique et Médicinale) for helpful discussions on β-lactam chemical stability.
This work was supported by the Fondation Roi Baudouin/Koning Boudewijn Stichting, Brussels, Belgium. H.S. is Boursier of the Belgian Fonds pour la Recherche dans l'Industrie et l'Agriculture. H.C. is Aspirant and M.-P.M.-L. is Maître de Recherches of the Belgian Fonds National de la Recherche Scientifique. We thank the drug suppliers for the kind gift of the corresponding clinical and/or investigational samples.
REFERENCES
- 1.Angus, B. J., M. D. Smith, Y. Suputtamongkol, H. Mattie, A. L. Walsh, V. Wuthiekanun, W. Chaowagul, and N. J. White. 2000. Pharmacokinetic-pharmacodynamic evaluation of ceftazidime continuous infusion versus intermittent bolus injection in septicaemic melioidosis. Br. J. Clin. Pharmacol. 50:184-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Augey, V., P. Y. Grosse, G. Albert, M. Audran, and F. Bressolle. 1996. High-performance liquid chromatographic determination of tazobactam and piperacillin in human plasma and urine. J. Chromatogr. B Biomed. Appl. 682:125-136. [DOI] [PubMed] [Google Scholar]
- 3.Benko, A. S., D. M. Cappelletty, J. A. Kruse, and M. J. Rybak. 1996. Continuous infusion versus intermittent administration of ceftazidime in critically ill patients with suspected gram-negative infections. Antimicrob. Agents Chemother. 40:691-695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boyd, D. B. 1984. Electronic structures of cephalosporins and penicillins. 15. Inductive effect of the three-position side chain in cephalosporins. J. Med. Chem. 27:63-66. [DOI] [PubMed] [Google Scholar]
- 5.Boyd, D. B. 1985. Elucidating the leaving group effect in the beta-lactam ring opening mechanism of cephalosporins. J. Organ. Chem. 50:886-888. [Google Scholar]
- 6.Craig, W. A. 1995. Antibiotic selection factors and description of a hospital-based outpatient antibiotic therapy program in the USA. Eur. J. Clin. Microbiol. Infect. Dis. 14:636-642. [DOI] [PubMed] [Google Scholar]
- 7.Craig, W. A., and S. C. Ebert. 1992. Continuous infusion of beta-lactam antibiotics. Antimicrob. Agents Chemother. 36:2577-2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Domenig, C., F. Traunmuller, S. Kozek, W. Wisser, W. Klepetko, R. Steininger, C. Spiss, and F. Thalhammer. 2001. Continuous beta-lactam antibiotic therapy in a double-lung transplanted patient with a multidrug-resistant Pseudomonas aeruginosa infection. Transplantation 71:744-745. [DOI] [PubMed] [Google Scholar]
- 9.Elkhaili, H., S. Niedergang, D. Pompei, L. Linger, D. Leveque, and F. Jehl. 1996. High-performance liquid chromatographic assay for meropenem in serum. J. Chromatogr. B Biomed. Appl. 686:19-26. [DOI] [PubMed] [Google Scholar]
- 10.Farina, A., R. Porra, V. Cotichini, and A. Doldo. 1999. Stability of reconstituted solutions of ceftazidime for injections: an HPLC and CE approach. J. Pharm. Biomed. Anal. 20:521-530. [DOI] [PubMed] [Google Scholar]
- 11.Favetta, P., C. Allombert, C. Breysse, C. Dufresne, J. Guitton, and J. Bureau. 2002. Fortum stability in different disposable infusion devices by pyridine assay. J. Pharm. Biomed. Anal. 27:873-879. [DOI] [PubMed] [Google Scholar]
- 12.Forgue, S. T., P. Kari, and R. Barbhaiya. 1987. N-oxidation of N-methylpyrrolidine released in vivo from cefepime. Drug Metab. Dispos. 15:808-815. [PubMed] [Google Scholar]
- 13.Fubara, J. O., and R. E. Notari. 1998. A kinetic oxymoron: concentration-dependent first-order rate constants for hydrolysis of ceftazidime. J. Pharm. Sci. 87:53-58. [DOI] [PubMed] [Google Scholar]
- 14.Fubara, J. O., and R. E. Notari. 1998. Influence of pH, temperature and buffers on cefepime degradation kinetics and stability predictions in aqueous solutions. J. Pharm. Sci. 87:1572-1576. [DOI] [PubMed] [Google Scholar]
- 15.Garcia-Capdevila, L., C. Lopez-Calull, C. Arroyo, M. A. Moral, M. A. Mangues, and J. Bonal. 1997. Determination of imipenem in plasma by high-performance liquid chromatography for pharmacokinetic studies in patients. J. Chromatogr. B Biomed. Sci. Appl. 692:127-132. [DOI] [PubMed] [Google Scholar]
- 16.Georges, B., M. Archambaud, S. Saivin, J. F. Decun, P. Cougot, M. Mazerolles, P. Andrieu, C. Suc, G. Houin, G. Chabanon, and C. Virenque. 1999. Perfusion continue versus administration discontinue de céfépime en réanimation: résultats préliminaires. Pathol. Biol. 47:483-485. [PubMed] [Google Scholar]
- 17.Ghuysen, J. M. 1991. Serine beta-lactamases and penicillin-binding proteins. Annu. Rev. Microbiol. 45:37-67. [DOI] [PubMed] [Google Scholar]
- 18.Grant, E. M., M. K. Zhong, P. G. Ambrose, D. P. Nicolau, C. H. Nightingale, and R. Quintiliani. 2000. Stability of meropenem in a portable infusion device in a cold pouch. Am. J. Health Syst. Pharm. 57:992-995. [DOI] [PubMed] [Google Scholar]
- 19.Jehl, F., P. Birckel, and H. Monteil. 1987. Hospital routine analysis of penicillins, third-generation cephalosporins and aztreonam by conventional and high-speed high-performance liquid chromatography. J. Chromatogr. 413:109-119. [DOI] [PubMed] [Google Scholar]
- 20.Leder, K., J. D. Turnidge, T. M. Korman, and M. L. Grayson. 1999. The clinical efficacy of continuous-infusion flucloxacillin in serious staphylococcal sepsis. J. Antimicrob. Chemother. 43:113-118. [DOI] [PubMed] [Google Scholar]
- 21.Lipman, J., C. D. Gomersall, T. Gin, G. M. Joynt, and R. J. Young. 1999. Continuous infusion ceftazidime in intensive care: a randomized controlled trial. J. Antimicrob. Chemother. 43:309-311. [DOI] [PubMed] [Google Scholar]
- 22.MacGowan, A. P., and K. E. Bowker. 1998. Continuous infusion of beta-lactam antibiotics. Clin. Pharmacokinet. 35:391-402. [DOI] [PubMed] [Google Scholar]
- 23.Mouton, J. W., and A. A. Vinks. 1996. Is continuous infusion of beta-lactam antibiotics worthwhile? Efficacy and pharmacokinetic considerations. J. Antimicrob. Chemother. 38:5-15. [DOI] [PubMed] [Google Scholar]
- 24.Nicolau, D. P., J. McNabb, M. K. Lacy, R. Quintiliani, and C. H. Nightingale. 2001. Continuous versus intermittent administration of ceftazidime in intensive care unit patients with nosocomial pneumonia. Int. J. Antimicrob. Agents 17:497-504. [DOI] [PubMed] [Google Scholar]
- 25.Page, M. I. 1992. The chemistry of beta-lactams. Blackie Academic and Professional, London, England.
- 26.Page, M. I., and P. Proctor. 1984. Mechanims of beta-lactam ring opening in cephalosporins. J. Am. Chem. Soc. 106:3820-3825. [Google Scholar]
- 27.Pilkiewicz, F. G., B. J. Remsburg, S. M. Fisher, and R. B. Sykes. 1983. High-pressure liquid chromatographic analysis of aztreonam in sera and urine. Antimicrob. Agents Chemother. 23:852-856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rabouan-Guyon, S. M., A. F. Guet, P. Y. Courtois, and D. M. C. Barthes. 1997. Stability study of cefepime in different infusion solutions. Int. J. Pharm. 154:185-190. [Google Scholar]
- 29.Rappaz, I., L. A. Decosterd, J. Bille, M. Pilet, N. Belaz, and M. Roulet. 2000. Continuous infusion of ceftazidime with a portable pump is as effective as thrice-a-day bolus in cystic fibrosis children. Eur. J. Pediatr. 159:919-925. [DOI] [PubMed] [Google Scholar]
- 30.Servais, H., and P. M. Tulkens. 2001. Stability and compatibility of ceftazidime administered by continuous infusion to intensive care patients. Antimicrob. Agents Chemother. 45:2643-2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smith, G. B., G. C. Dezeny, and A. W. Douglas. 1990. Stability and kinetics of degradation of imipenem in aqueous solution. J. Pharm. Sci. 79:732-740. [DOI] [PubMed] [Google Scholar]
- 32.Stendal, T. L., W. Klem, H. H. Tonnesen, and I. Kjonniksen. 1998. Drug stability and pyridine generation in ceftazidime injection stored in an elastomeric infusion device. Am. J. Health Syst. Pharm. 55:683-685. [DOI] [PubMed] [Google Scholar]
- 33.Strominger, J. L. 1968. Penicillin-sensitive enzymatic reactions in bacterial cell wall synthesis. Harvey Lecture 64:179-213. [PubMed] [Google Scholar]
- 34.Sugioka, T., T. Asano, Y. Chikaraishi, E. Suzuki, A. Sano, T. Kuriki, M. Shirotsuka, and K. Saito. 1990. Stability and degradation pattern of cefpirome (HR 810) in aqueous solution. Chem. Pharm. Bull. 38:1998-2002. [DOI] [PubMed] [Google Scholar]
- 35.Swanson, D. J., C. DeAngelis, I. L. Smith, and J. J. Schentag. 1986. Degradation kinetics of imipenem in normal saline and in human serum. Antimicrob. Agents Chemother. 29:936-937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Takeuchi, Y., M. Sunagawa, Y. Isobe, Y. Hamazume, and T. Noguchi. 1995. Stability of a 1β-methylcarbapenem antibiotic, meropenem (SM-7338) in aqueous solution. Chem. Pharm. Bull. 43:689-692. [DOI] [PubMed] [Google Scholar]
- 37.Thalhammer, F., F. Traunmuller, M. Frass, U. M. Hollenstein, G. J. Locker, B. Stoiser, T. Staudinger, R. Thalhammer-Scherrer, and H. Burgmann. 1999. Continuous infusion versus intermittent administration of meropenem in critically ill patients. J. Antimicrob. Chemother. 43:523-527. [DOI] [PubMed] [Google Scholar]
- 38.Turnidge, J. D. 1998. The pharmacodynamics of beta-lactams. Clin. Infect. Dis. 27:10-22. [DOI] [PubMed] [Google Scholar]
- 39.U.S. Pharmacopeial Convention. 1995. Pharmacopeia of the United States of America (the national formulary). U.S. Pharmacopeial Convention, Rockville, Md.
- 40.Vinks, A. A., R. W. Brimicombe, H. G. Heijerman, and W. Bakker. 1997. Continuous infusion of ceftazidime in cystic fibrosis patients during home treatment: clinical outcome, microbiology and pharmacokinetics. J. Antimicrob. Chemother. 40:125-133. [DOI] [PubMed] [Google Scholar]
- 41.Vondracek, T. G. 1995. Beta-lactam antibiotics: is continuous infusion the preferred method of administration? Ann. Pharmacother. 29:415-424. [DOI] [PubMed] [Google Scholar]
- 42.Zajac, M., J. Siwek, and I. Muszalska. 1998. The mechanism of ceftazidime degradation in aqueous solutions. Acta Pol. Pharm. 55:275-278. [PubMed] [Google Scholar]
- 43.Zhou, M., and R. E. Notari. 1995. Influence of pH, temperature, and buffers on the kinetics of ceftazidime degradation in aqueous solutions. J. Pharm. Sci. 84:534-538. [DOI] [PubMed] [Google Scholar]